Abstract
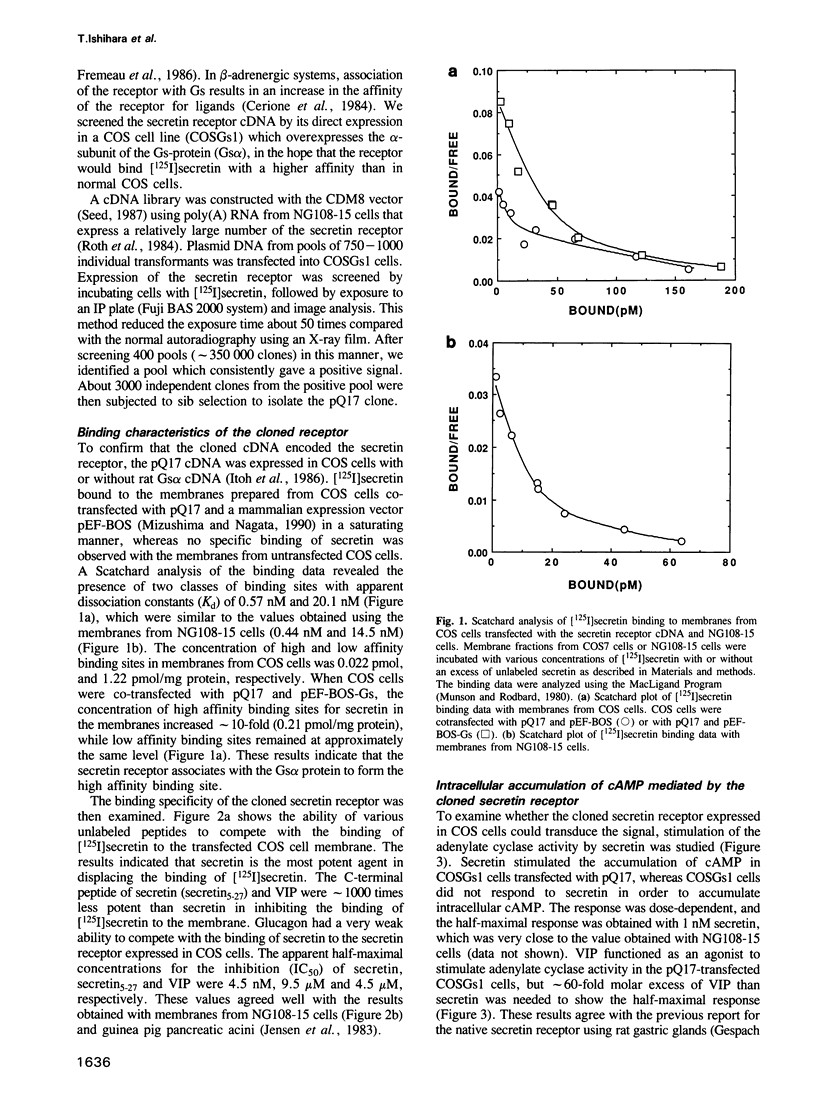
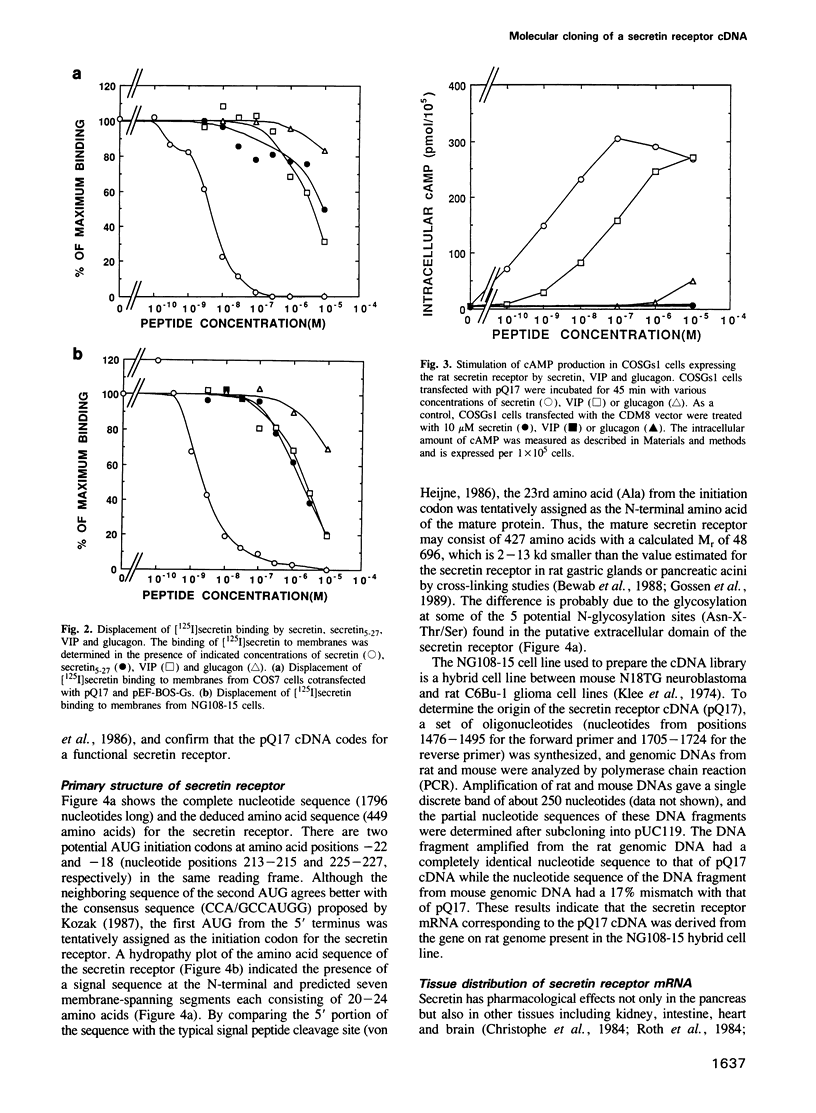
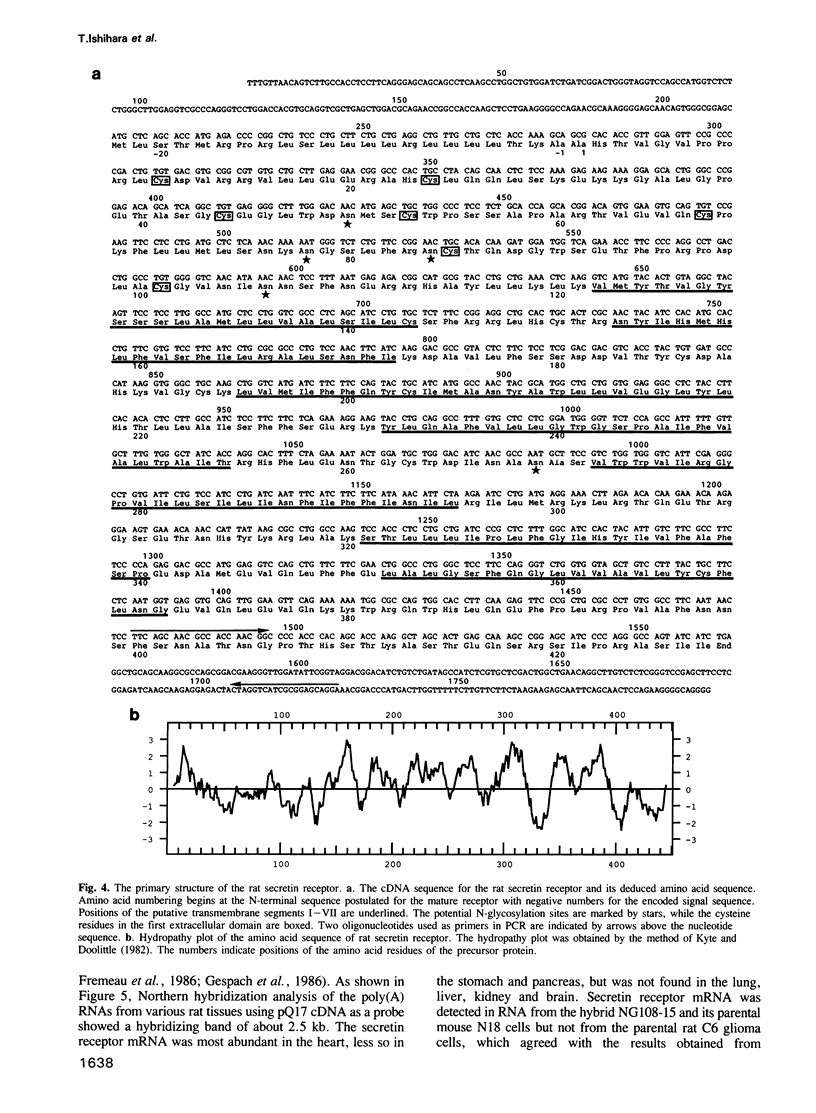
Secretin is a 27 amino acid peptide which stimulates the secretion of bicarbonate, enzymes and potassium ion from the pancreas. A complementary DNA encoding the rat secretin receptor was isolated from a CDM8 expression library of NG108-15 cell line. The secretin receptor expressed in COS cells could specifically bind the iodinated secretin with high and low affinities. Co-expression of the secretin receptor with the alpha-subunit of rat Gs protein increased the concentration of the high affinity receptor in the membrane fraction of the transfected COS cells. Secretin could stimulate accumulation of cAMP in COS cells expressing the cloned secretin receptor. The nucleotide sequence analysis of the cDNA has revealed that the secretin receptor consists of 449 amino acids with a calculated Mr of 48,696. The secretin receptor contains seven putative transmembrane segments, and belongs to a family of the G protein-coupled receptor. However, the amino acid sequence of the secretin receptor has no significant similarity with that of other G protein-coupled receptors. A 2.5 kb mRNA coding for the secretin receptor could be detected in NG108-15 cells, and rat heart, stomach and pancreatic tissue.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai H., Hori S., Aramori I., Ohkubo H., Nakanishi S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1990 Dec 20;348(6303):730–732. doi: 10.1038/348730a0. [DOI] [PubMed] [Google Scholar]
- Bawab W., Gespach C., Marie J. C., Chastre E., Rosselin G. Pharmacology and molecular identification of secretin receptors in rat gastric glands. Life Sci. 1988;42(7):791–798. doi: 10.1016/0024-3205(88)90652-2. [DOI] [PubMed] [Google Scholar]
- Bayliss W. M., Starling E. H. The mechanism of pancreatic secretion. J Physiol. 1902 Sep 12;28(5):325–353. doi: 10.1113/jphysiol.1902.sp000920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonnette B. M., Collen M. J., Adachi H., Jensen R. T., Gardner J. D. Receptors for vasoactive intestinal peptide and secretin on rat pancreatic acini. Am J Physiol. 1984 Jun;246(6 Pt 1):G710–G717. doi: 10.1152/ajpgi.1984.246.6.G710. [DOI] [PubMed] [Google Scholar]
- Cerione R. A., Codina J., Benovic J. L., Lefkowitz R. J., Birnbaumer L., Caron M. G. The mammalian beta 2-adrenergic receptor: reconstitution of functional interactions between pure receptor and pure stimulatory nucleotide binding protein of the adenylate cyclase system. Biochemistry. 1984 Sep 25;23(20):4519–4525. doi: 10.1021/bi00315a003. [DOI] [PubMed] [Google Scholar]
- Chang T. M., Chey W. Y. Radioimmunoassay of secretin. A critical review and current status. Dig Dis Sci. 1980 Jul;25(7):529–552. doi: 10.1007/BF01315215. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Christophe J., Waelbroeck M., Chatelain P., Robberecht P. Heart receptors for VIP, PHI and secretin are able to activate adenylate cyclase and to mediate inotropic and chronotropic effects. Species variations and physiopathology. Peptides. 1984 Mar-Apr;5(2):341–353. doi: 10.1016/0196-9781(84)90232-8. [DOI] [PubMed] [Google Scholar]
- Couvineau A., Voisin T., Guijarro L., Laburthe M. Purification of vasoactive intestinal peptide receptor from porcine liver by a newly designed one-step affinity chromatography. J Biol Chem. 1990 Aug 5;265(22):13386–13390. [PubMed] [Google Scholar]
- Fremeau R. T., Jr, Jensen R. T., Charlton C. G., Miller R. L., O'Donohue T. L., Moody T. W. Secretin: specific binding to rat brain membranes. J Neurosci. 1983 Aug;3(8):1620–1625. doi: 10.1523/JNEUROSCI.03-08-01620.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau R. T., Jr, Korman L. Y., Moody T. W. Secretin stimulates cyclic AMP formation in the rat brain. J Neurochem. 1986 Jun;46(6):1947–1955. doi: 10.1111/j.1471-4159.1986.tb08518.x. [DOI] [PubMed] [Google Scholar]
- Fukunaga R., Ishizaka-Ikeda E., Seto Y., Nagata S. Expression cloning of a receptor for murine granulocyte colony-stimulating factor. Cell. 1990 Apr 20;61(2):341–350. doi: 10.1016/0092-8674(90)90814-u. [DOI] [PubMed] [Google Scholar]
- Gespach C., Bataille D., Vauclin N., Moroder L., Wünsch E., Rosselin G. Secretin receptor activity in rat gastric glands. Binding studies, cAMP generation and pharmacology. Peptides. 1986;7 (Suppl 1):155–163. doi: 10.1016/0196-9781(86)90179-8. [DOI] [PubMed] [Google Scholar]
- Gossen D., Poloczek P., Svoboda M., Christophe J. Molecular architecture of secretin receptors: the specific covalent labelling of a 51 kDa peptide after cross-linking of [125I]iodosecretin to intact rat pancreatic acini. FEBS Lett. 1989 Jan 30;243(2):205–208. doi: 10.1016/0014-5793(89)80130-9. [DOI] [PubMed] [Google Scholar]
- Gozes I., Brenneman D. E. VIP: molecular biology and neurobiological function. Mol Neurobiol. 1989 Winter;3(4):201–236. doi: 10.1007/BF02740606. [DOI] [PubMed] [Google Scholar]
- Itoh H., Kozasa T., Nagata S., Nakamura S., Katada T., Ui M., Iwai S., Ohtsuka E., Kawasaki H., Suzuki K. Molecular cloning and sequence determination of cDNAs for alpha subunits of the guanine nucleotide-binding proteins Gs, Gi, and Go from rat brain. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3776–3780. doi: 10.1073/pnas.83.11.3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. T., Charlton C. G., Adachi H., Jones S. W., O'Donohue T. L., Gardner J. D. Use of 125I-secretin to identify and characterize high-affinity secretin receptors on pancreatic acini. Am J Physiol. 1983 Aug;245(2):G186–G195. doi: 10.1152/ajpgi.1983.245.2.G186. [DOI] [PubMed] [Google Scholar]
- Katada T., Amano T., Ui M. Modulation by islet-activating protein of adenylate cyclase activity in C6 glioma cells. J Biol Chem. 1982 Apr 10;257(7):3739–3746. [PubMed] [Google Scholar]
- Klee W. A., Nirenberg M. A neuroblastoma times glioma hybrid cell line with morphine receptors. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3474–3477. doi: 10.1073/pnas.71.9.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopin A. S., Wheeler M. B., Leiter A. B. Secretin: structure of the precursor and tissue distribution of the mRNA. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2299–2303. doi: 10.1073/pnas.87.6.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Luthman H., Magnusson G. High efficiency polyoma DNA transfection of chloroquine treated cells. Nucleic Acids Res. 1983 Mar 11;11(5):1295–1308. doi: 10.1093/nar/11.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K. C., Sprengel R., Phillips H. S., Köhler M., Rosemblit N., Nikolics K., Segaloff D. L., Seeburg P. H. Lutropin-choriogonadotropin receptor: an unusual member of the G protein-coupled receptor family. Science. 1989 Aug 4;245(4917):494–499. doi: 10.1126/science.2502842. [DOI] [PubMed] [Google Scholar]
- Mizushima S., Nagata S. pEF-BOS, a powerful mammalian expression vector. Nucleic Acids Res. 1990 Sep 11;18(17):5322–5322. doi: 10.1093/nar/18.17.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S., Maniatis T. Expression cloning of the murine interferon gamma receptor cDNA. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9248–9252. doi: 10.1073/pnas.86.23.9248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Mutt V., Jorpes J. E., Magnusson S. Structure of porcine secretin. The amino acid sequence. Eur J Biochem. 1970 Sep;15(3):513–519. doi: 10.1111/j.1432-1033.1970.tb01034.x. [DOI] [PubMed] [Google Scholar]
- O'Dowd B. F., Lefkowitz R. J., Caron M. G. Structure of the adrenergic and related receptors. Annu Rev Neurosci. 1989;12:67–83. doi: 10.1146/annurev.ne.12.030189.000435. [DOI] [PubMed] [Google Scholar]
- Robberecht P., Cauvin A., Gourlet P., Christophe J. Heterogeneity of VIP receptors. Arch Int Pharmacodyn Ther. 1990 Jan-Feb;303:51–66. [PubMed] [Google Scholar]
- Robberecht P., Waelbroeck M., Camus J. C., De Neef P., Christophe J. Importance of disulfide bonds in receptors for vasoactive intestinal peptide and secretin in rat pancreatic plasma membranes. Biochim Biophys Acta. 1984 Jun 27;773(2):271–278. doi: 10.1016/0005-2736(84)90091-9. [DOI] [PubMed] [Google Scholar]
- Roskoski R., Jr, White L., Knowlton R., Roskoski L. M. Regulation of tyrosine hydroxylase activity in rat PC12 cells by neuropeptides of the secretin family. Mol Pharmacol. 1989 Dec;36(6):925–931. [PubMed] [Google Scholar]
- Rosselin G. The receptors of the VIP family peptides (VIP, secretin, GRF, PHI, PHM, GIP, glucagon and oxyntomodulin). Specificities and identity. Peptides. 1986;7 (Suppl 1):89–100. doi: 10.1016/0196-9781(86)90170-1. [DOI] [PubMed] [Google Scholar]
- Roth B. L., Beinfeld M. C., Howlett A. C. Secretin receptors on neuroblastoma cell membranes: characterization of 125I-labeled secretin binding and association with adenylate cyclase. J Neurochem. 1984 Apr;42(4):1145–1152. doi: 10.1111/j.1471-4159.1984.tb12723.x. [DOI] [PubMed] [Google Scholar]
- Sakurai T., Yanagisawa M., Takuwa Y., Miyazaki H., Kimura S., Goto K., Masaki T. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature. 1990 Dec 20;348(6303):732–735. doi: 10.1038/348732a0. [DOI] [PubMed] [Google Scholar]
- Seed B. An LFA-3 cDNA encodes a phospholipid-linked membrane protein homologous to its receptor CD2. 1987 Oct 29-Nov 4Nature. 329(6142):840–842. doi: 10.1038/329840a0. [DOI] [PubMed] [Google Scholar]
- Shigemoto R., Yokota Y., Tsuchida K., Nakanishi S. Cloning and expression of a rat neuromedin K receptor cDNA. J Biol Chem. 1990 Jan 15;265(2):623–628. [PubMed] [Google Scholar]
- Zhou Q. Y., Grandy D. K., Thambi L., Kushner J. A., Van Tol H. H., Cone R., Pribnow D., Salon J., Bunzow J. R., Civelli O. Cloning and expression of human and rat D1 dopamine receptors. Nature. 1990 Sep 6;347(6288):76–80. doi: 10.1038/347076a0. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]



