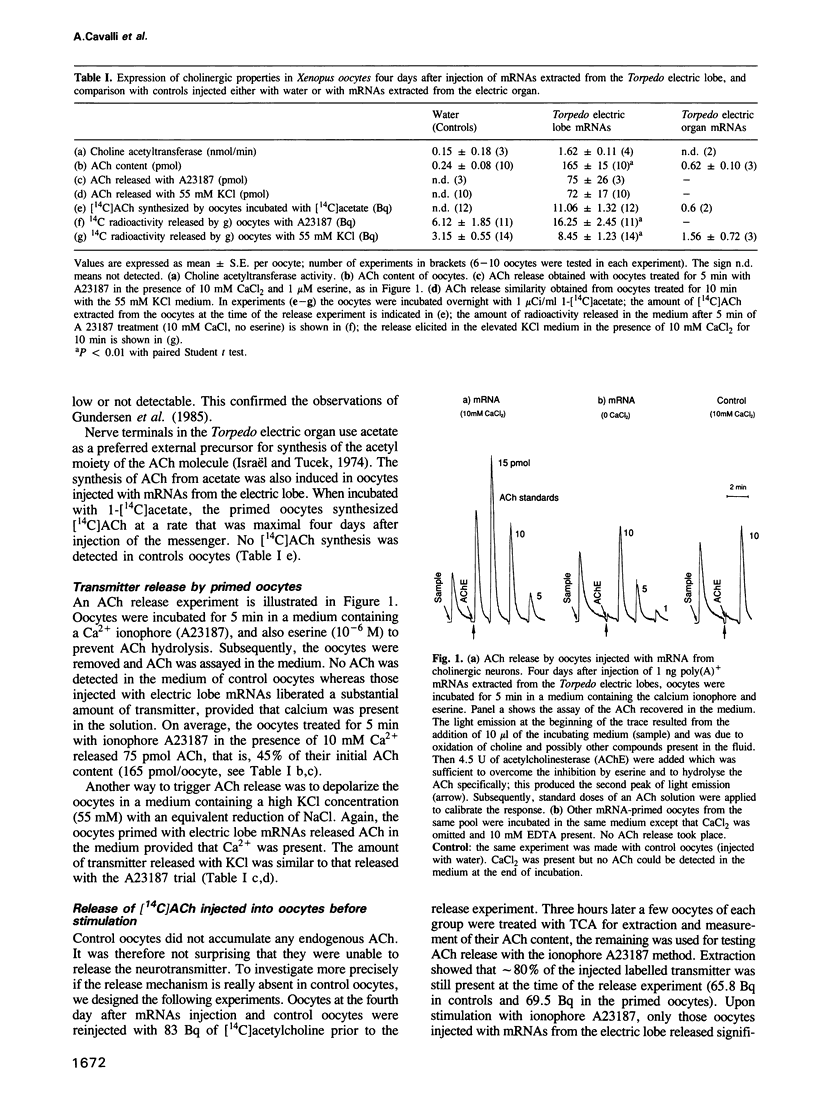
Abstract
Xenopus laevis oocytes were injected with poly(A)+ mRNAs extracted from the electric lobes of Torpedo marmorata. The electric lobes contain the perikarya of approximately 120,000 cholinergic neurons that innervate the electric organs and are homologous to motor neurons. The injected oocytes accumulated acetylcholine and were able to synthesize [14C]acetylcholine from 1-[14C]acetate. With KCl depolarization and upon treatment with a Ca2+ ionophore, they released their endogenous as well as the radiolabelled neurotransmitter in a Ca(2+)-dependent manner. No synthesis or release were obtained from control oocytes. With respect to their dependency upon Ca2+ concentration, the oocytes injected with Torpedo electric lobe mRNAs released acetylcholine in a manner which closely resembled that found in the native synapses. In contrast to the controls, primed oocytes were also able to release [14C]acetylcholine that was injected a few hours prior to the release trial. Immunoblot analysis demonstrated that the 15 kd proteolipid antigen of the purified mediatophore, a 200 kd presynaptic protein able to translocate acetylcholine, was expressed in the ACh-releasing oocytes but not in the controls. The present observation may provide a useful approach for investigating the proteins involved in the release of acetylcholine and of other neurotransmitter substances.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birman S., Israël M., Lesbats B., Morel N. Solubilization and partial purification of a presynaptic membrane protein ensuring calcium-dependent acetylcholine release from proteoliposomes. J Neurochem. 1986 Aug;47(2):433–444. doi: 10.1111/j.1471-4159.1986.tb04520.x. [DOI] [PubMed] [Google Scholar]
- Ceccarelli B., Hurlbut W. P. Vesicle hypothesis of the release of quanta of acetylcholine. Physiol Rev. 1980 Apr;60(2):396–441. doi: 10.1152/physrev.1980.60.2.396. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dunant Y., Eder L., Servetiadis-Hirt L. Acetylcholine release evoked by single or a few nerve impulses in the electric organ of Torpedo. J Physiol. 1980 Jan;298:185–203. doi: 10.1113/jphysiol.1980.sp013075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonnum F. Choline acetyltransferase binding to and release from membranes. Biochem J. 1968 Sep;109(3):389–398. doi: 10.1042/bj1090389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudry-Talarmain Y. M., Diebler M. F., Robba M., Lancelot J. C., Lesbats B., Israël M. Effect of cetiedil analogs on acetylcholine and choline fluxes in synaptosomes and vesicles. Eur J Pharmacol. 1989 Aug 3;166(3):427–433. doi: 10.1016/0014-2999(89)90355-5. [DOI] [PubMed] [Google Scholar]
- Gundersen C. B., Jenden D. J., Miledi R. Choline acetyltransferase and acetylcholine in Xenopus oocytes injected with mRNA from the electric lobe of Torpedo. Proc Natl Acad Sci U S A. 1985 Jan;82(2):608–611. doi: 10.1073/pnas.82.2.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israël M., Lesbats B. Continuous determination by a chemiluminescent method of acetylcholine release and compartmentation in Torpedo electric organ synaptosomes. J Neurochem. 1981 Dec;37(6):1475–1483. doi: 10.1111/j.1471-4159.1981.tb06317.x. [DOI] [PubMed] [Google Scholar]
- Israël M., Lesbats B., Morel N., Manaranche R., Gulik-Krzywicki T., Dedieu J. C. Reconstitution of a functional synaptosomal membrane possessing the protein constituents involved in acetylcholine translocation. Proc Natl Acad Sci U S A. 1984 Jan;81(1):277–281. doi: 10.1073/pnas.81.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israël M., Morel N., Lesbats B., Birman S., Manaranche R. Purification of a presynaptic membrane protein that mediates a calcium-dependent translocation of acetylcholine. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9226–9230. doi: 10.1073/pnas.83.23.9226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israël M., Tucek S. Utilization of acetate and pyruvate for the synthesis of 'total', 'bound' and 'free' acetylcholine in the electric organ of Torpedo. J Neurochem. 1974 Apr;22(4):487–491. doi: 10.1111/j.1471-4159.1974.tb06883.x. [DOI] [PubMed] [Google Scholar]
- Jones M. V. Cytochrome c linked nicotinic acid hydroxylase in Pseudomonas ovalis Chester. FEBS Lett. 1973 Jun 1;32(2):321–324. doi: 10.1016/0014-5793(73)80864-6. [DOI] [PubMed] [Google Scholar]
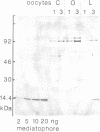
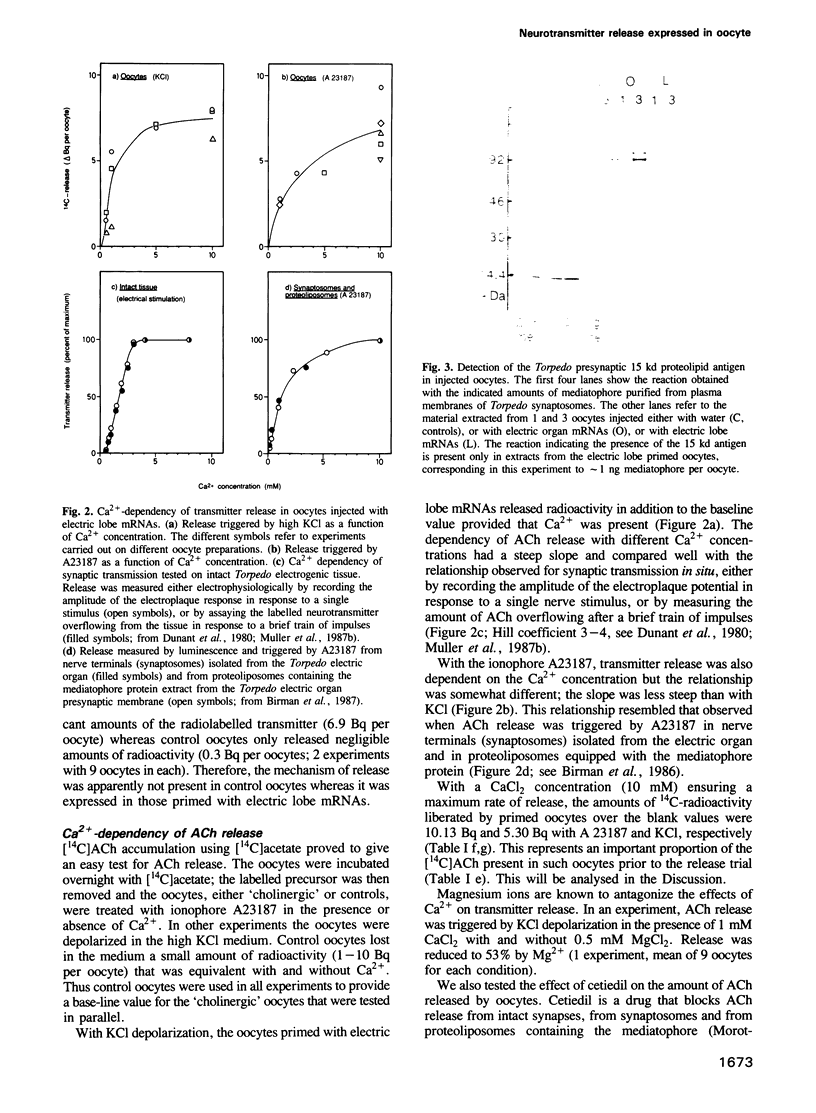
- Morel N., Synguelakis M., Le Gal la Salle G. Detection with monoclonal antibodies of a 15-kDa proteolipid in both presynaptic plasma membranes and synaptic vesicles in Torpedo electric organ. J Neurochem. 1991 Apr;56(4):1401–1408. doi: 10.1111/j.1471-4159.1991.tb11438.x. [DOI] [PubMed] [Google Scholar]
- Muller D., Garcia-Segura L. M., Parducz A., Dunant Y. Brief occurrence of a population of presynaptic intramembrane particles coincides with transmission of a nerve impulse. Proc Natl Acad Sci U S A. 1987 Jan;84(2):590–594. doi: 10.1073/pnas.84.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller D., Loctin F., Dunant Y. Inhibition of evoked acetylcholine release: two different mechanisms in the Torpedo electric organ. Eur J Pharmacol. 1987 Jan 13;133(2):225–234. doi: 10.1016/0014-2999(87)90154-3. [DOI] [PubMed] [Google Scholar]