Abstract
The natural history of cirrhosis can be divided into an initial stage, known as compensated cirrhosis, and an advanced stage which encompasses both decompensated cirrhosis and acute-on-chronic liver failure (ACLF). The latter syndrome has been recently described as an acute deterioration of liver function in patients with cirrhosis, which is usually triggered by a precipitating event and results in the failure of one or more organs and high short-term mortality rates. Each stage is characterized by distinctive clinical manifestations and prognoses. One of the key elements involved in cirrhosis physiopathology is systemic inflammation, recently described as one of the components in the cirrhosis-associated immune dysfunction syndrome. This syndrome refers to the combination of immune deficiency and exacerbated inflammation that coexist during the course of cirrhosis and relates to the appearance of clinical complications. Since systemic inflammation is often difficult to assess in cirrhosis patients, new objective, reproducible and readily-available markers are needed in order to optimize prognosis and lengthen survival. Thus, surrogate serum markers and clinical parameters of systemic inflammation have been sought to improve disease follow-up and management, especially in decompensated cirrhosis and ACLF. Leukocyte counts (evaluated as total leukocytes, total eosinophils or neutrophil:lymphocyte ratio) and plasma levels of procalcitonin or C-reactive protein have been proposed as prognostic markers, each with advantages and shortcomings. Research and prospective randomized studies that validate these and other markers are clearly warranted.
Keywords: Immune dysfunction, Cirrhosis, Acute-on-chronic liver failure, Prognosis, Systemic inflammation
Core tip: Due to the overwhelming evidence that sustains systemic inflammation influences the natural history of cirrhosis, a review of its current prognostic markers is necessary to highlight their strengths and weaknesses and stimulate further clinical research on this subject.
INTRODUCTION
Liver cirrhosis is the final phase of all progressive and chronic liver diseases. The natural history of cirrhosis occurs in stages: an initial stage termed compensated cirrhosis and an advanced stage that includes both decompensated cirrhosis and acute-on-chronic liver failure (ACLF), each aspect with distinct clinical manifestations and prognoses[1-4]. The physiopathology of cirrhosis is determined by multiple factors of varying importance, including oxidative stress, systemic inflammation, and organ dysfunction[5]. Systemic inflammation has traditionally been evaluated by the presence of the systemic inflammatory response syndrome (SIRS), a state in which clinical and biochemical parameters such as heart and respiratory rate, white cell count, and body temperature are altered. SIRS is associated with organ dysfunction in cirrhosis patients and with the outcome of ACLF[3,6]. Cirrhosis patients often exhibit systemic inflammation together with immune deficiency as part of the cirrhosis-associated immune dysfunction (CAID) syndrome[6]. Because systemic inflammation contributes to the evolution of cirrhosis, several serum markers and clinical parameters of inflammation have been evaluated as prognostic markers for the late stages of cirrhosis. In this article we outline the stages and physiopathology of cirrhosis, focusing on systemic inflammation, currently-described clinical and biochemical inflammation markers, and their potential utility as prognostic tools.
NATURAL HISTORY OF CIRRHOSIS: THE SLOW LANE AND THE SHORTCUT
If the natural history of cirrhosis is considered from a clinical point of view, the disease can be divided into sequential stages[7] of varying speeds (Figure 1). The traditional clinical classification defines an initial stage, termed compensated cirrhosis, characterized by the absence of complications such as variceal bleeding, ascites, and hepatic encephalopathy. Portal hypertension may already be present (evident by the presence of varices), though below the clinically-relevant threshold[2,8-10]. The initial stage has a low risk for decompensation (7%-10%) and death (1%-3.4%) which is associated with a lower hepatic venous pressure gradient (HPVG)[2,11]. The advanced stage of cirrhosis can be divided according to speed and severity. The “slow lane”, termed decompensated cirrhosis, is represented by the multi-step occurrence of cirrhosis-related complications[12]. Progression to decompensated cirrhosis occurs in 5%-7% of compensated cirrhosis patients per year[2]. Sub-classifications of this stage have been suggested by D’Amico et al[2], separating patients with ascites with or without esophageal varices that have never bled (associated mortality rate of 20% per year) from those who suffered gastrointestinal bleeding with or without ascites (associated mortality rate of 57% per year). The identified prognostic factors are not only associated with portal hypertension but also with liver function deterioration; thus, these factors include the Child Pugh score, the Model for End-Stage Liver Disease (MELD) score, patient age, and the HPVG. Despite the fact that hemodynamic and clinical variables are key determinants in cirrhosis-associated mortality[7], other events have been linked to poor prognoses. This is the case with bacterial infections, which increase the mortality rate four-fold independently of cirrhosis severity[13-15].
Figure 1.
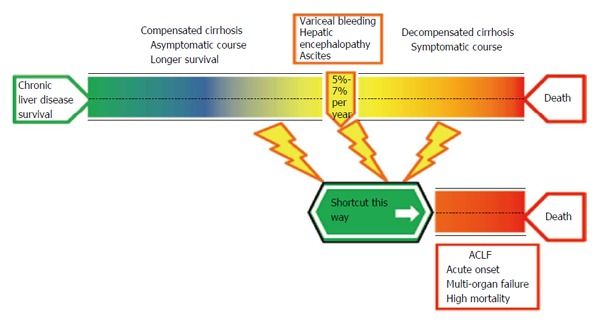
Natural history of cirrhosis. The classical compensated and decompensated phases of cirrhosis are divided by the presence of specific complications and marked by stable progression. A possible shortcut may occur after a decompensating event in any phase of cirrhosis, hastening the development of organ failure and a worse prognosis; this syndrome is termed acute-on-chronic liver failure (ACLF).
The “shortcut” in the advanced stage is represented by ACLF. This syndrome is defined as an acute deterioration of liver function in patients with cirrhosis, which is usually triggered by a precipitating event and results in the failure of one or more organs and high short-term mortality rates (up to 78% in a three-month period)[3,4,16,17]. ACLF does not always appear as a late or terminal event in cirrhosis, since it can occur in the absence of a prior history of decompensated cirrhosis or a few weeks after the first episode of decompensation. Furthermore, ACLF is not a temporally-fixed syndrome; patients may progress or improve in a dynamic fashion. The ACLF mortality risk increases remarkably with the number of organs that fail; hence, several independent prognostic scores are used to better assess mortality in these patients[4,16,18-21].
INFLAMMATION IS A KEY FACTOR IN THE PATHOGENESIS OF DECOMPENSATED CIRRHOSIS AND ACLF
Systemic inflammation and immune system dysregulation are now proposed to integrate the main physiopathological pathway involved in the natural history of cirrhosis[5,6,22,23]. The recently-described CAID syndrome refers to the combination of immune deficiency and systemic inflammation that occurs as a consequence of persistent immune cell activation through infectious and non-infectious stimuli. These two components coexist in a dynamic manner from the initial to the final stages of cirrhosis, though in different magnitudes along the way[3,6,23].
IMMUNE SYSTEM DAMAGE
The immune system alterations in cirrhosis are thought to be multifactorial and occur in a multi-step manner. The local injury takes place in the liver, where architectural disorganization caused by sinusoidal fibrosis impairs bacterial clearance[6,24]. Concomitantly, there is a diminished synthesis of innate immune system proteins and pattern recognition receptors (i.e., Toll-like receptors) that, together, reduce the bactericidal capacity of the cells of the innate immune system (e.g., stellate cells, neutrophils, natural killers, macrophages)[25,26]. As cirrhosis progresses, another key organ is affected: the gut. The gut-associated lymphoid tissue (GALT) is the first immunological barrier of defense against antigens and pathogens entering the organism from the intestine[27]. In advanced cirrhosis, the GALT is under the constant pressure of pathological bacterial translocation (BT) and bacterial products translocation that results from a leaky gut, an elevated enteric bacterial load, and changes in intestinal microbiota populations towards pathogenic species (dysbiosis)[22,28-34]. Finally, at a systemic level, immune cell function is compromised not only due to cytopenia, secondary to enlarged spleen sequestration when significant portal hypertension is present, but also affecting each cellular line individually[6,13]. In advanced cirrhosis, neutrophils have been shown to have deranged phagocytic activity of opsonized bacteria[35,36], as well as monocytes, that also exhibit impaired phagocytosis and diminished major histocompatibility complex class II protein expression when located in ascitic fluid[37]. B lymphocytes show particular dysfunctions in their memory cell subset, and T lymphocytes display specific depletions of their helper and cytotoxic subsets[38,39]. These alterations become more significant as liver cirrhosis progresses. Eventually, the long-lasting activation of immune cells causes their exhaustion and reprogramming into a transient state of unresponsiveness to further bacterial product challenge; this phenomenon is termed “endotoxin tolerance”[6,22,23,40].
SYSTEMIC INFLAMMATION: THE GUILTY PARTY
Damage to the immune system is only one half of the problem. Systemic inflammation is mediated through the activation of all innate and adaptive immune cells, resulting in an increased production of pro-inflammatory cytokines and upregulated expression of cell activation markers[13,15,41,42]. In compensated cirrhosis, ligands released from necrotic hepatocytes, known as damage-associated molecular patterns (DAMPs), may activate the immune system and cause sterile systemic inflammation. In decompensated cirrhosis, other ligands also appear. Systemic inflammation is thought to be primarily triggered by BT or bacterial products (e.g., lipopolysaccharide, methylated DNA) translocated from the intestinal lumen into the circulation. In this case, the culprits are termed pathogen-associated molecular patterns (PAMPs)[5,6,13,43]. At the decompensated stage, due to portal hypertension and the leaky gut, persistent BT further activates the immune system. In response to the continuous influx of PAMPs, the levels of pro-inflammatory cytokines and leukocyte activation antigens significantly increase[44-47]. Numerous cytokines and activation antigens are involved in this initial “pro-inflammatory” phenotype, such as tumor necrosis factor-α, interleukine-1 beta (IL-1β), IL-6, interferon-γ, IL-17, IL-18, ICAM-1, and VCAM-1. Concomitantly, the levels of anti-inflammatory cytokines (e.g., IL-10, transforming growth factor-β) are decreased[3,6,42,43,48]. In the more advanced stages of cirrhosis, the immune system is exhausted and unable to mount functional innate and adaptive immune responses, resembling an endotoxin tolerance scenario. At this point, an “immunodeficient” phenotype is observed, characterized by increased levels of anti-inflammatory cytokines and leukocyte inhibitory antigens and deteriorated immune cell function[6,23,40,49]. An extreme version of this scenario has been suggested to be the underlying mechanism in ACLF, in which an immune-paresis state similar to sepsis occurs[49].
These clinical stages may have a gradual (decompensated cirrhosis) or abrupt (ACLF) onset and a dynamic evolution[6]. The excessive activation of the immune system may contribute to the symptoms of cirrhosis because systemic inflammation and oxidative stress, modulated by glutaminase gene alterations, have been described as the underlying mechanisms for hepatic encephalopathy[50,51]. A similar scenario has been proposed for ascites, since pro-inflammatory cytokines are responsible for the local release of nitric oxide and other vasodilators; this leads to the hyperdynamic circulatory state found in decompensated cirrhosis, effective hypovolemia, activation of the renin angiotensin system, and ultimately ascites formation[5,43]. In the absence of an acute superimposed injury, these events and the progressive impairment of left ventricular function[44,52] eventually lead to circulatory and renal dysfunction. Several studies have described renal damage to be mediated specifically by pro-inflammatory cytokines, PAMPs, and DAMPs, which reduce the glomerular filtration rate and damage tubular epithelial cells[4,16,53]. In ACLF the extreme manifestations of CAID are observed. The associated prognosis is directly related to the severity of systemic inflammation and the number of organ failures[3,16-18].
CLINICAL IMPLICATIONS OF SYSTEMIC INFLAMMATION IN CIRRHOSIS: IDENTIFICATION OF PROGNOSTIC FACTORS
Due to the overwhelming evidence implicating systemic inflammation in the natural history of cirrhosis, several easily available serum markers and clinical parameters have been proposed as prognostic tools to improve follow-up and management, especially in decompensated cirrhosis and ACLF. These markers are summarized in Table 1 and described further.
Table 1.
Proposed inflammation-related prognostic markers in advanced cirrhosis
| Marker | Ref. | Prognostic implications | Study population | Limitations |
| SIRS | [52,53-55] | Portal hypertension-related complications and death | Decompensated cirrhosis patients admitted for acute decompensating events | Baseline elevated heart rate, respiratory frequency, and decreased PMN count in cirrhosis |
| Total leukocyte count | [16,48,56,57] | Development of ACLF, ACLF progression, ACLF related- mortality | ACLF | Hypersplenism possible cause of PMN count reduction, lack of clinically validated cut-off point |
| Absolute eosinophil count | [58] | Short-term mortality | Decompensated cirrhosis patients admitted for acute decompensating events | No external validation |
| Neutrophil:lymphocyte ratio | [59] | Short-term mortality | End-stage cirrhosis patients listed for liver transplant | No external validation |
| PCT | [62,63] | Infection, short-term mortality | Decompensated cirrhosis patients admitted for acute decompensating events | Lack of studies in non-infected cohorts |
| CRP | [62-68] | Infection, short-term mortality, HCC-related mortality | Decompensated cirrhosis patients admitted for acute decompensating events | Utility in organ allocation and HCC prognostic scores still to be validated |
SIRS: Systemic inflammatory response syndrome; PMN: Polymorphonuclear leukocyte; ACLF: Acute-on-chronic liver failure; PCT: Procalcitonin; CRP: C-reactive protein; HCC: Hepatocellular carcinoma.
SIRS
SIRS is defined by the presence of at least two of the following criteria: altered body temperature (> 38 °C or < 36 °C), elevated respiratory rate or hyperventilation (20 breaths/min or PaCO2 < 32 mmHg), tachycardia (heart rate > 90 beats/min), and altered leukocyte count (> 12000/mm3, < 4000/mm3, or > 10% immature forms)[42]. The presence of SIRS has been associated with worse outcomes in the setting of decompensated cirrhosis. In a study evaluating a cohort of cirrhosis patients admitted for acute renal failure, the presence of SIRS was found to be a major independent prognostic factor, independent of infection[54]. The presence of SIRS was also found to predict the development of portal hypertension-related complications and death in cirrhosis patients having an episode of acute decompensation[55]. In two similar studies with larger cohorts, SIRS was found to be an independent predictor of poor outcome[56,57]. Hence, SIRS could be considered an additional prognostic factor for the severity of liver disease. Unfortunately, this syndrome can be difficult to assess in cirrhosis patients. Hypersplenism, hyperventilation associated with encephalopathy, hyperkinetic circulatory syndrome, or the use of beta blockers may modify the clinical or biochemical parameters of SIRS[42]. New markers of SIRS that are less subject to heterogeneous findings would thus be particularly useful in cirrhosis.
LEUKOCYTE COUNT
Leukocyte count is an isolated element of SIRS frequently identified as a surrogate marker of this syndrome. In a large prospective observational study performed by the Chronic Liver Failure Consortium that aimed to describe the clinical features and prognostic factors of ACLF, leukocyte count was found to be an independent predictor of the development of ACLF, its severity, and its associated mortality[16,58]. In a large collaborative study in infected ACLF patients, leukocyte count was found to be an independent predictor of short-term mortality[59]. This finding has also been reported in ACLF patients without infectious decompensating events. In a study evaluating the relationship between portal hypertension and systemic inflammation in alcohol-related ACLF, disturbances in systemic and hepatic hemodynamics were associated with dysregulated inflammation, revealed by higher levels of leukocytes, C-reactive protein (CRP), and pro-inflammatory cytokines. These elevations, together with multi-organ failure and a marked activation of the sympathetic nervous system, were found to be predictors of higher mortality rates[51]. Leukocyte subsets and ratios have also been suggested as prognostic tools. In a study by Kotecha et al[60], that evaluated the role of absolute eosinophil count and procalcitonin (PCT) in predicting in-hospital mortality of admitted cirrhotic patients with SIRS, the baseline absolute eosinophil count of less than 104 cells/mm3 accurately predicted in-hospital mortality in critically-ill cirrhosis patients with SIRS, independent of the MELD score or serum sodium levels. In addition, the neutrophil:lymphocyte ratio was described as an independent risk factor for death in a cohort of end-stage cirrhosis patients listed for liver transplantation[61]. Despite the fact that leukocyte count has been consistently defined as a risk factor for mortality in severely-ill cirrhosis patients, there are some drawbacks to this marker. One is the lack of a cut-off point for individual patient evaluation (i.e., the specific mortality expected in an ACLF patient with a leukocyte count of 11000/mm3 is unknown), diminishing its utility in everyday practice; such a cut-off point has only been determined for eosinophil count, without further external validation. In addition, the majority of these studies were conducted completely or partially in infected or alcohol-related ACLF patients, two etiologies associated with higher leukocyte counts per se, with only subgroup results available for the uninfected ACLF cohorts.
CRP AND PROCALCITONIN
Both serum proteins are tightly associated with SIRS. CRP, the prototype human acute phase protein, is a well-known marker of inflammation and is one of the most frequently-quantified molecules in clinical medicine[62]. CRP is synthesized mainly in the liver. CRP and PCT, a prohormone used as a marker of bacterial infections, is produced by most parenchymal tissues throughout the body during the acute phase of infection by these microorganisms[63]. Assays for CRP and PCT are readily-available, inexpensive, and more accurate than clinical parameters of SIRS for the identification of systemic inflammation. Both proteins have been evaluated as prognostic markers for short-term mortality in cirrhosis patients, usually in the context of infection[64,65]. However, CRP has also been suggested to be a useful tool independent of infection. In the prospective study by Cervoni et al[66] where the utility of CRP as a mortality risk factor in cirrhosis inpatients was evaluated, CRP levels ≥ 29 mg/L were found to be independent predictors of short-term mortality in cirrhosis patients with Child-Pugh scores ≥ B8, independent of age, MELD score, and co-morbidities; in this regard, CRP performed better than the presence of infection or SIRS. Di Martino et al[67] included CRP variation over 15 d as an additional element in the MELD score to better assess short-term mortality in decompensated cirrhosis patients. The inclusion of CRP improved MELD score accuracy in severely-ill cirrhosis patients admitted for acute decompensating events, but not in cirrhosis patients with planned admissions due to endoscopic procedures, etc[67]. Although CRP may be a useful addition to the MELD score in the setting of decompensated cirrhosis, several factors (e.g., the usage of different CRP cut-off values according to the severity of cirrhosis and the need for two measurements of CRP in samples obtained 15 d apart) have reduced the utility of using CRP in organ allocation[68].
The use of CRP as a surrogate marker of survival has been studied in the setting of hepatocellular carcinoma (HCC). The presence of CRP levels > 6.3 mg/L, together with a neutrophil:lymphocyte ratio > 2.3, was identified as an independent risk factor for lower survival in HCC patients[69]. Similar findings were attained when CRP levels were compared to the levels of serum albumin: a CRP:albumin ratio of ≥ 0.037 was found to be an independent survival factor in HCC patients and correlated with tumor progression and reduced liver functional reserve[70]. Furthermore, it has been proposed that the addition of CRP to the currently-validated staging systems for HCC (e.g., the Cancer Liver Italian Program, Japan Integrated Staging, Barcelona Clinic Liver Cancer classification system, Tokyo score, and tumor node metastasis classification) could improve their prognostic abilities[71].
FUTURE DIRECTIONS
The crucial role of systemic inflammation in the pathophysiology and prognosis of cirrhosis patients has been thoroughly described. Since SIRS is often difficult to assess in cirrhosis patients, new objective, reproducible and readily-available surrogate markers are needed in order to optimize prognosis and lengthen survival. Leukocyte count, neutrophil:lymphocyte ratio, and absolute eosinophil count have been proposed, though with no clear cut-off points or extensive validation so far. PCT has also been suggested, yet its utility appears to apply exclusively to infected patients. CRP is useful as a prognostic marker in decompensated cirrhosis patients and ACLF despite the presence of infection, as well as in HCC. However, the value of adding CRP to current prognostic scores remains to be confirmed. Further basic research and prospective randomized studies that validate these and other markers are clearly warranted.
Footnotes
P- Reviewer: Dos Santos JL, Gong ZJ, Higuera-de la Tijera F, Zhu X S- Editor: Song XX L- Editor: A E- Editor: Liu SQ
Conflict-of-interest statement: None of the authors have received fees for serving as a speaker or consultant, nor have they received financial or non-financial support related to this manuscript. The authors have no commercial, personal, political, intellectual, or religious interests to declare.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 16, 2015
First decision: June 3, 2015
Article in press: July 2, 2015
References
- 1.Asrani SK, Kamath PS. Natural history of cirrhosis. Curr Gastroenterol Rep. 2013;15:308. doi: 10.1007/s11894-012-0308-y. [DOI] [PubMed] [Google Scholar]
- 2.D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 3.Jalan R, Gines P, Olson JC, Mookerjee RP, Moreau R, Garcia-Tsao G, Arroyo V, Kamath PS. Acute-on chronic liver failure. J Hepatol. 2012;57:1336–1348. doi: 10.1016/j.jhep.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 4.Moreau R, Arroyo V. Acute-on-chronic liver failure: a new clinical entity. Clin Gastroenterol Hepatol. 2015;13:836–841. doi: 10.1016/j.cgh.2014.02.027. [DOI] [PubMed] [Google Scholar]
- 5.Arroyo V, García-Martinez R, Salvatella X. Human serum albumin, systemic inflammation, and cirrhosis. J Hepatol. 2014;61:396–407. doi: 10.1016/j.jhep.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385–1396. doi: 10.1016/j.jhep.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Tsao G, Friedman S, Iredale J, Pinzani M. Now there are many (stages) where before there was one: In search of a pathophysiological classification of cirrhosis. Hepatology. 2010;51:1445–1449. doi: 10.1002/hep.23478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albilllos A, Garcia-Tsao G. Classification of cirrhosis: the clinical use of HVPG measurements. Dis Markers. 2011;31:121–128. doi: 10.3233/DMA-2011-0834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Franchis R, Primignani M. Natural history of portal hypertension in patients with cirrhosis. Clin Liver Dis. 2001;5:645–663. doi: 10.1016/s1089-3261(05)70186-0. [DOI] [PubMed] [Google Scholar]
- 10.European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397–417. doi: 10.1016/j.jhep.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Ripoll C, Groszmann R, Garcia-Tsao G, Grace N, Burroughs A, Planas R, Escorsell A, Garcia-Pagan JC, Makuch R, Patch D, et al. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133:481–488. doi: 10.1053/j.gastro.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 12.Dienstag JL, Ghany MG, Morgan TR, Di Bisceglie AM, Bonkovsky HL, Kim HY, Seeff LB, Szabo G, Wright EC, Sterling RK, et al. A prospective study of the rate of progression in compensated, histologically advanced chronic hepatitis C. Hepatology. 2011;54:396–405. doi: 10.1002/hep.24370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruns T, Zimmermann HW, Stallmach A. Risk factors and outcome of bacterial infections in cirrhosis. World J Gastroenterol. 2014;20:2542–2554. doi: 10.3748/wjg.v20.i10.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arvaniti V, D’Amico G, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, Burroughs AK. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139:1246–1256, 1256.e1-e5. doi: 10.1053/j.gastro.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 15.Tandon P, Garcia-Tsao G. Bacterial infections, sepsis, and multiorgan failure in cirrhosis. Semin Liver Dis. 2008;28:26–42. doi: 10.1055/s-2008-1040319. [DOI] [PubMed] [Google Scholar]
- 16.Arroyo V, Moreau R, Jalan R, Ginès P. Acute-on-chronic liver failure: A new syndrome that will re-classify cirrhosis. J Hepatol. 2015;62:S131–S143. doi: 10.1016/j.jhep.2014.11.045. [DOI] [PubMed] [Google Scholar]
- 17.Asrani SK, O’Leary JG. Acute-on-chronic liver failure. Clin Liver Dis. 2014;18:561–574. doi: 10.1016/j.cld.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jalan R, Pavesi M, Saliba F, Amorós A, Fernandez J, Holland-Fischer P, Sawhney R, Mookerjee R, Caraceni P, Moreau R, et al. The CLIF Consortium Acute Decompensation score (CLIF-C ADs) for prognosis of hospitalised cirrhotic patients without acute-on-chronic liver failure. J Hepatol. 2015;62:831–840. doi: 10.1016/j.jhep.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 19.Planas R, Ballesté B, Alvarez MA, Rivera M, Montoliu S, Galeras JA, Santos J, Coll S, Morillas RM, Solà R. Natural history of decompensated hepatitis C virus-related cirrhosis. A study of 200 patients. J Hepatol. 2004;40:823–830. doi: 10.1016/j.jhep.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Yi ZQ, Lu MH, Xu XW, Fu XY, Tan DM. A novel prognostic score for acute-on-chronic hepatitis B liver failure. J Huazhong Univ Sci Technolog Med Sci. 2015;35:87–92. doi: 10.1007/s11596-015-1394-5. [DOI] [PubMed] [Google Scholar]
- 21.Shi Y, Yang Y, Hu Y, Wu W, Yang Q, Zheng M, Zhang S, Xu Z, Wu Y, Yan H, et al. Acute-on-chronic liver failure precipitated by hepatic injury is distinct from that precipitated by extrahepatic insults. Hepatology. 2015;62:232–242. doi: 10.1002/hep.27795. [DOI] [PubMed] [Google Scholar]
- 22.Giannelli V, Di Gregorio V, Iebba V, Giusto M, Schippa S, Merli M, Thalheimer U. Microbiota and the gut-liver axis: bacterial translocation, inflammation and infection in cirrhosis. World J Gastroenterol. 2014;20:16795–16810. doi: 10.3748/wjg.v20.i45.16795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sipeki N, Antal-Szalmas P, Lakatos PL, Papp M. Immune dysfunction in cirrhosis. World J Gastroenterol. 2014;20:2564–2577. doi: 10.3748/wjg.v20.i10.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selmi C, Mackay IR, Gershwin ME. The immunological milieu of the liver. Semin Liver Dis. 2007;27:129–139. doi: 10.1055/s-2007-979466. [DOI] [PubMed] [Google Scholar]
- 25.Aoyama T, Paik YH, Seki E. Toll-like receptor signaling and liver fibrosis. Gastroenterol Res Pract. 2010;2010:pii: 192543. doi: 10.1155/2010/192543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamoto N, Kanai T. Role of toll-like receptors in immune activation and tolerance in the liver. Front Immunol. 2014;5:221. doi: 10.3389/fimmu.2014.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wiest R, Lawson M, Geuking M. Pathological bacterial translocation in liver cirrhosis. J Hepatol. 2014;60:197–209. doi: 10.1016/j.jhep.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 28.Muñoz L, José Borrero M, Ubeda M, Lario M, Díaz D, Francés R, Monserrat J, Pastor O, Aguado-Fraile E, Such J, et al. Interaction between intestinal dendritic cells and bacteria translocated from the gut in rats with cirrhosis. Hepatology. 2012;56:1861–1869. doi: 10.1002/hep.25854. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Tsao G, Lee FY, Barden GE, Cartun R, West AB. Bacterial translocation to mesenteric lymph nodes is increased in cirrhotic rats with ascites. Gastroenterology. 1995;108:1835–1841. doi: 10.1016/0016-5085(95)90147-7. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Tsao G, Wiest R. Gut microflora in the pathogenesis of the complications of cirrhosis. Best Pract Res Clin Gastroenterol. 2004;18:353–372. doi: 10.1016/j.bpg.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Knolle PA, Gerken G. Local control of the immune response in the liver. Immunol Rev. 2000;174:21–34. doi: 10.1034/j.1600-0528.2002.017408.x. [DOI] [PubMed] [Google Scholar]
- 32.Shawcross DL. Is it time to target gut dysbiosis and immune dysfunction in the therapy of hepatic encephalopathy? Expert Rev Gastroenterol Hepatol. 2015;9:539–542. doi: 10.1586/17474124.2015.1035257. [DOI] [PubMed] [Google Scholar]
- 33.Wiest R, Das S, Cadelina G, Garcia-Tsao G, Milstien S, Groszmann RJ. Bacterial translocation in cirrhotic rats stimulates eNOS-derived NO production and impairs mesenteric vascular contractility. J Clin Invest. 1999;104:1223–1233. doi: 10.1172/JCI7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wiest R, Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology. 2005;41:422–433. doi: 10.1002/hep.20632. [DOI] [PubMed] [Google Scholar]
- 35.Tritto G, Bechlis Z, Stadlbauer V, Davies N, Francés R, Shah N, Mookerjee RP, Such J, Jalan R. Evidence of neutrophil functional defect despite inflammation in stable cirrhosis. J Hepatol. 2011;55:574–581. doi: 10.1016/j.jhep.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 36.Mookerjee RP, Stadlbauer V, Lidder S, Wright GA, Hodges SJ, Davies NA, Jalan R. Neutrophil dysfunction in alcoholic hepatitis superimposed on cirrhosis is reversible and predicts the outcome. Hepatology. 2007;46:831–840. doi: 10.1002/hep.21737. [DOI] [PubMed] [Google Scholar]
- 37.Fagan KJ, Rogers GB, Melino M, Arthur DM, Costello ME, Morrison M, Powell EE, Irvine KM. Ascites bacterial burden and immune cell profile are associated with poor clinical outcomes in the absence of overt infection. PLoS One. 2015;10:e0120642. doi: 10.1371/journal.pone.0120642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernsmeier C, Pop OT, Singanayagam A, Triantafyllou E, Patel VC, Weston CJ, Curbishley S, Sadiq F, Vergis N, Khamri W, et al. Patients with acute-on-chronic liver failure have increased numbers of regulatory immune cells expressing the receptor tyrosine kinase MERTK. Gastroenterology. 2015;148:603–615.e14. doi: 10.1053/j.gastro.2014.11.045. [DOI] [PubMed] [Google Scholar]
- 39.Yee RM, Lehil MS, Rongey C, Shen H, Cozen ML, Monto A, Ryan JC. Impaired lymphocyte reactivity measured by immune function testing in untransplanted patients with cirrhosis. Clin Vaccine Immunol. 2013;20:526–529. doi: 10.1128/CVI.00595-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tiegs G, Lohse AW. Immune tolerance: what is unique about the liver. J Autoimmun. 2010;34:1–6. doi: 10.1016/j.jaut.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 41.Leber B, Spindelboeck W, Stadlbauer V. Infectious complications of acute and chronic liver disease. Semin Respir Crit Care Med. 2012;33:80–95. doi: 10.1055/s-0032-1301737. [DOI] [PubMed] [Google Scholar]
- 42.Wong F, Bernardi M, Balk R, Christman B, Moreau R, Garcia-Tsao G, Patch D, Soriano G, Hoefs J, Navasa M. Sepsis in cirrhosis: report on the 7th meeting of the International Ascites Club. Gut. 2005;54:718–725. doi: 10.1136/gut.2004.038679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jalan R, Fernandez J, Wiest R, Schnabl B, Moreau R, Angeli P, Stadlbauer V, Gustot T, Bernardi M, Canton R, et al. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol. 2014;60:1310–1324. doi: 10.1016/j.jhep.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 44.Fukui H. Gut-liver axis in liver cirrhosis: How to manage leaky gut and endotoxemia. World J Hepatol. 2015;7:425–442. doi: 10.4254/wjh.v7.i3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ilan Y. Leaky gut and the liver: a role for bacterial translocation in nonalcoholic steatohepatitis. World J Gastroenterol. 2012;18:2609–2618. doi: 10.3748/wjg.v18.i21.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lutz P, Nischalke HD, Strassburg CP, Spengler U. Spontaneous bacterial peritonitis: The clinical challenge of a leaky gut and a cirrhotic liver. World J Hepatol. 2015;7:304–314. doi: 10.4254/wjh.v7.i3.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seo YS, Shah VH. The role of gut-liver axis in the pathogenesis of liver cirrhosis and portal hypertension. Clin Mol Hepatol. 2012;18:337–346. doi: 10.3350/cmh.2012.18.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Attar BM, Moore CM, George M, Ion-Nedelcu N, Turbay R, Zachariah A, Ramadori G, Fareed J, Van Thiel DH. Procalcitonin, and cytokines document a dynamic inflammatory state in non-infected cirrhotic patients with ascites. World J Gastroenterol. 2014;20:2374–2382. doi: 10.3748/wjg.v20.i9.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wasmuth HE, Kunz D, Yagmur E, Timmer-Stranghöner A, Vidacek D, Siewert E, Bach J, Geier A, Purucker EA, Gressner AM, et al. Patients with acute on chronic liver failure display “sepsis-like” immune paralysis. J Hepatol. 2005;42:195–201. doi: 10.1016/j.jhep.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 50.Romero-Gómez M, Montagnese S, Jalan R. Hepatic encephalopathy in patients with acute decompensation of cirrhosis and acute-on-chronic liver failure. J Hepatol. 2015;62:437–447. doi: 10.1016/j.jhep.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 51.Mehta G, Mookerjee RP, Sharma V, Jalan R. Systemic inflammation is associated with increased intrahepatic resistance and mortality in alcohol-related acute-on-chronic liver failure. Liver Int. 2015;35:724–734. doi: 10.1111/liv.12559. [DOI] [PubMed] [Google Scholar]
- 52.Karagiannakis DS, Vlachogiannakos J, Anastasiadis G, Vafiadis-Zouboulis I, Ladas SD. Frequency and severity of cirrhotic cardiomyopathy and its possible relationship with bacterial endotoxemia. Dig Dis Sci. 2013;58:3029–3036. doi: 10.1007/s10620-013-2693-y. [DOI] [PubMed] [Google Scholar]
- 53.Shah N, Dhar D, El Zahraa Mohammed F, Habtesion A, Davies NA, Jover-Cobos M, Macnaughtan J, Sharma V, Olde Damink SW, Mookerjee RP, et al. Prevention of acute kidney injury in a rodent model of cirrhosis following selective gut decontamination is associated with reduced renal TLR4 expression. J Hepatol. 2012;56:1047–1053. doi: 10.1016/j.jhep.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 54.Thabut D, Massard J, Gangloff A, Carbonell N, Francoz C, Nguyen-Khac E, Duhamel C, Lebrec D, Poynard T, Moreau R. Model for end-stage liver disease score and systemic inflammatory response are major prognostic factors in patients with cirrhosis and acute functional renal failure. Hepatology. 2007;46:1872–1882. doi: 10.1002/hep.21920. [DOI] [PubMed] [Google Scholar]
- 55.Cazzaniga M, Dionigi E, Gobbo G, Fioretti A, Monti V, Salerno F. The systemic inflammatory response syndrome in cirrhotic patients: relationship with their in-hospital outcome. J Hepatol. 2009;51:475–482. doi: 10.1016/j.jhep.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 56.Abdel-Khalek EE, El-Fakhry A, Helaly M, Hamed M, Elbaz O. Systemic inflammatory response syndrome in patients with liver cirrhosis. Arab J Gastroenterol. 2011;12:173–177. doi: 10.1016/j.ajg.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 57.Behroozian R, Bayazidchi M, Rasooli J. Systemic Inflammatory Response Syndrome and MELD Score in Hospital Outcome of Patients with Liver Cirrhosis. Middle East J Dig Dis. 2012;4:168–172. [PMC free article] [PubMed] [Google Scholar]
- 58.Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426–1437, 1437.e1-e9. doi: 10.1053/j.gastro.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 59.Bajaj JS, O’Leary JG, Reddy KR, Wong F, Biggins SW, Patton H, Fallon MB, Garcia-Tsao G, Maliakkal B, Malik R, et al. Survival in infection-related acute-on-chronic liver failure is defined by extrahepatic organ failures. Hepatology. 2014;60:250–256. doi: 10.1002/hep.27077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kotecha HL, Arora A, Chawlani R, Toshniwal J, Bansal N, Tyagi P, Sharma P, Kumar M, Kumar A. Low eosinophil count predicts in-hospital mortality in cirrhosis with systemic inflammatory response syndrome. Eur J Gastroenterol Hepatol. 2013;25:676–682. doi: 10.1097/MEG.0b013e32835eb8f7. [DOI] [PubMed] [Google Scholar]
- 61.Leithead JA, Rajoriya N, Gunson BK, Ferguson JW. Neutrophil-to-lymphocyte ratio predicts mortality in patients listed for liver transplantation. Liver Int. 2015;35:502–509. doi: 10.1111/liv.12688. [DOI] [PubMed] [Google Scholar]
- 62.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 63.Rahimkhani M, Einollahi N, Khavari Daneshvar H, Dashti N. Survey of serum procalcitonin in cirrhotic patients. Acta Med Iran. 2013;51:153–156. [PubMed] [Google Scholar]
- 64.Lazzarotto C, Ronsoni MF, Fayad L, Nogueira CL, Bazzo ML, Narciso-Schiavon JL, de Lucca Schiavon L, Dantas-Corrêa EB. Acute phase proteins for the diagnosis of bacterial infection and prediction of mortality in acute complications of cirrhosis. Ann Hepatol. 2013;12:599–607. [PubMed] [Google Scholar]
- 65.Huang WP, Jiang WQ, Hu B, Ye H, Zeng HK. [Significance of serum procalcitonin levels in the evaluation of severity and prognosis of patients with systemic inflammatory response syndrome] Zhongguo Weizhongbing Jijiu Yixue. 2012;24:294–297. [PubMed] [Google Scholar]
- 66.Cervoni JP, Thévenot T, Weil D, Muel E, Barbot O, Sheppard F, Monnet E, Di Martino V. C-reactive protein predicts short-term mortality in patients with cirrhosis. J Hepatol. 2012;56:1299–1304. doi: 10.1016/j.jhep.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 67.Di Martino V, Coutris C, Cervoni JP, Dritsas S, Weil D, Richou C, Vanlemmens C, Thevenot T. Prognostic value of C-reactive protein levels in patients with cirrhosis. Liver Transpl. 2015;21:753–760. doi: 10.1002/lt.24088. [DOI] [PubMed] [Google Scholar]
- 68.Ruf AE, Villamil FG. C-reactive protein and model for end-stage liver disease score: Have we found the fifth element? Liver Transpl. 2015;21:713–715. doi: 10.1002/lt.24140. [DOI] [PubMed] [Google Scholar]
- 69.Oh BS, Jang JW, Kwon JH, You CR, Chung KW, Kay CS, Jung HS, Lee S. Prognostic value of C-reactive protein and neutrophil-to-lymphocyte ratio in patients with hepatocellular carcinoma. BMC Cancer. 2013;13:78. doi: 10.1186/1471-2407-13-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kinoshita A, Onoda H, Imai N, Iwaku A, Oishi M, Tanaka K, Fushiya N, Koike K, Nishino H, Matsushima M. The C-reactive protein/albumin ratio, a novel inflammation-based prognostic score, predicts outcomes in patients with hepatocellular carcinoma. Ann Surg Oncol. 2015;22:803–810. doi: 10.1245/s10434-014-4048-0. [DOI] [PubMed] [Google Scholar]
- 71.Kinoshita A, Onoda H, Imai N, Iwaku A, Oishi M, Tanaka K, Fushiya N, Koike K, Nishino H, Matsushima M, et al. The addition of C-reactive protein to validated staging systems improves their prognostic ability in patients with hepatocellular carcinoma. Oncology. 2014;86:308–317. doi: 10.1159/000360704. [DOI] [PubMed] [Google Scholar]


