Abstract
Marine no-take zones can have positive impacts for target species and are increasingly important management tools. However, whether they indirectly benefit higher order predators remains unclear. The endangered African penguin (Spheniscus demersus) depends on commercially exploited forage fish. We examined how chick survival responded to an experimental 3-year fishery closure around Robben Island, South Africa, controlling for variation in prey biomass and fishery catches. Chick survival increased by 18% when the closure was initiated, which alone led to a predicted 27% higher population compared with continued fishing. However, the modelled population continued to decline, probably because of high adult mortality linked to poor prey availability over larger spatial scales. Our results illustrate that small no-take zones can have bottom-up benefits for highly mobile marine predators, but are only one component of holistic, ecosystem-based management regimes.
Keywords: breeding success, fishing closures, fisheries–seabird interactions, marine conservation, marine protected areas
1. Introduction
Anthropogenic actions, including industrial fishing, have profoundly altered marine ecosystems and rapid action is required to rehabilitate the oceans [1]. Marine protected areas (MPAs) are increasingly designated to protect benthic habitats and species, but their efficacy for highly mobile species is unclear [2,3]. This problem is exacerbated when fisheries closures are designed to benefit mobile, upper trophic level predators by protecting their prey [4]. In particular, behaviourally mediated change or unrelated natural fluctuations in prey may mask population-level responses to closures [4–6].
The endangered African penguin Spheniscus demersus could benefit from MPAs [7]. This southern African endemic, a short-range (20–40 km) forager when breeding [6], feeds on commercially exploited forage fish (sardine Sardinops sagax and anchovy Engraulis encrasicolus) [8]. Decreased availability of these fish off western South Africa has been linked to a 69% reduction in penguin numbers between 2001 and 2013 [9]. Purse-seine fisheries may deplete stocks [10,11] and without spatial management, the South African fishery can remove adult sardine and anchovy recruits from waters adjacent to penguin colonies [6]. The species' worsening conservation status led to the implementation of experimental fishing closures around four colonies between 2008 and 2014. An initial ban at St Croix Island (33°48′ S, 25°46′ E) reduced penguin foraging effort but did not influence breeding success, adult body mass or chick growth [6,7]. Therefore, the efficacy of these closures at the population-level and whether they should continue, have been the subject of much debate [12].
From 2011 to 2013, a 20 km radius around Robben Island (33°48′ S, 18°22′ E), South Africa, was closed to purse-seine fishing. Chick survival is heavily influenced by the rate and amount of food delivered to the nest, so should respond if closure increases prey availability above baseline levels [6]. We examined whether penguin chick survival varied between years with (2011–2013) and without (2001–2010) fisheries closure and used a demographic model to examine the impact on population growth. Crucially, we used biomass estimates to account for variation in prey availability, penguin population estimates to control for density-dependent effects and catch data from outside the closure to control for changes in fishing activity over larger spatial scales.
2. Material and methods
(a). Penguin data
Data were from 1054 African penguin nests monitored at Robben Island between 2001 and 2010 and 447 nests between 2011 and 2013 (electronic supplementary material, table S1). We calculated the number of days each chick was exposed to potential mortality (nestling days), then estimated failure rates and s.e. for each year independently using parametric survival models in R v. 3.0.2. We used nest identity as a shared frailty term, an exponential error distribution [13] and an exponential distribution to transform the failure rates to annual estimates of chick survival [8]. An island-wide census in May each year estimated the annual breeding population [14].
(b). Fish biomass and catch data
To account for changing prey availability, we used hydro-acoustic survey estimates of the adult biomass (excluding age 0 juveniles) of sardine west of Cape Agulhas during November prior to penguin breeding and the recruit (age 0) biomass of anchovy in May of the breeding season from 2001 to 2013. Although no catches were taken within the closed area, fishing continued outside (figure 1). To account for possible effects of this on closure efficacy [6], we used annual sardine and anchovy catch data from the 30 nautical mile (55.6 km) fishing blocks around Robben Island (see the electronic supplementary material).
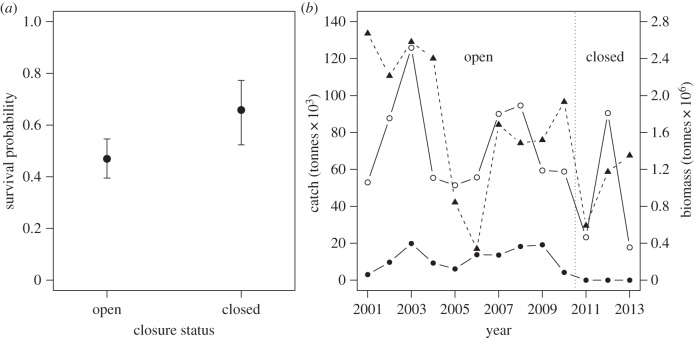
Figure 1.
(a) Mean (±95% credible intervals) chick survival during 2001–2010 (open) and 2011–2013 (closed) from model 7 (table 1). (b) Combined sardine (November surveys) and anchovy (May surveys) biomass off western South Africa (filled triangles) and combined catches within 10 nautical miles (entirely encompassed by the closure; filled circles) and 30 nautical miles of Robben Island (open circles). The vertical line indicates the onset of closure.
(c). Analysis of closure effect
We considered candidate models similar in form to linear models, with additive fixed effects and normally distributed residuals (table 1 and electronic supplementary material, table S2). The annual chick survival estimates (ϕc,y), transformed to the logit scale, formed the response variable. As these were estimated rather than observed directly, we modelled them as originating from a latent normal distribution so that logit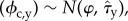 where φ is the unknown true mean survival and τ̂y is the standard error for year y. The ‘Closure’ variable (open = 0, closed = 1) was included in each candidate model (except the null model), with the catch, biomass and census data added to account for changing conditions experienced by the breeding population over time. Models were fitted using Monte Carlo Markov Chain estimation using the ‘rjags' and ‘coda’ libraries for R v. 3.0.2, non-informative priors and three chains of length 1 000 000 (first 10 000 samples discarded as burn-in, no thinning). Models were compared using penalized expected deviance (PED) and considered well supported if their ΔPED was smaller than the associated s.e. under repeated sampling [15].
where φ is the unknown true mean survival and τ̂y is the standard error for year y. The ‘Closure’ variable (open = 0, closed = 1) was included in each candidate model (except the null model), with the catch, biomass and census data added to account for changing conditions experienced by the breeding population over time. Models were fitted using Monte Carlo Markov Chain estimation using the ‘rjags' and ‘coda’ libraries for R v. 3.0.2, non-informative priors and three chains of length 1 000 000 (first 10 000 samples discarded as burn-in, no thinning). Models were compared using penalized expected deviance (PED) and considered well supported if their ΔPED was smaller than the associated s.e. under repeated sampling [15].
Table 1.
Model selection results for analyses relating African penguin chick survival to closure status. 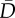 , expected deviance; Popt, optimism penalty applied to model; PED, penalized expected deviance (
, expected deviance; Popt, optimism penalty applied to model; PED, penalized expected deviance (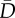 + Popt); ΔPED, difference in PED; s.e., standard error associated with ΔPED; ratio of ΔPED/s.e., indicating model support; AB, anchovy biomass; SB, sardine biomass; SC, sardine catch; AC, anchovy catch; C, closure status. The top five and the null model are shown. n.a., not applicable.
+ Popt); ΔPED, difference in PED; s.e., standard error associated with ΔPED; ratio of ΔPED/s.e., indicating model support; AB, anchovy biomass; SB, sardine biomass; SC, sardine catch; AC, anchovy catch; C, closure status. The top five and the null model are shown. n.a., not applicable.
| model no. | model | 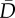 |
Popt | PED | ΔPED | s.e. | ΔPED/s.e. | closure effect |
|---|---|---|---|---|---|---|---|---|
| 1 | AB + SB + C | −8.16 | 112.1 | 103.9 | 0.0 | 0.00 | 0.00 | + |
| 7 | SB + C | −8.79 | 122.2 | 113.4 | 9.5 | 11.91 | 0.80 | + |
| 5 | SB + AC + C | −8.74 | 122.1 | 113.4 | 9.5 | 10.74 | 0.88 | + |
| 6 | SB + SC + C | −8.93 | 129.7 | 120.8 | 16.9 | 14.45 | 1.17 | + |
| 3 | AB + SC + C | −8.73 | 134.5 | 125.8 | 21.9 | 17.74 | 1.23 | + |
| 17 | null model | −8.90 | 215.3 | 206.4 | 102.5 | 33.48 | 3.06 | n.a. |
(d). Demographic model structure
We constructed a matrix model with one juvenile, three immature and one adult stage classes. We assumed a post-breeding census and that all individuals mature at 4 years [16]. The model was
| 2.1 |
where Nt is a vector holding the numbers in each stage at time t, and A is the population projection matrix:
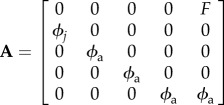 |
2.2 |
For A, ϕj = first year survival (0.343) and ϕa = immature and adult survival (0.743), as studies suggest they are equivalent [17]. Fecundity (F) = P × f × R × ϕa, where P = breeding probability (assumed to be 1); f = proportion of females in the population (assumed to be 0.5) and R = E × B × ϕe × ϕc, where E = clutch size (1.86 eggs) [18], B = breeding frequency (1.27 clutches per annum) [18], ϕe = egg survival (0.548) [18] and ϕc = chick survival. Using a starting population of 8512 pairs in 2004 [14], we first modelled the observed population trajectory for 2005–2013. We then simulated the population trajectory over 10 years (2014–2023) in the presence and absence of closure by modifying the ϕc component of F with the mean closure effect from the best supported model above.
3. Results
Three models were well supported (ΔPED/s.e. < 1), all containing positive closure effects (table 1 and electronic supplementary material, figure S1). The model with the lowest PED (model 1, table 1) and the third best model (model 5) were nested in the simpler model 7 (table 1), which accounted for changes in sardine biomass and closure status. Based on this (most parsimonious) model, chick survival in ‘Closed’ years was 0.658 (95% credible intervals: 0.523–0.773) versus 0.470 (0.395–0.546) in ‘Open’ years at mean sardine biomass (figure 1).
The demographic model reproduced the decline at Robben Island (figure 2), predicting 1349 pairs in 2013 (1.06% below the census figure). Without closure (ϕc = 0.470), the population growth rate (λ) = 0.815 and the 2023 population = 175 pairs. With closure (ϕc = 0.658), λ = 0.835 and the 2023 population = 222 pairs, a 26.9% increase. However, the projected population continued to decline in both cases and the difference (47 pairs) represented 3.5% of the 2013 population.
Figure 2.
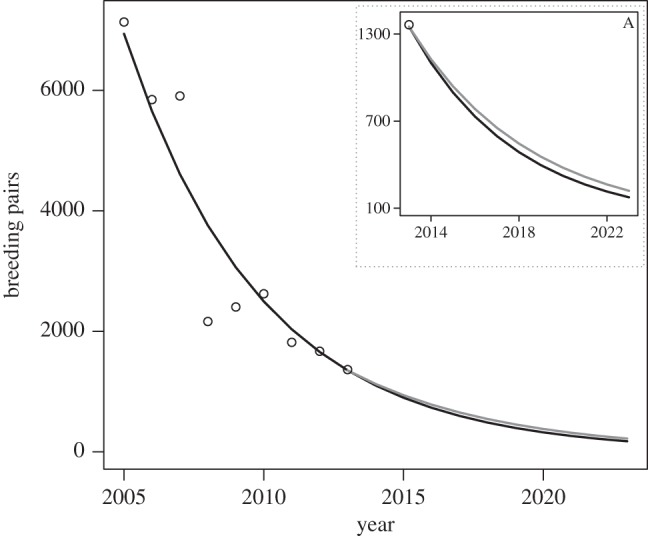
Observed (open circles) and modelled African penguin breeding population (pairs) if fishing continued for 2005–2023 (black line) and if fishing was excluded within 20 km of the island from 2014 to 2023 (grey line). (A) 2014–2023 projections on a scale from 100 to 1400 pairs.
4. Discussion
After controlling for long-term variation in prey availability, our results demonstrate that small-scale fishing closures can provide demographic benefits for penguins. Although the closure was relatively small, and catches continued at its boundary, chick survival was 18% higher on average when fishing was excluded, likely because of decreased prey depletion within the foraging range of breeding birds [5–7,10]. The population difference predicted to accrue over time supports the continuation of this closures programme [6].
Although our analysis suggests that if current conditions on the west coast prevail these closures will be insufficient to allow population recovery (figure 2), we only modelled an impact on chick survival. Population dynamics in long-lived vertebrates are often least sensitive to variation in fecundity. Thus, a key question remains whether small-scale closures can improve adult or juvenile survival. For African penguins elsewhere, closures decreased energy expenditure during provisioning [7], which may improve survival over time. Detecting such effects would require analysis of capture–mark–recapture data and a longer period of closures. In turn, this would allow for robust assessment of the magnitude of the population-level impacts of small-scale no-take zones.
Assessments of this kind are important to fully elucidate the role for targeted, small-scale fisheries closures in marine conservation. MPAs can contribute towards the conservation of marine predators, but rarely protect highly mobile species throughout their life cycle [2,3,19]. African penguins feed far from colonies when not breeding and have suffered poor adult survival over the last decade as the regional abundance of sardine fell below a critical threshold [17,20]. It is becoming increasingly clear that fishing can exacerbate forage fish population collapses [11], with consequences for predators [21]. The recent adult mortality observed in African penguins easily offsets the improved chick survival noted here. As a consequence, the conservation of African penguins (and many other marine predators) is likely to require strategies to maintain forage fish populations above critical thresholds [11,20,21] and spatial protection at various scales (i.e. MPA networks) [2]. In summary, our results support the use of small-scale fishing closures to conserve marine predators [4–6] but highlight the importance of integrating them into holistic, ecosystem-based management regimes.
Supplementary Material
Acknowledgements
The Department of Environmental Affairs and Robben Island Museum granted permission for our work. We thank all who assisted with penguin monitoring and Barbara Barham, Astrid Jarre, Tim Reid, Lynne Shannon and Florian Weller for useful discussions.
Data accessibility
The data are in the electronic supplementary material or Dryad digital repository: http://dx.doi.org/10.5061/dryad.t446r.
Authors' contributions
R.B.S., R.J.M.C. and C.D.v.d.L. contributed data. R.B.S. wrote the first draft and analysed the data with help from H.W., R.A. and S.C.V. All authors contributed to interpretation and revisions and approved the final version.
Competing interests
We have no competing interests.
Funding
We thank the Earthwatch Institute, Bristol Zoological Society, Leiden Conservation Foundation and National Research Foundation.
References
- 1.McCauley DJ, Pinsky ML, Palumbi SR, Estes JA, Joyce FH, Warner RR. 2015. Marine defaunation: animal loss in the global ocean. Science 347, 1255641 ( 10.1126/science.1255641) [DOI] [PubMed] [Google Scholar]
- 2.Lascelles B, et al. 2014. Migratory marine species: their status, threats and conservation management needs. Aquat. Conserv. Mar. Freshw. Ecosyst. 24, 111–127. ( 10.1002/aqc.2512) [DOI] [Google Scholar]
- 3.Hays GC, Mortimer JA, Ierodiaconou D, Esteban N. 2014. Use of long-distance migration patterns of an endangered species to inform conservation planning for the world's largest marine protected area. Conserv. Biol. 28, 1636–1644. ( 10.1111/cobi.12325) [DOI] [PubMed] [Google Scholar]
- 4.Daunt F, Wanless S, Greenstreet SPR, Jensen H, Hamer KC, Harris MP. 2008. The impact of the sandeel fishery closure on seabird food consumption, distribution, and productivity in the northwestern North Sea. Can. J. Fish. Aquat. Sci. 381, 362–381. ( 10.1139/F07-164) [DOI] [Google Scholar]
- 5.Frederiksen M, Jensen H, Daunt F, Mavor RA, Wanless S. 2008. Differential effects of a local industrial sand lance fishery on seabird breeding performance. Ecol. Appl. 18, 701–710. ( 10.1890/07-0797.1) [DOI] [PubMed] [Google Scholar]
- 6.Pichegru L, Ryan PG, van Eeden R, Reid T, Grémillet D, Wanless R. 2012. Industrial fishing, no-take zones and endangered penguins. Biol. Conserv. 156, 117–125. ( 10.1016/j.biocon.2011.12.013) [DOI] [Google Scholar]
- 7.Pichegru L, Grémillet D, Crawford RJM, Ryan PG. 2010. Marine no-take zone rapidly benefits endangered penguin. Biol. Lett. 6, 498–501. ( 10.1098/rsbl.2009.0913) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sherley RB, Underhill LG, Barham BJ, Barham PJ, Coetzee JC, Crawford RJM, Dyer BM, Leshoro TM, Upfold L. 2013. Influence of local and regional prey availability on breeding performance of African penguins Spheniscus demersus. Mar. Ecol. Prog. Ser. 473, 291–301. ( 10.3354/meps10070) [DOI] [Google Scholar]
- 9.Crawford RJM, Makhado AB, Waller LJ, Whittington PA. 2014. Winners and losers – response to recent environmental change by South African seabirds that compete with purse-seine fisheries for food. Ostrich 85, 111–117. ( 10.2989/00306525.2014.955141) [DOI] [Google Scholar]
- 10.Bertrand S, Joo R, Arbulu Smet C, Tremblay Y, Barbraud C, Weimerskirch H. 2012. Local depletion by a fishery can affect seabird foraging. J. Appl. Ecol. 49, 1168–1177. ( 10.1111/j.1365-2664.2012.02190.x) [DOI] [Google Scholar]
- 11.Essington TE, Moriarty PE, Froehlich HE, Hodgson EE, Koehn LE, Oken KL, Siple MC, Stawitz CC. 2015. Fishing amplifies forage fish population collapses. Proc. Natl. Acad. Sci. USA 112, 6648–6652. ( 10.1073/pnas.1422020112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cherry M. 2014. African penguins put researchers in a flap. Nature 514, 283 ( 10.1038/514283a) [DOI] [PubMed] [Google Scholar]
- 13.Therneau TM, Grambsch PM, Pankratz VS. 2003. Penalized survival models and frailty. J. Comput. Graph. Stat. 12, 156–175. ( 10.1198/1061860031365) [DOI] [Google Scholar]
- 14.Sherley RB, Barham PJ, Barham BJ, Crawford RJM, Dyer BM, Leshoro TM, Makhado AB, Upfold L, Underhill LG. 2014. Growth and decline of a penguin colony and the influence on nesting density and reproductive success. Popul. Ecol. 56, 119–128. ( 10.1007/s10144-013-0394-1) [DOI] [Google Scholar]
- 15.Plummer M. 2008. Penalized loss functions for Bayesian model comparison. Biostatistics 9, 523–539. ( 10.1093/biostatistics/kxm049) [DOI] [PubMed] [Google Scholar]
- 16.Whittington PA, Klages NTW, Crawford RJM, Wolfaardt AC, Kemper J. 2005. Age at first breeding of the African penguin. Ostrich 76, 14–20. ( 10.2989/00306520509485468) [DOI] [Google Scholar]
- 17.Sherley RB, Abadi F, Ludynia K, Barham BJ, Clark AE, Altwegg R. 2014. Age-specific survival and movement among major African penguin Spheniscus demersus colonies. Ibis 156, 716–728. ( 10.1111/ibi.12189) [DOI] [Google Scholar]
- 18.Shannon LJ, Crawford RJM. 1999. Management of the African penguin Spheniscus demersus—insights from modelling. Mar. Ornithol. 27, 119–128. [Google Scholar]
- 19.Boersma PD, Parrish JK. 1999. Limiting abuse: marine protected areas, a limited solution. Ecol. Econ. 31, 287–304. ( 10.1016/S0921-8009(99)00085-3) [DOI] [Google Scholar]
- 20.Robinson WML, Butterworth DS, Plaganyi EE. 2015. Quantifying the projected impact of the South African sardine fishery on the Robben Island penguin colony. ICES J. Mar. Sci. (In press.) ( 10.1093/icesjms/fsv035) [DOI] [Google Scholar]
- 21.Cury PM, et al. 2011. Global seabird response to forage fish depletion—one-third for the birds. Science 334, 1703–1706. ( 10.1126/science.1212928) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are in the electronic supplementary material or Dryad digital repository: http://dx.doi.org/10.5061/dryad.t446r.