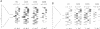Abstract
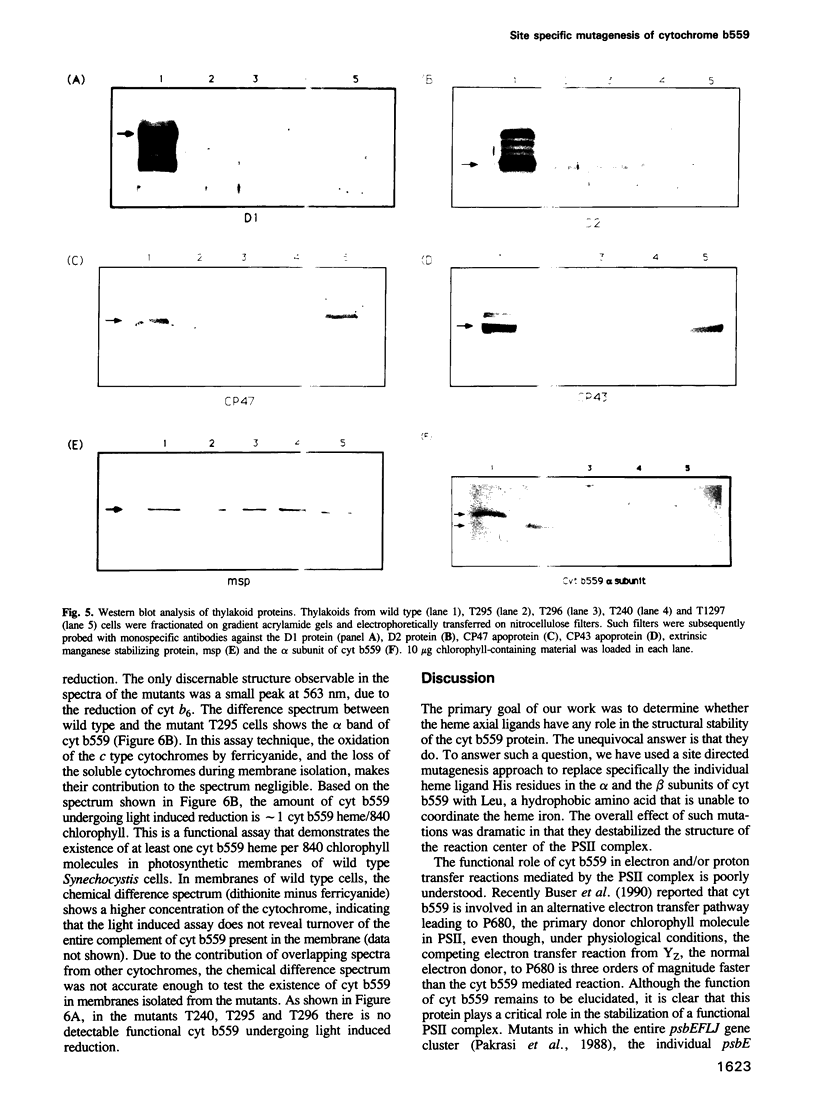
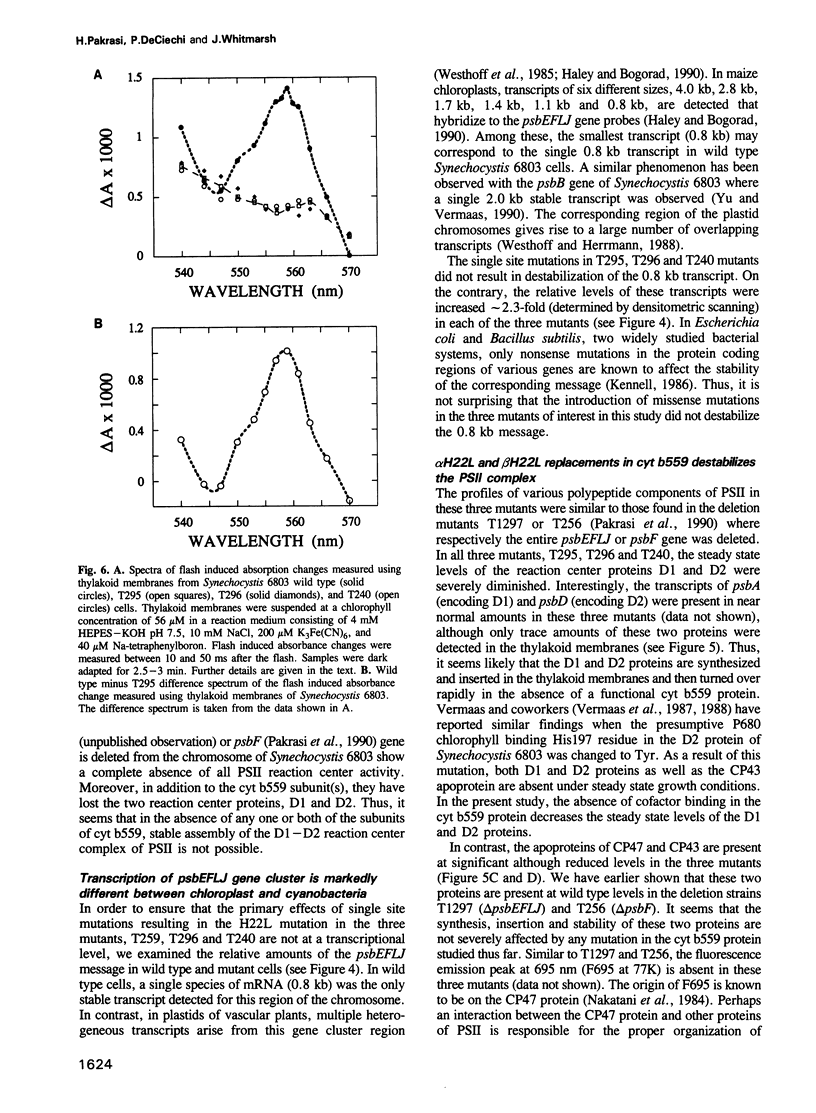
Cytochrome (cyt) b559, an integral membrane protein, is an essential component of the photosystem II (PSII) complex in the thylakoid membranes of oxygenic photosynthetic organisms. Cyt b559 has two subunits, alpha and beta, each with one predicted membrane spanning alpha-helical domain. The heme cofactor of this cytochrome is coordinated between two histidine residues. Each of the two subunit polypeptides of cyt b559 has one His residue. To investigate the influence of these His residues on the structure of cyt b559 and the PSII complex, we used a site directed mutagenesis approach to replace each His residue with a Leu residue. Introduction of these missense mutations in the transformable unicellular cyanobacterium, Synechocystis 6803, resulted in complete loss of PSII activity. Northern blot analysis showed that these mutations did not affect the stability of the polycistronic mRNA that encompasses both the psbE and the psbF genes, encoding the alpha and the beta subunits, respectively. Moreover, both of the single His mutants showed the presence of the alpha subunit which was 1.5 kd smaller than the same polypeptide in wild type cells. A secondary effect of such a structural change was that D1 and D2, two proteins that form the catalytic core (reaction center) of PSII, were also destabilized. Our results demonstrate that proper axial coordination of the heme cofactor in cyt b559 is important for the structural integrity of the reaction center of PSII.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babcock G. T., Widger W. R., Cramer W. A., Oertling W. A., Metz J. G. Axial ligands of chloroplast cytochrome b-559: identification and requirement for a heme-cross-linked polypeptide structure. Biochemistry. 1985 Jul 2;24(14):3638–3645. doi: 10.1021/bi00335a036. [DOI] [PubMed] [Google Scholar]
- Burnap R. L., Sherman L. A. Deletion mutagenesis in Synechocystis sp. PCC6803 indicates that the Mn-stabilizing protein of photosystem II is not essential for O2 evolution. Biochemistry. 1991 Jan 15;30(2):440–446. doi: 10.1021/bi00216a020. [DOI] [PubMed] [Google Scholar]
- Buser C. A., Thompson L. K., Diner B. A., Brudvig G. W. Electron-transfer reactions in manganese-depleted photosystem II. Biochemistry. 1990 Sep 25;29(38):8977–8985. doi: 10.1021/bi00490a014. [DOI] [PubMed] [Google Scholar]
- Falkenberg F. W., Mai U., Puppe C., Afifi M. S., Risse P., Grimm V., Harmann B., Herrmann G., Mondorf A. W. Urinary kidney-derived antigens determined by tests built on monoclonal antibodies: new markers for kidney damage. Uremia Invest. 1985;9(2):103–110. doi: 10.3109/08860228509088197. [DOI] [PubMed] [Google Scholar]
- Fang H., Lin R. J., Gennis R. B. Location of heme axial ligands in the cytochrome d terminal oxidase complex of Escherichia coli determined by site-directed mutagenesis. J Biol Chem. 1989 May 15;264(14):8026–8032. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fridén H., Hederstedt L. Role of His residues in Bacillus subtilis cytochrome b558 for haem binding and assembly of succinate: quinone oxidoreductase (complex II). Mol Microbiol. 1990 Jun;4(6):1045–1056. doi: 10.1111/j.1365-2958.1990.tb00677.x. [DOI] [PubMed] [Google Scholar]
- Gyllensten U. B., Erlich H. A. Generation of single-stranded DNA by the polymerase chain reaction and its application to direct sequencing of the HLA-DQA locus. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7652–7656. doi: 10.1073/pnas.85.20.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley J., Bogorad L. Alternative promoters are used for genes within maize chloroplast polycistronic transcription units. Plant Cell. 1990 Apr;2(4):323–333. doi: 10.1105/tpc.2.4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer B., Kramer W., Fritz H. J. Different base/base mismatches are corrected with different efficiencies by the methyl-directed DNA mismatch-repair system of E. coli. Cell. 1984 Oct;38(3):879–887. doi: 10.1016/0092-8674(84)90283-6. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Labarre J., Chauvat F., Thuriaux P. Insertional mutagenesis by random cloning of antibiotic resistance genes into the genome of the cyanobacterium Synechocystis strain PCC 6803. J Bacteriol. 1989 Jun;171(6):3449–3457. doi: 10.1128/jb.171.6.3449-3457.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanba O., Satoh K. Isolation of a photosystem II reaction center consisting of D-1 and D-2 polypeptides and cytochrome b-559. Proc Natl Acad Sci U S A. 1987 Jan;84(1):109–112. doi: 10.1073/pnas.84.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson F., Andersson B., Jansson C. Photosystem II characteristics of a constructed Synechocystis 6803 mutant lacking synthesis of the D1 polypeptide. Plant Mol Biol. 1990 Jun;14(6):1051–1054. doi: 10.1007/BF00019402. [DOI] [PubMed] [Google Scholar]
- Pakrasi H. B., Diner B. A., Williams JGK., Arntzen C. J. Deletion Mutagenesis of the Cytochrome b559 Protein Inactivates the Reaction Center of Photosystem II. Plant Cell. 1989 Jun;1(6):591–597. doi: 10.1105/tpc.1.6.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakrasi H. B., Nyhus K. J., Granok H. Targeted deletion mutagenesis of the beta subunit of cytochrome b559 protein destabilizes the reaction center of photosystem II. Z Naturforsch C. 1990 May;45(5):423–429. doi: 10.1515/znc-1990-0519. [DOI] [PubMed] [Google Scholar]
- Pakrasi H. B., Williams J. G., Arntzen C. J. Targeted mutagenesis of the psbE and psbF genes blocks photosynthetic electron transport: evidence for a functional role of cytochrome b559 in photosystem II. EMBO J. 1988 Feb;7(2):325–332. doi: 10.1002/j.1460-2075.1988.tb02816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki P., Krisch H. M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984 Sep;29(3):303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Reddy K. J., Webb R., Sherman L. A. Bacterial RNA isolation with one hour centrifugation in a table-top ultracentrifuge. Biotechniques. 1990 Mar;8(3):250–251. [PubMed] [Google Scholar]
- Tae G. S., Black M. T., Cramer W. A., Vallon O., Bogorad L. Thylakoid membrane protein topography: transmembrane orientation of the chloroplast cytochrome b-559 psbE gene product. Biochemistry. 1988 Dec 27;27(26):9075–9080. doi: 10.1021/bi00426a002. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Westhoff P., Herrmann R. G. Complex RNA maturation in chloroplasts. The psbB operon from spinach. Eur J Biochem. 1988 Feb 1;171(3):551–564. doi: 10.1111/j.1432-1033.1988.tb13824.x. [DOI] [PubMed] [Google Scholar]
- Whitmarsh J., Ort D. R. Stoichiometries of electron transport complexes in spinach chloroplasts. Arch Biochem Biophys. 1984 Jun;231(2):378–389. doi: 10.1016/0003-9861(84)90401-6. [DOI] [PubMed] [Google Scholar]
- Yarger J. G., Armilei G., Gorman M. C. Transcription terminator-like element within a Saccharomyces cerevisiae promoter region. Mol Cell Biol. 1986 Apr;6(4):1095–1101. doi: 10.1128/mcb.6.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Vermaas WFJ. Transcript Levels and Synthesis of Photosystem II Components in Cyanobacterial Mutants with Inactivated Photosystem II Genes. Plant Cell. 1990 Apr;2(4):315–322. doi: 10.1105/tpc.2.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]