Abstract
Flower-visiting insects exhibit innate preferences for particular colours. A previous study demonstrated that naive Papilio xuthus females prefer yellow and red, whereas males are more attracted to blue. Here, we demonstrate that the innate colour preference can be modified by olfactory stimuli in a sexually dimorphic manner. Naive P. xuthus were presented with four coloured discs: blue, green, yellow and red. The innate colour preference (i.e. the colour first landed on) of the majority of individuals was blue. When scent from essential oils of either orange flower or lily was introduced to the room, females’ tendency to select the red disc increased. Scents of lavender and flowering potted Hibiscus rosa-sinensis, however, were less effective. Interestingly, the odour of the non-flowering larval host plant, Citrus unshiu, shifted the preference to green in females. In males, however, all plant scents were less effective than in females, such that blue was always the most favoured colour. These observations indicate that interactions between visual and olfactory cues play a more prominent role in females.
Keywords: colour vision, Lepidoptera, foraging, olfaction, innate preference, sexual dimorphism
1. Introduction
Innate colour preference has been reported in many flower-visiting insects. Honeybees, bumblebees, flies, hawkmoths and butterflies are generally attracted to blue [1,2], though some butterflies appear to prefer either yellow or reddish colours [3,4]. We have previously found that the innately preferred colour of the Japanese yellow swallowtail butterfly, Papilio xuthus, is sexually dimorphic: females prefer yellow and red, whereas males prefer blue [5]. This colour preference can be altered of course by visits to rewarding flowers.
Besides visual information such as colour and shape, floral scents are another crucial cue for flower visitors [3]. Hymenopterans can detect and learn a wide range of floral volatiles from various plant species. Nocturnal moths visit flowers that have strong smells but pale colours, and floral scents play vital roles in various aspects of their foraging behaviour. In diurnal Lepidoptera, floral scents may play a lesser but still important role in triggering searching behaviour, or in attraction at close range. For butterflies, some floral volatiles have a greater behavioural influence than others, even though their antennae can detect a wide variety of volatiles [6].
The interaction between scent and colour has been extensively studied in hawkmoths [3] and honeybees [7]. The importance of these cues is context-dependent in both groups. In general, diurnal butterflies and hawkmoths rely on visual cues over olfactory ones when foraging, whereas the opposite is true of nocturnal moths. In naive Manduca sexta, both visual and olfactory cues are required to elicit proper foraging behaviour [6].
Why is the innate colour preference of P. xuthus sexually dimorphic? We addressed this question by testing their colour preference in a laboratory where light and chemical conditions were carefully controlled. Our results indicate that the basic innate colour preference is blue in both sexes, but that sexual dimorphism in innate colour preference arises from differences in the effects of particular plant scents between sexes.
2. Material and methods
(a). Animals
We used laboratory-raised summer form P. xuthus of both sexes. Each newly emerged adult was kept individually in a box covered with gauze, without feeding, for a few days until the experiment. All tested butterflies were naive in terms of foraging behaviour, and unmated. In experiments to confirm that the antennae detect odour, we blocked both antennae by coating them with clear mascara on the day of emergence.
(b). Colour preference test
All experiments were performed in a cage (H 46 × W 62 × D 42 cm, figure 1a). The room temperature was around 30°C. Light intensity in the cage was between 6000 and 8000 lux, produced by 12 halogen lamps, six fluorescent tubes and four xenon lamps (figure 1a).
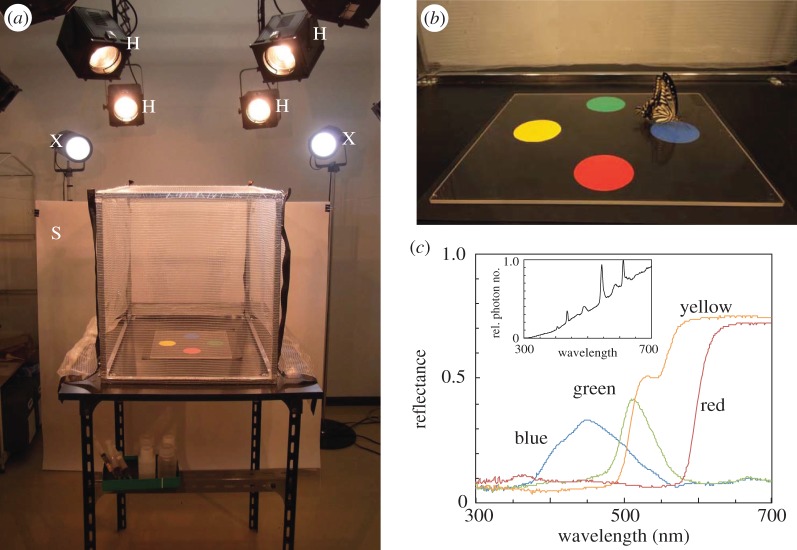
Figure 1.
(a) Experimental set-up. A small cage was illuminated by halogen lamps (H), fluorescent tubes and xenon lamps (X). A screen (S) was set at one side of the cage. (b) Colour preference test. A starved P. xuthus lands on one of four coloured discs without any training. (c) Visual stimuli reflectance spectra of the four coloured papers and irradiation spectrum of the illumination (inset).
In all experiments, one butterfly was released into the cage where the four coloured discs were presented on the floor (figure 1b). The colour of the disc upon which the butterfly first landed and extended its proboscis was recorded as its innate preference. If an individual did not visit any disc within 5 min of release, we retested it later that day or the following day.
(c). Stimuli
The visual stimulus consisted of four coloured discs (blue, green, yellow and red, diameter 5 cm), which are clearly discriminable for foraging P. xuthus [5], with 5 cm spacing, on a black background and covered with quartz glass (figure 1b). One of three different arrangements was selected at random for each butterfly. Figure 1c shows the reflectance spectra of the coloured discs and the irradiation spectrum of the illumination.
As odourant sources, we used essential oils of three different flowers, potted plants of two species, and two synthetic mimics. The essential oils were Neroli: bitter orange, Citrus aurantium; Lirio: Lilium spp. and Lavender: Lavender angustifolia. Six potted plants of either Citrus unshiu or flowering Hibiscus rosa-sinensis were used. We produced two synthetic mimics: (Neroli and C. unshiu tree scent) from authentic samples of the volatile organic compounds (VOCs) identified by gas chromatography–mass spectrometry analyses (electronic supplementary material, S1 and tables S1 and S2). Quantity and delivery method for each odourant was determined according to preliminary experiments (electronic supplementary material, S1). All odourant sources were hidden from the tested animals. After experiments with one particular odourant, we ventilated the room for at least 5 days. Before commencing the next experiment, we performed a control test to ensure that there was no lingering effect of the previous odourant.
(d). Statistical analysis
We used multinominal tests to determine whether the distribution of visits among the four colours was random. Fisher's exact test was used to compare the distributions of visits among the four colours under a particular odourant versus the control. We used an analogous approach to compare the distributions of visits between sexes (electronic supplementary material, S1).
3. Result and discussion
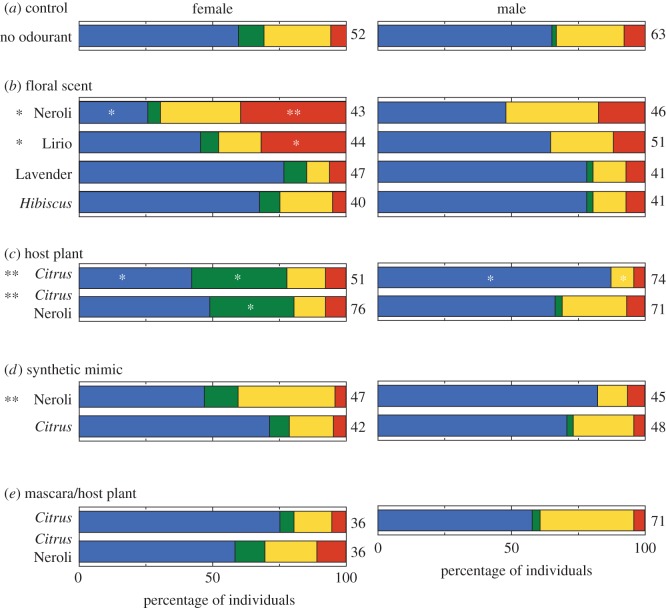
Each starved naive P. xuthus landed on a coloured disc shortly after being released into a small cage (figure 1b). When no particular scent was present in the room, more than half of both males and females selected blue (figure 2a and electronic supplementary material, table S3), which is a common behaviour across insect species [1].
Figure 2.
Innate colour preference of intact individuals in different ambient odour conditions; (a) no particular plant scent (control), (b) floral scent, (c) larval host plants, (d) synthetic mimics and (e) innate colour preference with host plants in individuals whose antennae were blocked with mascara. White asterisks indicate significant differences in proportion of visits to each colour between the odourless (control) and various plant scent conditions (Fisher's exact test; *p < 0.05, **p < 0.001). Black asterisks on the left show between-sex comparisons of the distribution of visits over all four colours (Fisher's exact test; *p < 0.05, **p < 0.001). Numbers on the right side indicate numbers of tested individuals.
This innate preference for blue in females is at odds with our previous study, where female P. xuthus preferred yellow and red [5]. We therefore hypothesized that plant scents, another important cue for foraging, might have affected their innate colour preference [3,6].
We first tested the effects of floral scents using either essential oils—Neroli, Lirio and Lavender—or flowering potted H. rosa-sinensis plants (figure 2b). In the Neroli and Lirio conditions, innate colour preference in females differed significantly from the odourless control (Fisher's exact test; p < 0.01 in Neroli condition and p = 0.009 for Lirio condition). With Neroli, the frequency of visits to the red disc increased, whereas that to blue decreased. Similarly, Lirio odour increased the number of females selecting red. However, no effect of Lavender nor Hibiscus was found (electronic supplementary material, table S3). This odour specificity in the colour preference effects presumably relates to the fact that some particular floral volatiles appear to be important for attracting butterflies [6].
Second, we introduced larval host plants, C. unshiu, to the room. The scent of Citrus trees, non-flowering, significantly modified the innate colour preference of females (Fisher's exact test; p = 0.004), which visited the green disc more and the blue one less frequently than in the control (figure 2c). Interestingly, a similar effect was observed even when Neroli was presented together with Citrus trees (Fisher's exact test; p = 0.025); the Citrus tree scent appeared to mask the effect of Neroli. Ovipositing butterflies are generally attracted to greenish colours [8], but none of the (unmated) females in this study displayed oviposition behaviour towards the green disc. Presumably, attraction to green triggered by Citrus tree odour is advantageous even for virgin female P. xuthus, as it would encourage them to stay in the vicinity of the host plant.
Plant scents can be characterized according to their concentrations of various VOCs. We attempted to ascertain whether the observed colour preference effects are linked to particular VOCs by using synthetic mimics of Neroli and C. unshiu scent. These mimics, however, did not reproduce the colour preference effects of natural scents (figure 2d), suggesting that minor VOC components might be necessary for this behaviour, as in other butterfly species [6].
With particular plant scents, a clear sexual dimorphism in innate colour preference emerged (figure 2: black asterisks). Compared with females, effects of all odours were rather limited in males (figure 2: right column). In females whose antennae were blocked, the effects of both Citrus trees and Neroli disappeared (figure 2e), demonstrating that the scents affecting colour preference are indeed detected via the antennae. Because no clear sexual dimorphism in the visual abilities or retinal organization of P. xuthus has been detected [9], this behavioural sex difference may be attributed to the olfactory system, or to the system integrating multi-modal information. In fact, three glomeruli among about 60 in the antennal lobe of female P. xuthus are enlarged compared with those of males [10]. These sexually dimorphic glomeruli are presumably analogous to those of M. sexta that respond to the major volatiles of host plants [11].
Because the mushroom body (MB) of P. xuthus receives prominent visual inputs as well as olfactory ones [12], it might facilitate effective foraging by integrating visual and olfactory information to produce the effect we observed. A similar organization of the MB in bees has led to the suggestion that the structure is involved in the integration of multiple sensory modalities, as well as olfactory learning [13]. In addition, certain colours either enhance or suppress MB activity in response to particular scents in M. sexta [7]. Papilio xuthus appears to have poor olfactory memory (unpublished data, 2010), but can be readily trained to colours. We speculate, therefore, that the MB of P. xuthus may be involved in colour learning and integration of multi-sensory modalities.
In the wild, P. xuthus often feed on reddish flowers [14]. Females might initially feed successfully from reddish flowers thanks to their innate preference being modified by floral scent. Subsequent learning would then reinforce this tendency to visit reddish flowers. How floral scents influence foraging behaviour of P. xuthus in the wild, and in particular, how males come to be attracted to reddish flowers, remain open questions and may be revealed by future studies.
Supplementary Material
Acknowledgements
We thank Dr H Ohtsuki for assistance with statistical analysis and Dr F Stewart for useful comments and English editing.
Ethics
The experiments were performed under the MEXT guideline for proper conduct of animal experiments and related activities in academic research institutions.
Data accessibility
Detailed odourant stimuli, chemical analysis of plant scents, statistical analysis and three tables can be found in electronic supplementary material.
Authors' contributions
M.K., M.Y. and K.A. designed the experiment. M.K. and M.Y. carried out the behavioural experiments. Y.I. and H.O. measured the VOCs and made synthetic mimics. M.K., M.Y., K.A., Y.I. and H.O. wrote the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by JSPS KAKENHI for Scientific Research no. 24570084 to M.K. and no. 26251036 to K.A. and the NARO grant for SIP ‘Technologies for creating next-generation agriculture, forestry and fisheries' to K.A.
References
- 1.Lunau K, Maier EJ. 1995. Innate colour preferences of flower visitors. J. Comp. Physiol. A 177, 1–19. ( 10.1007/s00114-013-1060-3) [DOI] [Google Scholar]
- 2.Kelber A. 1997. Innate preferences for flower features in the hawkmoth Macroglossum stellatarum. J. Exp. Biol. 200, 827–836. [DOI] [PubMed] [Google Scholar]
- 3.Dobson HEM. 2006. Relationship between floral fragrance composition and type of pollinator. In Biology of floral scent (eds Dudareva N, Pichersky E.), pp. 147–198. Boca Raton, FL: Taylor & Francis. [Google Scholar]
- 4.Blackiston D, Briscoe AD, Weiss MR. 2011. Color vision and learning in the monarch butterfly, Danaus plexippus (Nymphalidae). J. Exp. Biol. 214, 509–520. ( 10.1242/jeb.048728) [DOI] [PubMed] [Google Scholar]
- 5.Kinoshita M, Shimada N, Arikawa K. 1999. Colour vision of the foraging swallowtail butterfly Papilio xuthus. J. Exp. Biol. 202, 95–102. [DOI] [PubMed] [Google Scholar]
- 6.Anderson S. 2006. Floral scent and butterfly pollinators. In Biology of floral scent (eds Dudareva N, Pichersky E.), pp. 199–217. Boca Raton, FL: Taylor & Francis. [Google Scholar]
- 7.Leonard AS, Masek P. 2014. Multisensory integration of colors and scents: insights from bees and flowers. J. Comp. Physiol. A 200, 463–474. ( 10.1007/s00359-014-0904-4) [DOI] [PubMed] [Google Scholar]
- 8.Kelber A. 1999. Ovipositing butterflies use a red receptor to see green. J. Exp. Biol. 202, 2619–2630. [DOI] [PubMed] [Google Scholar]
- 9.Kinoshita M, Arikawa K. 2014. Color and polarization vision in foraging Papilio. J. Comp. Physiol. A 200, 513–526. ( 10.1007/s00359-014-0903-5) [DOI] [PubMed] [Google Scholar]
- 10.Kinoshita M, Yoshida M, Arikawa K. 2012. Odor of Citrus trees causes sexual dimorphism in innate color preference of swallowtail butterflies. Front. Behav. Neurosci. Conf. Abstract: Tenth International Congress of Neuroethology. ( 10.3389/conf.fnbeh.2012.27.00222) [DOI] [Google Scholar]
- 11.King JR, Christensen TA, Hildebrand JG. 2000. Response characteristics of an identified, sexually dimorphic olfactory glomerulus. J. Neurosci. 20, 2391–2399. (doi:10270-6474/00/202391-09$15.00/0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kinoshita M, Shimohigashi M, Tominaga Y, Arikawa K, Homberg U. 2015. Topographically distinct visual and olfactory inputs to the mushroom body in the swallowtail butterfly, Papilio xuthus. J. Comp. Neurol. 523, 162–182. ( 10.1002/cne.23674) [DOI] [PubMed] [Google Scholar]
- 13.Farris SM. 2013. Evolution of complex higher brain centers and behaviors: behavioral correlates of mushroom body elaboration in insects. Brain Behav. Evol. 82, 9–18. ( 10.1159/000352057) [DOI] [PubMed] [Google Scholar]
- 14.Hirota SK, Nitta K, Kim Y, Kato A, Kawakubo N, Yasumoto AA, Yahara T. 2012. Relative role of flower color and scent on pollinator attraction: experimental tests using F1 and F2 hybrids of daylily and nightlily. PLoS ONE 7, e39010 ( 10.1371/journal.pone.0039010) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Detailed odourant stimuli, chemical analysis of plant scents, statistical analysis and three tables can be found in electronic supplementary material.