Abstract
Rising levels of anthropogenic carbon dioxide in the atmosphere are acidifying the oceans and producing diverse and important effects on marine ecosystems, including the production of fatty acids (FAs) by primary producers and their transfer through food webs. FAs, particularly essential FAs, are necessary for normal structure and function in animals and influence composition and trophic structure of marine food webs. To test the effect of ocean acidification (OA) on the FA composition of fish, we conducted a replicated experiment in which larvae of the marine fish red drum (Sciaenops ocellatus) were reared under a climate change scenario of elevated CO2 levels (2100 µatm) and under current control levels (400 µatm). We found significantly higher whole-body levels of FAs, including nine of the 11 essential FAs, and altered relative proportions of FAs in the larvae reared under higher levels of CO2. Consequences of this effect of OA could include alterations in performance and survival of fish larvae and transfer of FAs through food webs.
Keywords: ocean acidification, fatty acids, larval fish, Sciaenops ocellatus
1. Introduction
Anthropogenic CO2 in the atmosphere is dissolving into the oceans and acidifying them [1–3]. This decline in pH is expected to be greater in coastal areas where the effects will be especially important because of the high biodiversity, presence of areas of special conservation interest (e.g. coral reefs), or importance to seafood production [2,3]. Ocean acidification (OA) has been demonstrated to affect fundamental processes of the early stages of fish, such as growth and survival [4], behaviour [5,6], auditory and olfactory function [7,8], otolith calcification [9], and even cause tissue damage [10]. The effect of OA on the synthesis or metabolic pathways of important biomolecules is less known.
Fatty acids (FAs) are biomolecules that are structural components of cell membranes, metabolized for energy, or stored for future use. FAs are designated as X:YωZ, where X is the number of carbon atoms, Y is the number of double bonds and ωZ indicates the position of the first double bond from the methyl terminus [11,12]. Some FAs can be assembled from precursors, but most animals cannot synthesize de novo enough of the long-chain (≥18 carbon atoms) FAs that contain multiple double bonds to meet their physiological requirements [12]. These highly unsaturated FAs are manufactured by primary producers, and animals obtain them almost exclusively from their diet. For that reason, they are known as essential FAs (EFAs) [11]. Some EFAs (e.g. eicosapentaenoic acid (EPA, 20 : 5ω3) and arachidonic acid (ARA, 20 : 4ω6)) are precursors of other important biomolecules, such as eicosanoids and prostaglandins. Moreover, EFAs are indispensable for the development of neural and retinal tissues and proper neural functioning in many animals, including humans [13].
Changes in the EFA composition of organisms at lower trophic levels due to OA are currently under scrutiny [14,15]. For example the majority of primary production in the oceans is expected to shift from diatoms to other microalgae (e.g. Phaeocystis) [16], and as a result EFA production in the oceans is expected to decrease [12,16]. Beyond this shift in availability of EFAs, OA may alter the way that animals process and store FAs obtained from their diet, which would have consequences for the animal's survival and the transfer of FAs to higher trophic levels [17–18]. We selected the marine fish red drum (Sciaenops ocellatus) as a model species to conduct the first experiments on the potential effect of OA on FA composition of fish larvae. Red drum is a species of high economic importance in aquaculture and recreational fisheries, inhabiting estuarine and coastal areas on the east coast of North America, which are endangered by global change and OA [2], and the species has been the subject of intense research on the dynamics and ecological significance of variations in FA composition of eggs and larvae [17–18].
2. Material and methods
(a). Ocean acidification experiment
Two batches of fertilized red drum eggs were collected from natural spawns from a single tank of broodstock. Each batch was divided into two treatment levels: control CO2 level (400 µatm) and high CO2 level (2100 µatm), and reared at a constant temperature (27.4 ± 0.3°C) and salinity (36.6 ± 0.9 ppt). Both high CO2 and control groups (two tanks per group) were fed equally with the same highly enriched FA diet. At day 23 post-hatching, all the fish surviving in each tank were euthanized and measured. For each tank, all the fish were then combined, lyophilized and homogenized, and then three samples were analysed for FAs composition using gas chromatography [18], measuring a total of 27 FAs.
(b). Statistical analyses
The number of fish remaining in each tank was compared between groups using a Mann–Whitney U-test; fish length distribution in each tank was compared across treatments using PERMANOVA, and a t-test was used to compare total FA content. For each FA, ANOVA or a Wilcoxon signed-rank test was used on raw or log-transformed FA concentrations and relative percentages for comparisons of the control and high CO2 groups (see electronic supplementary material, tables S1 and S2). A redundancy analysis (RDA) was performed on the complete FA composition for control and high CO2 groups, including egg batch as a factor. Statistical analyses were performed using the R package (www.r-project.org) [19].
3. Results
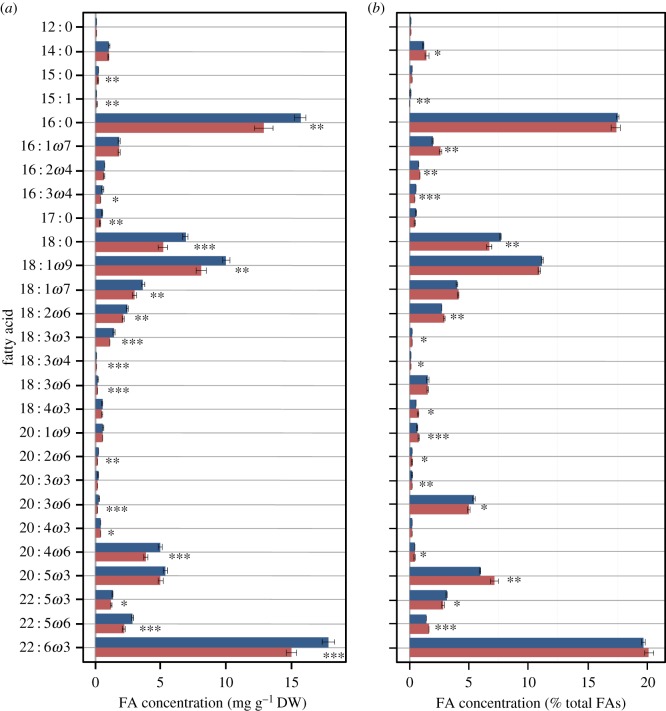
There was a significant effect of OA treatment on the number of fish remaining (Mann–Whitney U-test, p ≤ 0.01), with 61.7% more fish in the high CO2 group. Mean fish length was significantly smaller (25.3%) in fish reared under high CO2 conditions (PERMANOVA, Pseudo-F: 26.7; p ≤ 0.001). Total FA content was significantly higher in the high CO2 group (t-test, t9.77: −4.2, p ≤ 0.01). Analyses of individual FAs (expressed as mg g−1 dry weight) showed that 19 of the 27 FAs had significantly higher values under high CO2 (figure 1a; electronic supplementary material, table S1), including higher levels for nine of the 11 EFAs. Expressed on a relative basis (% total FAs), nine FAs were disproportionately higher under elevated CO2; nine were disproportionately lower; and nine remained relatively unchanged (figure 1b; electronic supplementary material, table S2).
Figure 1.
Mean concentrations of FAs (mg FA g−1 dry weight) (a) and mean relative % of FAs (b); in red drum larvae reared under control (red) and high CO2 (blue) conditions. Error bars are 1 s.e.. Asterisks indicate significant differences (see electronic supplementary material, tables S1 and S2) (*p < 0.05; **p < 0.01; ***p < 0.001). (Online version in colour.)
The RDA interaction model (model adjusted R2 = 50%) showed that CO2 level was a significant factor but egg batch was not (CO2 level: F1,8: 10.1, p ≤ 0.001; egg batch: F1,8: 1.4, p > 0.05) (figure 2; electronic supplementary material, table S3). Only the first RDA axis was significant, explaining 54.3% of the model variance (figure 2; electronic supplementary material, table S3).
Figure 2.
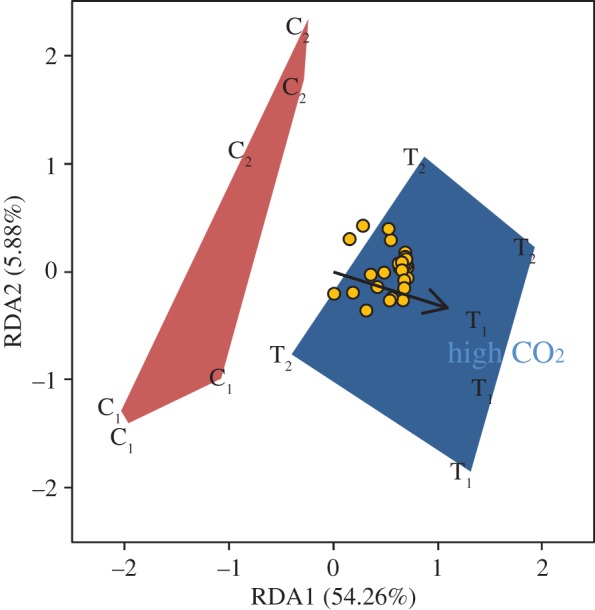
Ordination plot of RDA results. Letters and numbers identify CO2 level and replicate number (C = control (400 µatm); T = high CO2 (2100 µatm)). Circles represent the FAs measured in red drum larvae. Coloured polygons enclose each treatment group. Arrow shows the direction and intensity of the effect of high CO2 on FA composition. (Online version in colour.)
4. Discussion
Our results identify a strong effect of elevated CO2 levels (2100 µatm of CO2, predicted for the year 2300 [1]) on the FA content of larval fish. This agrees with recent work that shows an increase in total lipid content of cod (Gadus morhua) larvae under high levels of CO2 (4200 µatm) [10]. That prior study reported no differences in the composition of the lipids, while we found 19 of the 27 FAs analysed to be significantly elevated at only 50% of the level of CO2 used in the prior study. Further, the relative amounts of different FAs varied under elevated CO2, with some FAs increasing significantly and others decreasing significantly. While OA usually jeopardizes larval survival [4,6], it is worth noting that the increase in some of these FAs may improve ecological performance of the larvae as recent studies of red drum larvae have shown that higher levels of some EFAs are positively correlated with larval escape performance [17,20]. Three EFAs—DHA, EPA and ARA—are especially important to larval fish physiology [11] and were expected to be closely regulated, regardless of OA. Surprisingly, DHA and ARA increased on a weight basis, and EPA and ARA decreased on a percentage basis under OA conditions. These changes could have significant impacts on physiological functions.
Higher tissue levels of EFAs could potentially result from increased absorption, synthesis, biotransformation and/or storage. Some marine fish can manufacture EFAs but their capacity is limited [11,18,21]. Increased absorption of ingested EFAs is an unlikely explanation because absorption of other nutrients would have increased as well, leading to more growth, but larval growth decreased while tissue levels of EFAs increased. Rather, we suggest that red drum larvae under this stressor deposit a larger portion of the ingested FAs in tissues. Nevertheless, the mechanism through which this response to OA operates is unknown.
OA can affect organisms and ecosystems by altering FA production (e.g. changes in communities of primary producers [22]) or through effects on uptake of FAs by higher trophic levels [23]. We showed that storage of many FAs by red drum larvae increases and that FA proportions differ under OA. The consequences of these changes in FAs in tissues on ecological performance of fish larvae and on food web structure and function need to be explored for a more complete understanding of the impacts of OA on marine ecosystems.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Andrew J. Esbaugh, Benjamin Walther, John Mohan, Jeff Kaiser, Andrew Kang and Genesok Oh for their assistance.
Ethics
Procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Texas at Austin under Animal Use Protocols AUP-2013-00041, AUP-2013-00155 and AUP-2012-00133.
Authors' contributions
C.D.G., I.A.C., M.P., C.F. and L.A.F. designed the experiment; C.D.G. performed the experiment. C.D.G. and C.F. carried out the FA composition analysis; L.A.F., M.P. and C.D.G. analysed data. C.D.G., I.A.C., M.P. and L.A.F. wrote the manuscript. All authors discussed the results and contributed to the final version of the manuscript.
Competing interests
Authors declare no competing interest.
Funding
C.D.G. was funded by FPI-INIA-2012, this manuscript was financed by the research project REC2 (grant no. CTM2011-23835). Contribution 1705 of the University of Texas Marine Science Institute.
References
- 1.Caldeira K, Wickett ME. 2005. Ocean model predictions of chemistry changes from carbon dioxide emissions to the atmosphere and ocean. J. Geophys. Res. 110, C09S04 ( 10.1029/2004JC002671) [DOI] [Google Scholar]
- 2.Rhein M, et al. 2013. Observations: ocean. In IPCC climate change 2013: the physical science basis. Contribution of working group I to the fifth assessment report of the Intergovernmental Panel on Climate Change (eds Stocker TF, et al.), pp. 255–316. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 3.Doney SC, et al. 2012. Climate change impacts on marine ecosystems. Annu. Rev. Mar. Sci. 4, 11–37. ( 10.1146/annurev-marine-041911-111611) [DOI] [PubMed] [Google Scholar]
- 4.Baumann H, Talmage SC, Gobler CJ. 2012. Reduced early life growth and survival in a fish in direct response to increased carbon dioxide. Nat. Clim. Change 2, 38–41. ( 10.1038/nclimate1291) [DOI] [Google Scholar]
- 5.Welch MJ, Watson S-A, Welsh JQ, McCormick MI, Munday PL. 2014. Effects of elevated CO2 on fish behaviour undiminished by transgenerational acclimation. Nat. Clim. Change 4, 1086–1089. ( 10.1038/nclimate2400) [DOI] [Google Scholar]
- 6.Dixson DL, Munday PL, Jones GP. 2010. Ocean acidification disrupts the innate ability of fish to detect predator olfactory cues. Ecol. Lett. 13, 68–75. ( 10.1111/j.1461-0248.2009.01400.x) [DOI] [PubMed] [Google Scholar]
- 7.Simpson SD, Munday PL, Wittenrich ML, Manassa R, Dixson DL, Gagliano M, Yan HY. 2011. Ocean acidification erodes crucial auditory behaviour in a marine fish. Biol. Lett. 7, 917–920. ( 10.1098/rsbl.2011.0293) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munday PL, Dixson DL, Donelson JM, Jones GP, Pratchett MS, Devitsina GV, Døving KB. 2009. Ocean acidification impairs olfactory discrimination and homing ability of a marine fish. Proc. Natl Acad. Sci. USA 106, 1848–1852. ( 10.1073/pnas.0809996106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Checkley DM, Dickson AG, Takahashi M, Radich JA, Eisenkolb N, Asch R. 2009. Elevated CO2 enhances otolith growth in young fish. Science 324, 1683 ( 10.1126/science.1169806) [DOI] [PubMed] [Google Scholar]
- 10.Frommel AY, Maneja R, Lowe D, Malzahn AM, Geffen AJ, Folkvord A, Piatkowski U, Reusch TBH, Clemmesen C. 2011. Severe tissue damage in Atlantic cod larvae under increasing ocean acidification. Nat. Clim. Change 2, 42–46. ( 10.1038/nclimate1324) [DOI] [Google Scholar]
- 11.Tocher DR. 2003. Metabolism and functions of lipids and fatty acids in teleost fish. Rev. Fish. Sci. 11, 107–184. ( 10.1080/713610925) [DOI] [Google Scholar]
- 12.Brett MT, Müller-Navarra DC. 1997. The role of highly unsaturated fatty acids in aquatic foodweb processes. Freshw. Biol. 38, 483–499. ( 10.1046/j.1365-2427.1997.00220.x) [DOI] [Google Scholar]
- 13.Crawford MA, Broadhurst CL. 2012. The role of docosahexaenoic and the marine food web as determinants of evolution and hominid brain development: the challenge for human sustainability. Nutr. Health 21, 17–39. ( 10.1177/0260106012437550) [DOI] [PubMed] [Google Scholar]
- 14.Torstensson A, Hedblom M, Andersson J, Andersson MX, Wulff A. 2013. Synergism between elevated pCO2 and temperature on the Antarctic sea ice diatom Nitzschia lecointei. Biogeosciences 10, 6391–6401. ( 10.5194/bg-10-6391-2013) [DOI] [Google Scholar]
- 15.Leu E, Daase M, Schulz KG, Stuhr A, Riebesell U. 2013. Effect of ocean acidification on the fatty acid composition of a natural plankton community. Biogeosciences 10, 1143–1153. ( 10.5194/bg-10-1143-2013) [DOI] [Google Scholar]
- 16.Desvilettes C, Bec A. 2009. Formation and transfter of fatty acids in aquatic microbial food webs: role of heterotrophic protists. In Lipids in aquatic organisms (eds Arts MT, Brett MT, Kainz MJ.), pp. 25–42. New York, NY: Springer. [Google Scholar]
- 17.Fuiman LA, Ojanguren AF. 2011. Fatty acid content of eggs determines antipredator performance of fish larvae. J. Exp. Mar. Biol. Ecol. 407, 155–165. ( 10.1016/j.jembe.2011.06.004) [DOI] [Google Scholar]
- 18.Faulk CK, Holt GJ. 2008. Biochemical composition and quality of captive-spawned cobia Rachycentron canadum eggs. Aquaculture 279, 70–76. ( 10.1016/j.aquaculture.2008.03.050) [DOI] [Google Scholar]
- 19.Team RDC. 2011. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 20.Perez KO, Fuiman LA. 2015. Maternal diet and larval diet influence survival skills of larval red drum Sciaenops ocellatus. J. Fish Biol. 86, 1286–1304. ( 10.1111/jfb.12637) [DOI] [PubMed] [Google Scholar]
- 21.Norambuena F, Morais S, Estévez A, Bell JG, Tocher DR, Navarro JC, Cerdà J, Duncan N. 2013. Dietary modulation of arachidonic acid metabolism in senegalese sole (Solea senegalensis) broodstock reared in captivity. Aquaculture 372–375, 80–88. ( 10.1016/j.aquaculture.2012.10.035) [DOI] [Google Scholar]
- 22.Wynn-Edwards C, King R, Davidson A, Wright S, Nichols P, Wotherspoon S, Kawaguchi S, Virtue P. 2014. Species-specific variations in the nutritional quality of Southern Ocean phytoplankton in response to elevated pCO2. Water 6, 1840–1859. ( 10.3390/w6061840) [DOI] [Google Scholar]
- 23.Litzow MA, Bailey KM, Prahl FG, Heintz R. 2006. Climate regime shifts and reorganization of fish communities: the essential fatty acid limitation hypothesis. Mar. Ecol. Prog. Ser. 315, 1–11. ( 10.3354/meps315001) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.