Abstract
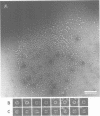
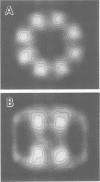
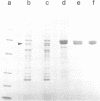
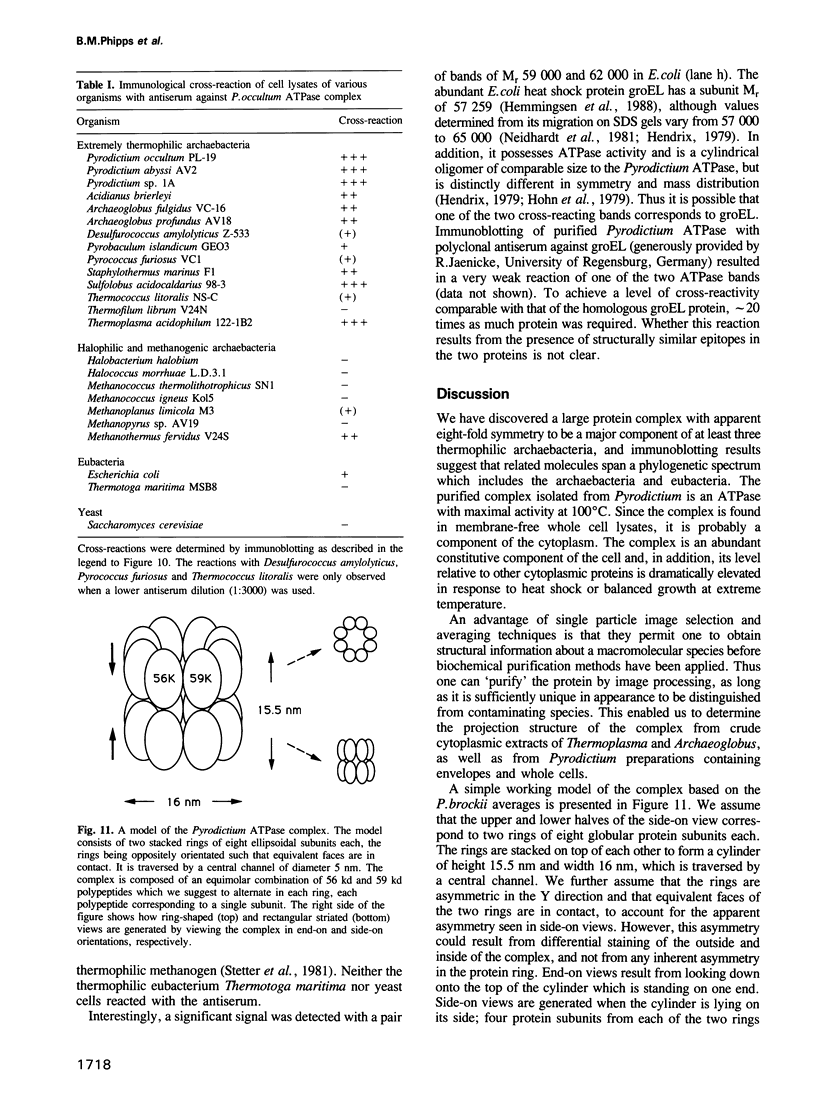
We have discovered a large cylindrical protein complex which is an abundant component of the cytoplasm of extremely thermophilic archaebacteria. Structural analysis by image processing of electron micrographs suggests that the complex is composed of two stacked rings of eight subunits each; the rings enclose a central channel. The complex purified from the hyperthermophile Pyrodictium occultum is composed of equal quantities of two polypeptides of Mr 56,000 and 59,000. It exhibits an extremely thermostable ATPase activity with a temperature optimum of 100 degrees C. The basal level of the ATPase complex in the cell is high, and it becomes highly enriched as a result of heat shock (shift from 102 degrees C to 108 degrees C) or balanced growth at temperatures near the physiological upper limit. Immunoblotting results indicate that a related protein is present in most thermophilic archaebacteria and in Escherichia coli. This protein complex may play an important role in the adaptation of thermophilic archaebacteria to life at high temperature.
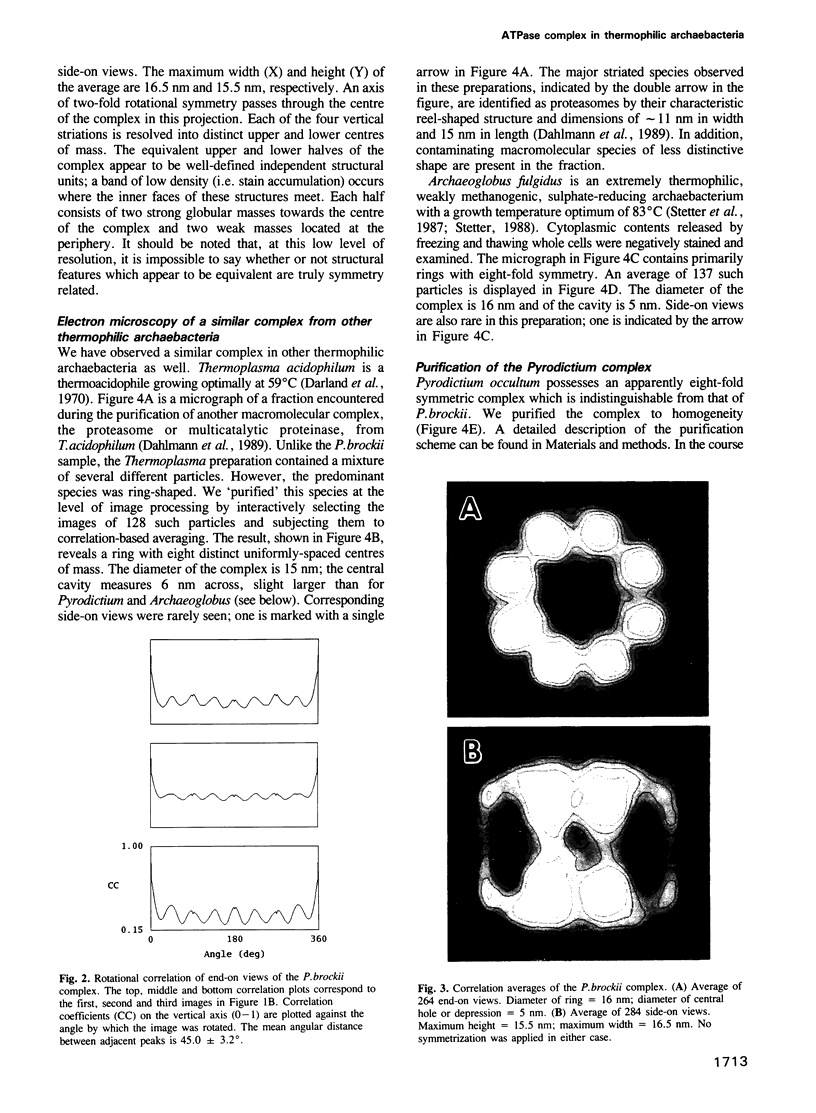
Full text
PDF
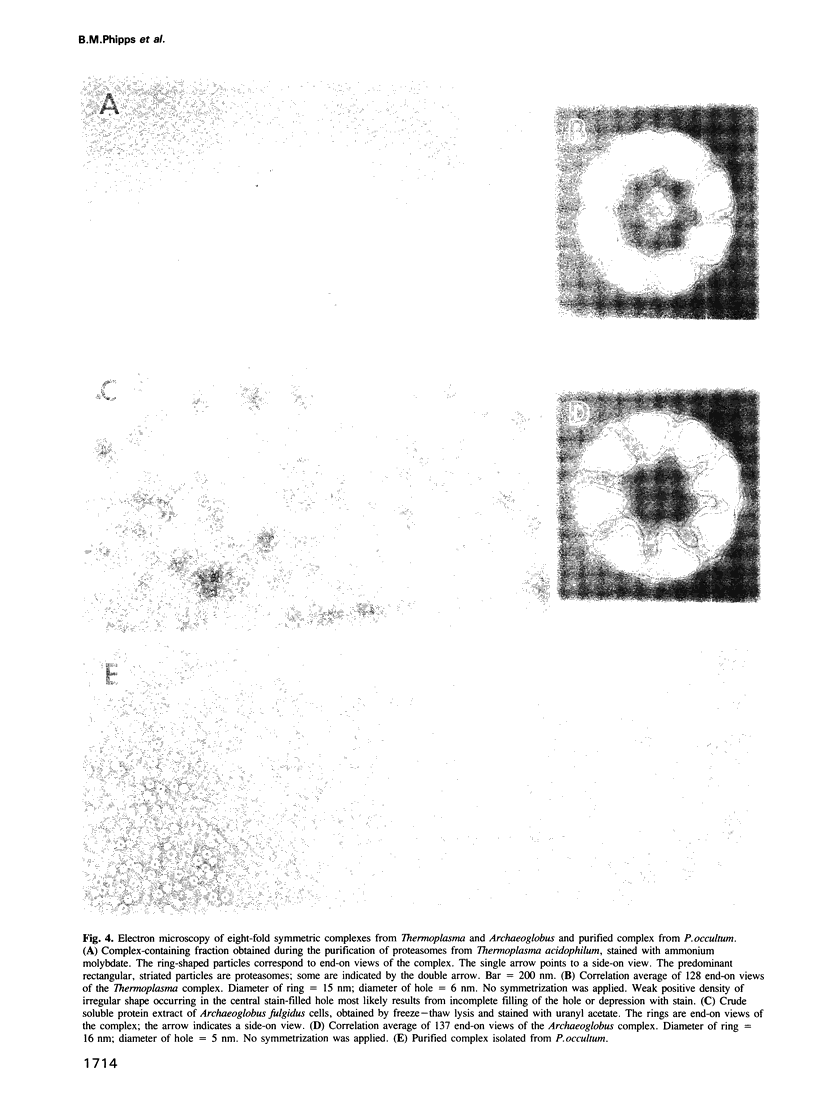





Images in this article
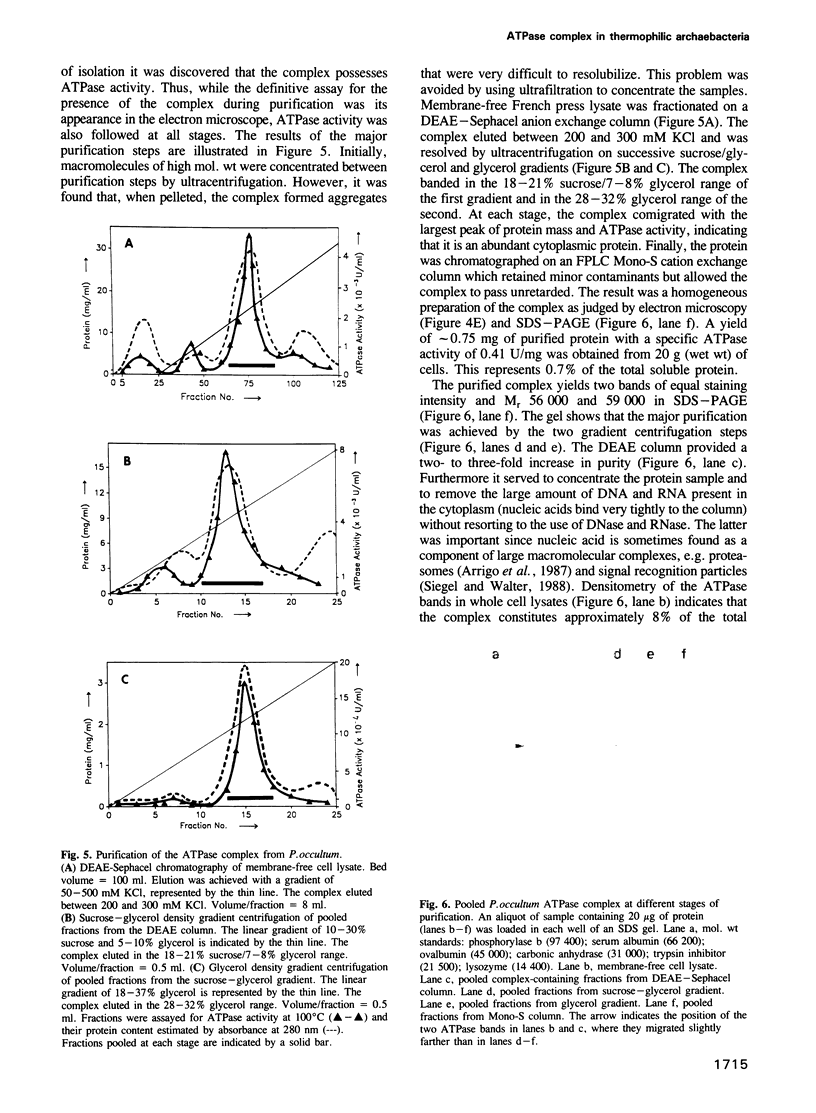
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
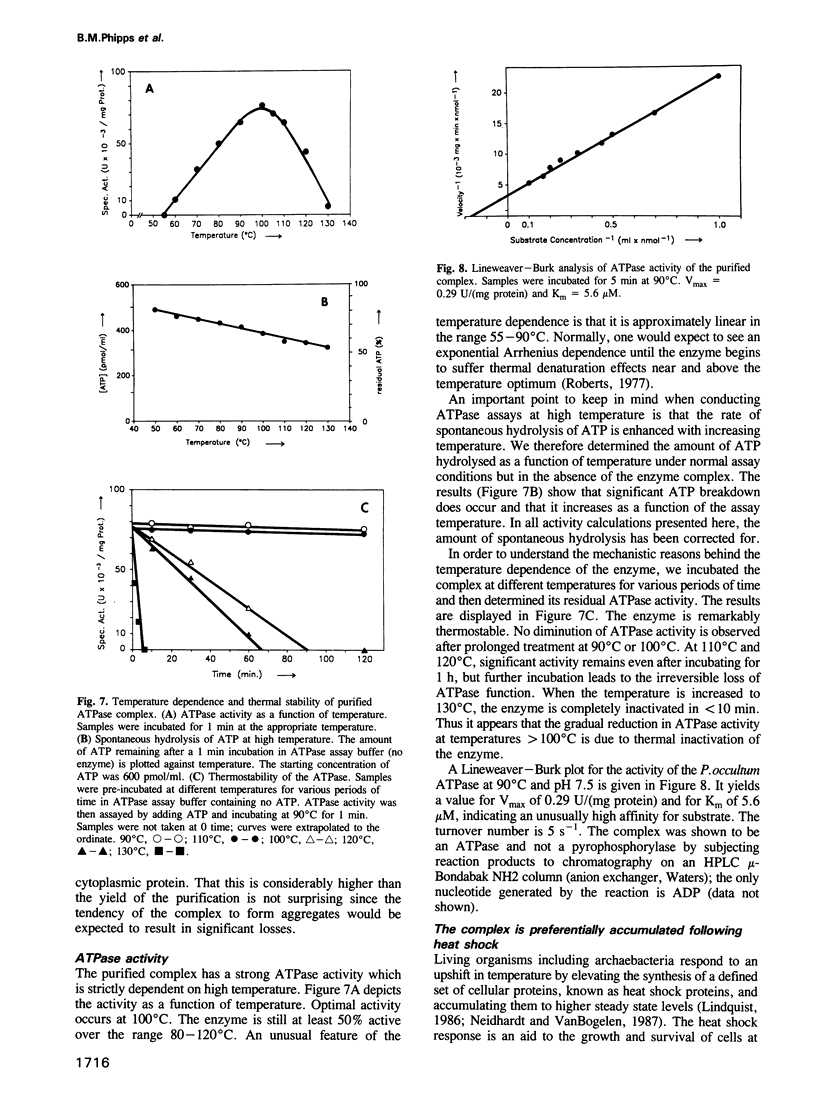
- Arrigo A. P., Simon M., Darlix J. L., Spahr P. F. A 20S particle ubiquitous from yeast to human. J Mol Evol. 1987;25(2):141–150. doi: 10.1007/BF02101756. [DOI] [PubMed] [Google Scholar]
- Baumeister W., Dahlmann B., Hegerl R., Kopp F., Kuehn L., Pfeifer G. Electron microscopy and image analysis of the multicatalytic proteinase. FEBS Lett. 1988 Dec 5;241(1-2):239–245. doi: 10.1016/0014-5793(88)81069-x. [DOI] [PubMed] [Google Scholar]
- Bochkareva E. S., Lissin N. M., Girshovich A. S. Transient association of newly synthesized unfolded proteins with the heat-shock GroEL protein. Nature. 1988 Nov 17;336(6196):254–257. doi: 10.1038/336254a0. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Costantino H. R., Brown S. H., Kelly R. M. Purification and characterization of an alpha-glucosidase from a hyperthermophilic archaebacterium, Pyrococcus furiosus, exhibiting a temperature optimum of 105 to 115 degrees C. J Bacteriol. 1990 Jul;172(7):3654–3660. doi: 10.1128/jb.172.7.3654-3660.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlmann B., Kopp F., Kuehn L., Niedel B., Pfeifer G., Hegerl R., Baumeister W. The multicatalytic proteinase (prosome) is ubiquitous from eukaryotes to archaebacteria. FEBS Lett. 1989 Jul 17;251(1-2):125–131. doi: 10.1016/0014-5793(89)81441-3. [DOI] [PubMed] [Google Scholar]
- Darland G., Brock T. D., Samsonoff W., Conti S. F. A thermophilic, acidophilic mycoplasma isolated from a coal refuse pile. Science. 1970 Dec 25;170(3965):1416–1418. doi: 10.1126/science.170.3965.1416. [DOI] [PubMed] [Google Scholar]
- Feldman S. R., Gonias S. L., Pizzo S. V. Model of alpha 2-macroglobulin structure and function. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5700–5704. doi: 10.1073/pnas.82.17.5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goloubinoff P., Christeller J. T., Gatenby A. A., Lorimer G. H. Reconstitution of active dimeric ribulose bisphosphate carboxylase from an unfoleded state depends on two chaperonin proteins and Mg-ATP. Nature. 1989 Dec 21;342(6252):884–889. doi: 10.1038/342884a0. [DOI] [PubMed] [Google Scholar]
- Goloubinoff P., Gatenby A. A., Lorimer G. H. GroE heat-shock proteins promote assembly of foreign prokaryotic ribulose bisphosphate carboxylase oligomers in Escherichia coli. Nature. 1989 Jan 5;337(6202):44–47. doi: 10.1038/337044a0. [DOI] [PubMed] [Google Scholar]
- Hegerl R., Altbauer A. The "EM" program system. Ultramicroscopy. 1982;9(1-2):109–116. doi: 10.1016/0304-3991(82)90233-9. [DOI] [PubMed] [Google Scholar]
- Heil A., Zillig W. Reconstitution of bacterial DNA-dependent RNA-polymerase from isolated subunits as a tool for the elucidation of the role of the subunits in transcription. FEBS Lett. 1970 Dec;11(3):165–168. doi: 10.1016/0014-5793(70)80519-1. [DOI] [PubMed] [Google Scholar]
- Hemmingsen S. M., Woolford C., van der Vies S. M., Tilly K., Dennis D. T., Georgopoulos C. P., Hendrix R. W., Ellis R. J. Homologous plant and bacterial proteins chaperone oligomeric protein assembly. Nature. 1988 May 26;333(6171):330–334. doi: 10.1038/333330a0. [DOI] [PubMed] [Google Scholar]
- Hendrix R. W. Purification and properties of groE, a host protein involved in bacteriophage assembly. J Mol Biol. 1979 Apr 15;129(3):375–392. doi: 10.1016/0022-2836(79)90502-3. [DOI] [PubMed] [Google Scholar]
- Herendeen S. L., VanBogelen R. A., Neidhardt F. C. Levels of major proteins of Escherichia coli during growth at different temperatures. J Bacteriol. 1979 Jul;139(1):185–194. doi: 10.1128/jb.139.1.185-194.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn T., Hohn B., Engel A., Wurtz M., Smith P. R. Isolation and characterization of the host protein groE involved in bacteriophage lambda assembly. J Mol Biol. 1979 Apr 15;129(3):359–373. doi: 10.1016/0022-2836(79)90501-1. [DOI] [PubMed] [Google Scholar]
- Hutchinson E. G., Tichelaar W., Hofhaus G., Weiss H., Leonard K. R. Identification and electron microscopic analysis of a chaperonin oligomer from Neurospora crassa mitochondria. EMBO J. 1989 May;8(5):1485–1490. doi: 10.1002/j.1460-2075.1989.tb03532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenicke R., Závodszky P. Proteins under extreme physical conditions. FEBS Lett. 1990 Aug 1;268(2):344–349. doi: 10.1016/0014-5793(90)81283-t. [DOI] [PubMed] [Google Scholar]
- Kopp F., Steiner R., Dahlmann B., Kuehn L., Reinauer H. Size and shape of the multicatalytic proteinase from rat skeletal muscle. Biochim Biophys Acta. 1986 Aug 15;872(3):253–260. doi: 10.1016/0167-4838(86)90278-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lecker S., Lill R., Ziegelhoffer T., Georgopoulos C., Bassford P. J., Jr, Kumamoto C. A., Wickner W. Three pure chaperone proteins of Escherichia coli--SecB, trigger factor and GroEL--form soluble complexes with precursor proteins in vitro. EMBO J. 1989 Sep;8(9):2703–2709. doi: 10.1002/j.1460-2075.1989.tb08411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaux P. G., Herendeen S. L., Bloch P. L., Neidhardt F. C. Transient rates of synthesis of individual polypeptides in E. coli following temperature shifts. Cell. 1978 Mar;13(3):427–434. doi: 10.1016/0092-8674(78)90317-3. [DOI] [PubMed] [Google Scholar]
- Lilley K. S., Davison M. D., Rivett A. J. N-terminal sequence similarities between components of the multicatalytic proteinase complex. FEBS Lett. 1990 Mar 26;262(2):327–329. doi: 10.1016/0014-5793(90)80220-d. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Martel R., Cloney L. P., Pelcher L. E., Hemmingsen S. M. Unique composition of plastid chaperonin-60: alpha and beta polypeptide-encoding genes are highly divergent. Gene. 1990 Oct 15;94(2):181–187. doi: 10.1016/0378-1119(90)90385-5. [DOI] [PubMed] [Google Scholar]
- McMullin T. W., Hallberg R. L. A highly evolutionarily conserved mitochondrial protein is structurally related to the protein encoded by the Escherichia coli groEL gene. Mol Cell Biol. 1988 Jan;8(1):371–380. doi: 10.1128/mcb.8.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullin T. W., Hallberg R. L. A normal mitochondrial protein is selectively synthesized and accumulated during heat shock in Tetrahymena thermophila. Mol Cell Biol. 1987 Dec;7(12):4414–4423. doi: 10.1128/mcb.7.12.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musgrove J. E., Johnson R. A., Ellis R. J. Dissociation of the ribulosebisphosphate-carboxylase large-subunit binding protein into dissimilar subunits. Eur J Biochem. 1987 Mar 16;163(3):529–534. doi: 10.1111/j.1432-1033.1987.tb10900.x. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., Phillips T. A., VanBogelen R. A., Smith M. W., Georgalis Y., Subramanian A. R. Identity of the B56.5 protein, the A-protein, and the groE gene product of Escherichia coli. J Bacteriol. 1981 Jan;145(1):513–520. doi: 10.1128/jb.145.1.513-520.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostermann J., Horwich A. L., Neupert W., Hartl F. U. Protein folding in mitochondria requires complex formation with hsp60 and ATP hydrolysis. Nature. 1989 Sep 14;341(6238):125–130. doi: 10.1038/341125a0. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Speculations on the functions of the major heat shock and glucose-regulated proteins. Cell. 1986 Sep 26;46(7):959–961. doi: 10.1016/0092-8674(86)90693-8. [DOI] [PubMed] [Google Scholar]
- Pihl T. D., Schicho R. N., Kelly R. M., Maier R. J. Characterization of hydrogen-uptake activity in the hyperthermophile Pyrodictium brockii. Proc Natl Acad Sci U S A. 1989 Jan;86(1):138–141. doi: 10.1073/pnas.86.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prangishvilli D., Zillig W., Gierl A., Biesert L., Holz I. DNA-dependent RNA polymerase of thermoacidophilic archaebacteria. Eur J Biochem. 1982 Mar 1;122(3):471–477. doi: 10.1111/j.1432-1033.1982.tb06461.x. [DOI] [PubMed] [Google Scholar]
- Reading D. S., Hallberg R. L., Myers A. M. Characterization of the yeast HSP60 gene coding for a mitochondrial assembly factor. Nature. 1989 Feb 16;337(6208):655–659. doi: 10.1038/337655a0. [DOI] [PubMed] [Google Scholar]
- Rothman J. E. Polypeptide chain binding proteins: catalysts of protein folding and related processes in cells. Cell. 1989 Nov 17;59(4):591–601. doi: 10.1016/0092-8674(89)90005-6. [DOI] [PubMed] [Google Scholar]
- Siegel V., Walter P. Functional dissection of the signal recognition particle. Trends Biochem Sci. 1988 Aug;13(8):314–316. doi: 10.1016/0968-0004(88)90127-2. [DOI] [PubMed] [Google Scholar]
- Stetter K. O., Lauerer G., Thomm M., Neuner A. Isolation of extremely thermophilic sulfate reducers: evidence for a novel branch of archaebacteria. Science. 1987 May 15;236(4803):822–824. doi: 10.1126/science.236.4803.822. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trent J. D., Osipiuk J., Pinkau T. Acquired thermotolerance and heat shock in the extremely thermophilic archaebacterium Sulfolobus sp. strain B12. J Bacteriol. 1990 Mar;172(3):1478–1484. doi: 10.1128/jb.172.3.1478-1484.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viitanen P. V., Lubben T. H., Reed J., Goloubinoff P., O'Keefe D. P., Lorimer G. H. Chaperonin-facilitated refolding of ribulosebisphosphate carboxylase and ATP hydrolysis by chaperonin 60 (groEL) are K+ dependent. Biochemistry. 1990 Jun 19;29(24):5665–5671. doi: 10.1021/bi00476a003. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Kandler O., Wheelis M. L. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zillig W., Holz I., Janekovic D., Klenk H. P., Imsel E., Trent J., Wunderl S., Forjaz V. H., Coutinho R., Ferreira T. Hyperthermus butylicus, a hyperthermophilic sulfur-reducing archaebacterium that ferments peptides. J Bacteriol. 1990 Jul;172(7):3959–3965. doi: 10.1128/jb.172.7.3959-3965.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]