Abstract
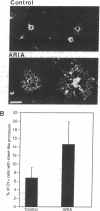
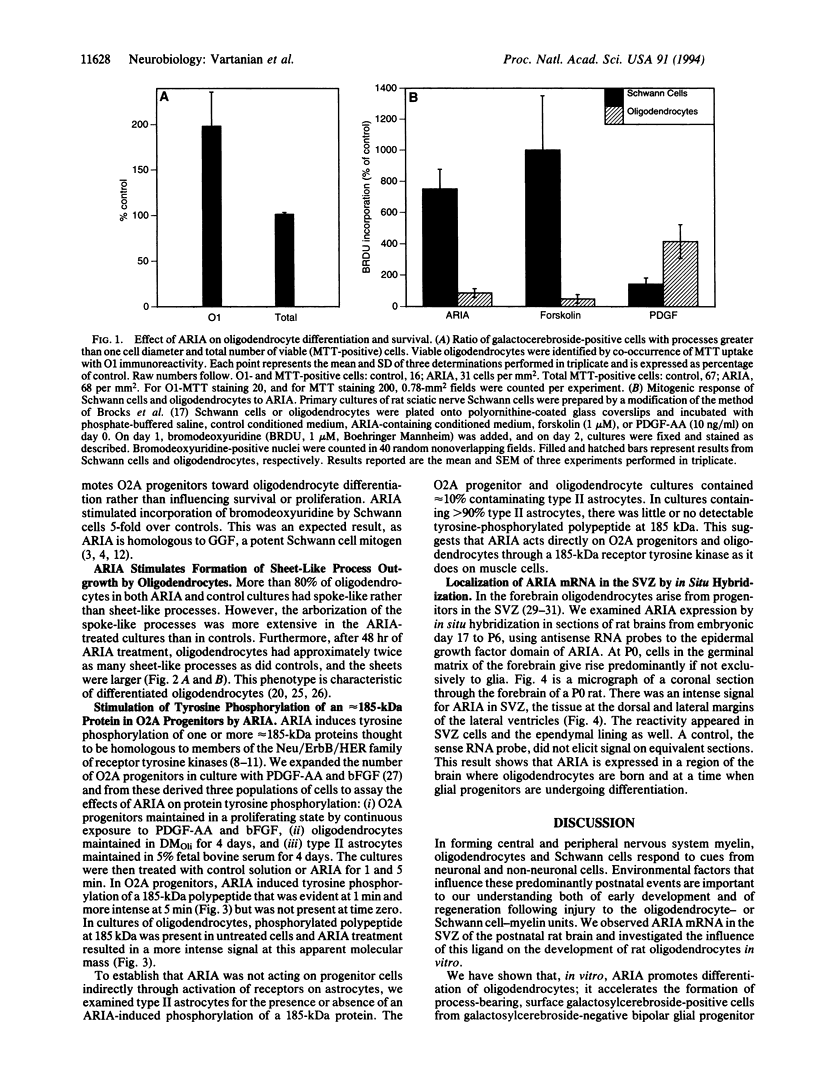
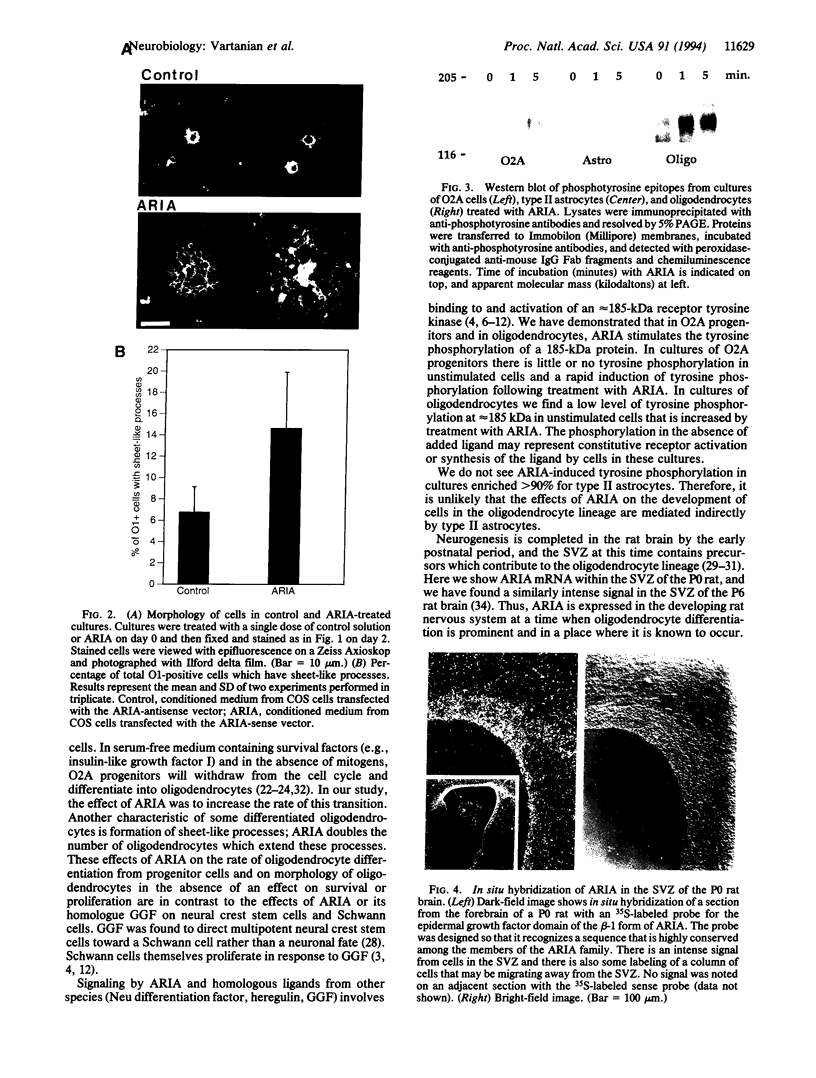
ARIA acetylcholine receptor-inducing activity protein, is a member of a family of ligands that includes the Neu differentiation factor, heregulin, and glial growth factor. These ligands all act through one or more receptor tyrosine kinases of approximately 185 kDa. In some conditions these ligands promote proliferation, whereas in others they induce differentiation. ARIA was originally isolated from chick brain on the basis of its ability to induce synthesis of nicotinic acetylcholine receptors in skeletal muscle. In this paper we show that ARIA is expressed in the subventricular zone of the rat brain and that it enhances the development of oligodendrocytes from bipotential (O2A) glial progenitor cells. We have also found that ARIA induces tyrosine phosphorylation of a 185-kDa protein in O2A progenitor cells. ARIA does not increase bromodeoxyuridine incorporation by oligodendrocytes but is mitogenic when added to Schwann cells in vitro. Thus, ARIA accelerates the formation of oligodendrocytes in vitro and is expressed where it could exercise the same influence in vivo.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong R. C., Dorn H. H., Kufta C. V., Friedman E., Dubois-Dalcq M. E. Pre-oligodendrocytes from adult human CNS. J Neurosci. 1992 Apr;12(4):1538–1547. doi: 10.1523/JNEUROSCI.12-04-01538.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres B. A., Hart I. K., Coles H. S., Burne J. F., Voyvodic J. T., Richardson W. D., Raff M. C. Cell death and control of cell survival in the oligodendrocyte lineage. Cell. 1992 Jul 10;70(1):31–46. doi: 10.1016/0092-8674(92)90531-g. [DOI] [PubMed] [Google Scholar]
- Barres B. A., Raff M. C. Control of oligodendrocyte number in the developing rat optic nerve. Neuron. 1994 May;12(5):935–942. doi: 10.1016/0896-6273(94)90305-0. [DOI] [PubMed] [Google Scholar]
- Brockes J. P., Fields K. L., Raff M. C. Studies on cultured rat Schwann cells. I. Establishment of purified populations from cultures of peripheral nerve. Brain Res. 1979 Apr 6;165(1):105–118. doi: 10.1016/0006-8993(79)90048-9. [DOI] [PubMed] [Google Scholar]
- Carmichael J., DeGraff W. G., Gazdar A. F., Minna J. D., Mitchell J. B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987 Feb 15;47(4):936–942. [PubMed] [Google Scholar]
- Corfas G., Falls D. L., Fischbach G. D. ARIA, a protein that stimulates acetylcholine receptor synthesis, also induces tyrosine phosphorylation of a 185-kDa muscle transmembrane protein. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1624–1628. doi: 10.1073/pnas.90.4.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corfas G., Fischbach G. D. The number of Na+ channels in cultured chick muscle is increased by ARIA, an acetylcholine receptor-inducing activity. J Neurosci. 1993 May;13(5):2118–2125. doi: 10.1523/JNEUROSCI.13-05-02118.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer C. A., Benjamins J. A. Antibody to galactocerebroside alters organization of oligodendroglial membrane sheets in culture. J Neurosci. 1988 Nov;8(11):4307–4318. doi: 10.1523/JNEUROSCI.08-11-04307.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falls D. L., Rosen K. M., Corfas G., Lane W. S., Fischbach G. D. ARIA, a protein that stimulates acetylcholine receptor synthesis, is a member of the neu ligand family. Cell. 1993 Mar 12;72(5):801–815. doi: 10.1016/0092-8674(93)90407-h. [DOI] [PubMed] [Google Scholar]
- Gard A. L., Pfeiffer S. E. Oligodendrocyte progenitors isolated directly from developing telencephalon at a specific phenotypic stage: myelinogenic potential in a defined environment. Development. 1989 May;106(1):119–132. doi: 10.1242/dev.106.1.119. [DOI] [PubMed] [Google Scholar]
- Giulian D., Baker T. J. Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci. 1986 Aug;6(8):2163–2178. doi: 10.1523/JNEUROSCI.06-08-02163.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodearl A. D., Davis J. B., Mistry K., Minghetti L., Otsu M., Waterfield M. D., Stroobant P. Purification of multiple forms of glial growth factor. J Biol Chem. 1993 Aug 25;268(24):18095–18102. [PubMed] [Google Scholar]
- Hardy R., Reynolds R. Proliferation and differentiation potential of rat forebrain oligodendroglial progenitors both in vitro and in vivo. Development. 1991 Apr;111(4):1061–1080. doi: 10.1242/dev.111.4.1061. [DOI] [PubMed] [Google Scholar]
- Holmes W. E., Sliwkowski M. X., Akita R. W., Henzel W. J., Lee J., Park J. W., Yansura D., Abadi N., Raab H., Lewis G. D. Identification of heregulin, a specific activator of p185erbB2. Science. 1992 May 22;256(5060):1205–1210. doi: 10.1126/science.256.5060.1205. [DOI] [PubMed] [Google Scholar]
- LeVine S. M., Goldman J. E. Embryonic divergence of oligodendrocyte and astrocyte lineages in developing rat cerebrum. J Neurosci. 1988 Nov;8(11):3992–4006. doi: 10.1523/JNEUROSCI.08-11-03992.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke G. E., Brockes J. P. Identification and purification of glial growth factor. J Neurosci. 1984 Jan;4(1):75–83. doi: 10.1523/JNEUROSCI.04-01-00075.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levison S. W., Goldman J. E. Both oligodendrocytes and astrocytes develop from progenitors in the subventricular zone of postnatal rat forebrain. Neuron. 1993 Feb;10(2):201–212. doi: 10.1016/0896-6273(93)90311-e. [DOI] [PubMed] [Google Scholar]
- Marchionni M. A., Goodearl A. D., Chen M. S., Bermingham-McDonogh O., Kirk C., Hendricks M., Danehy F., Misumi D., Sudhalter J., Kobayashi K. Glial growth factors are alternatively spliced erbB2 ligands expressed in the nervous system. Nature. 1993 Mar 25;362(6418):312–318. doi: 10.1038/362312a0. [DOI] [PubMed] [Google Scholar]
- Mayer M., Bögler O., Noble M. The inhibition of oligodendrocytic differentiation of O-2A progenitors caused by basic fibroblast growth factor is overridden by astrocytes. Glia. 1993 May;8(1):12–19. doi: 10.1002/glia.440080103. [DOI] [PubMed] [Google Scholar]
- McCarthy K. D., de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980 Jun;85(3):890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peles E., Bacus S. S., Koski R. A., Lu H. S., Wen D., Ogden S. G., Levy R. B., Yarden Y. Isolation of the neu/HER-2 stimulatory ligand: a 44 kd glycoprotein that induces differentiation of mammary tumor cells. Cell. 1992 Apr 3;69(1):205–216. doi: 10.1016/0092-8674(92)90131-u. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Lillien L. E., Richardson W. D., Burne J. F., Noble M. D. Platelet-derived growth factor from astrocytes drives the clock that times oligodendrocyte development in culture. Nature. 1988 Jun 9;333(6173):562–565. doi: 10.1038/333562a0. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Miller R. H., Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983 Jun 2;303(5916):390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Mirsky R., Fields K. L., Lisak R. P., Dorfman S. H., Silberberg D. H., Gregson N. A., Leibowitz S., Kennedy M. C. Galactocerebroside is a specific cell-surface antigenic marker for oligodendrocytes in culture. Nature. 1978 Aug 24;274(5673):813–816. [PubMed] [Google Scholar]
- Richardson W. D., Pringle N., Mosley M. J., Westermark B., Dubois-Dalcq M. A role for platelet-derived growth factor in normal gliogenesis in the central nervous system. Cell. 1988 Apr 22;53(2):309–319. doi: 10.1016/0092-8674(88)90392-3. [DOI] [PubMed] [Google Scholar]
- SLATER T. F., SAWYER B., STRAEULI U. STUDIES ON SUCCINATE-TETRAZOLIUM REDUCTASE SYSTEMS. III. POINTS OF COUPLING OF FOUR DIFFERENT TETRAZOLIUM SALTS. Biochim Biophys Acta. 1963 Nov 8;77:383–393. doi: 10.1016/0006-3002(63)90513-4. [DOI] [PubMed] [Google Scholar]
- Schechter A. L., Stern D. F., Vaidyanathan L., Decker S. J., Drebin J. A., Greene M. I., Weinberg R. A. The neu oncogene: an erb-B-related gene encoding a 185,000-Mr tumour antigen. Nature. 1984 Dec 6;312(5994):513–516. doi: 10.1038/312513a0. [DOI] [PubMed] [Google Scholar]
- Shah N. M., Marchionni M. A., Isaacs I., Stroobant P., Anderson D. J. Glial growth factor restricts mammalian neural crest stem cells to a glial fate. Cell. 1994 May 6;77(3):349–360. doi: 10.1016/0092-8674(94)90150-3. [DOI] [PubMed] [Google Scholar]
- Wen D., Peles E., Cupples R., Suggs S. V., Bacus S. S., Luo Y., Trail G., Hu S., Silbiger S. M., Levy R. B. Neu differentiation factor: a transmembrane glycoprotein containing an EGF domain and an immunoglobulin homology unit. Cell. 1992 May 1;69(3):559–572. doi: 10.1016/0092-8674(92)90456-m. [DOI] [PubMed] [Google Scholar]
- Wood P. M., Bunge R. P. Evidence that sensory axons are mitogenic for Schwann cells. Nature. 1975 Aug 21;256(5519):662–664. doi: 10.1038/256662a0. [DOI] [PubMed] [Google Scholar]