Abstract
The origin and evolution of clitellate annelids—earthworms, leeches and their relatives—is poorly understood, partly because body fossils of these delicate organisms are exceedingly rare. The distinctive egg cases (cocoons) of Clitellata, however, are relatively common in the fossil record, although their potential for phylogenetic studies has remained largely unexplored. Here, we report the remarkable discovery of fossilized spermatozoa preserved within the secreted wall layers of a 50-Myr-old clitellate cocoon from Antarctica, representing the oldest fossil animal sperm yet known. Sperm characters are highly informative for the classification of extant Annelida. The Antarctic fossil spermatozoa have several features that point to affinities with the peculiar, leech-like ‘crayfish worms' (Branchiobdellida). We anticipate that systematic surveys of cocoon fossils coupled with advances in non-destructive analytical methods may open a new window into the evolution of minute, soft-bodied life forms that are otherwise only rarely observed in the fossil record.
Keywords: Annelida, Clitellata, fossilization, spermatozoa, taphonomy, Antarctica
1. Background
Despite recent advances in molecular phylogenetics [1–4], the evolutionary history of Clitellata—earthworms, leeches and their relatives—is still poorly understood. This is in part because the delicate bodies of clitellates consist almost entirely of soft tissues, and can thus become fossilized only under exceptional circumstances [5]. Nevertheless, these organisms have left a peculiar presence in the fossil record in the form of dispersed egg cases (cocoons), which are very resistant to physical and chemical decay [6]. Clitellate cocoons are common, though sporadically illustrated, components of plant micro- and mesofossil assemblages obtained via bulk dissolution of clastic sedimentary rocks [6–9] as old as Middle Triassic [10]. This potential source of information about the origin and evolutionary history of clitellates has, however, received little scientific attention thus far, because it would appear impossible to determine the systematic affinities of the cocoon producers in any greater detail based on morphology alone.
Here, we report the occurrence of fossil spermatozoa that are preserved embedded in the wall layers of a 50-Myr-old (early Eocene) clitellate cocoon from Antarctica. These are the oldest fossil animal spermatozoa yet identified. Some morphological features of the spermatozoa are reminiscent of those of extant Branchiobdellida, a peculiar group of leech-like worms whose extant representatives are ectosymbionts on freshwater crayfish.
2. Material and methods
The fossil Clitellata cocoons were collected from marginal-marine deposits of the La Meseta Formation, Seymour/Marambio Island, Antarctic Peninsula (e.g. [11]; electronic supplementary material, figure S1). Individual cocoons were picked from dry-sieved sediment samples of poorly consolidated, shelly conglomerate informally referred to as the ‘Natica horizon’. The age of this deposit is considered to be approximately 50 Ma (Ypresian, early Eocene) based on strontium isotope dating and mammal biostratigraphy (e.g. [11]; see the electronic supplementary material). The fossils were analysed via light and scanning electron microscopy and via synchrotron-radiation-based X-ray tomographic microscopy (electronic supplementary material).
3. Results
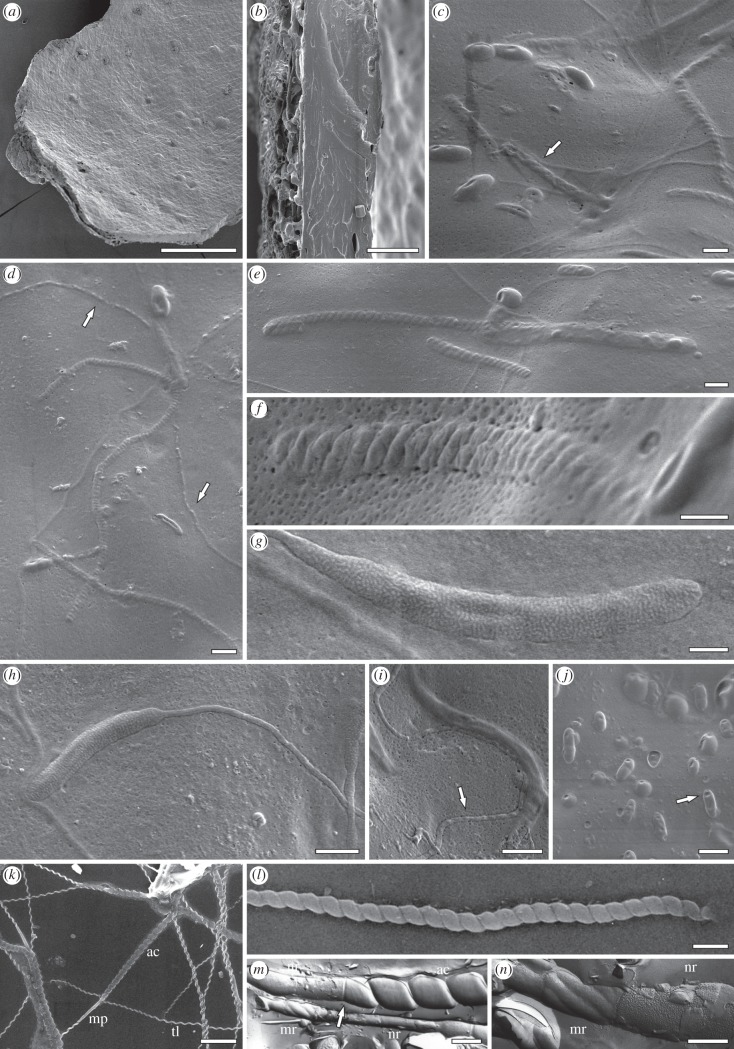
A single, approximately 1.5 × 0.8 mm, small annelid-cocoon fragment (specimen NRM-S089729) exposes the inner surface of the cocoon wall (figure 1a). The wall consists of a more than 25-µm-thick solid inner layer enveloped by a 5–10-µm-thick spongy outer layer composed of a loosely amalgamated network of interwoven ‘cables' up to approximately 5 µm in diameter [12] (figure 1b).
Figure 1.
Scanning electron micrographs of the Antarctic annelid-cocoon fossils showing cocoon structure and included spermatozoan fragments and bacteria (a–j), with images of extant branchiobdellid spermatozoa for comparison (k–n). (a) Overview of cocoon fragment. (b) Fracture surface showing spongy outer (left) and compact inner (right) wall layers. (c) Encased bacteria and spermatozoan fragments; note element showing multiple coils (arrow). (d) Encased spermatozoan fragments with tail portions (arrows). (e) Encased spermatozoan fragments resembling acrosomes. (f) Encased spermatozoan fragment tangentially encased in the cocoon wall. (g) Spermatozoan fragment showing granular texture. (h) Spermatozoan fragment showing granular texture and attached tail portion. (i) Isolated tail portion showing beaded structure (arrow). (j) Encased rod-shaped bacilli with characteristic dimples (arrow). (k–n) Spermatozoa of Branchiobdella sp. showing ‘drill-bit’ type acrosomes (ac), mid-pieces (mp) with nuclear (nr) and mitochondrial regions (mr), and tail regions (tl); note the suture between acrosome and nuclear region in (m) (arrow). Scale bars: (a) = 250 µm; (b) = 20 µm; (c–e,h,i,m) = 1 µm; (f,g,n) = 500 nm; (j,l) = 2 µm; (k) = 10 µm.
SEM analysis revealed various micro- and nano-inclusions embedded in the inner layer of the cocoon wall (figure 1c). The most conspicuous biological inclusions are fragments of straight or variably bent, narrowly cylindrical elements that reach approximately 18 µm long by approximately 600 nm wide and have a characteristic, helical ‘drill-bit’ structure, in some cases containing more than 80 gyres each with indistinct transverse striations (figure 1d–f). Other elements are rod-shaped, up to 12 µm long and approximately 500 nm wide, and with a finely granular texture (figure 1g,h). These commonly bear a whip-like tail up to approximately 250 nm thick and more than 30 µm long (figure 1h). Where these tails are well preserved and sharply defined, they reveal a regular beaded morphology reminiscent of a helical anatomical structure (figure 1i). In addition, we found a few approximately 5-µm-long and approximately 400-nm-wide, isolated elements that are composed of several intertwined, coiled fibres (figure 1c) that have a significantly lesser angle of coiling than the rather strongly compressed, simple spiral of the ‘drill-bit’ elements (figure 1d–f). Associated fossil bacteria consist mostly of rod-shaped, approximately 2-µm-long and approximately 0.8-µm-wide bacilli (figure 1j); many bear a characteristic dimple on the surface (figure 1j, arrow), and some occur in chains or clusters. Additionally, we attempted synchrotron-radiation-based X-ray tomographic microscopy on a portion of a cocoon wall; possible spermatozoa were detectable, but only at the limit of the instrument's resolution (see electronic supplementary material, figure S2).
4. Discussion
Similarities in dimensions, structure and texture indicate that the isolated elements described above represent the various components of the specialized filiform spermatozoa typical of clitellate annelids. Being very short-lived and delicate structures, spermatozoa are very rare in the fossil record. Perhaps the best-documented examples are spermatozoids of early land plants from the Devonian Rhynie Chert [13] and of gymnosperms from the Permian of Australia [14]. Preservation of animal spermatozoa is exceedingly rare, with just single pre-Quaternary records of collembolan spermatozoa from late Eocene Baltic amber [15] and of phosphatized giant spermatozoa of ostracods from Miocene cave deposits of Australia [16]. The clitellate spermatozoa described herein thus constitute the oldest fossil animal spermatozoa yet recorded, predating the previous oldest occurrence (late Eocene [15]) by at least 10 Myr.
Interestingly, of all morphological features that the annelid body plan exhibits, those that are currently considered among the most informative for resolving systematic relationships relate to the morphology and ultrastructure of the spermatozoa [2,17–20]. However, detailed comparisons of the fossils with extant taxa are difficult at present for several reasons: first, although progressively more living taxa are being sampled [21], our knowledge of the structural diversity of extant clitellate spermatozoa is still very incomplete; second, the fossil material consists mostly of disarticulated remains that yield limited information about the architecture of the complete spermatozoa; and third, the study of extant material has relied largely on TEM analysis to resolve ultrastructural features, whereas little is known about the gross morphology and texture of spermatozoa when studied using SEM, as is the case with the fossil material. We surmise that future detailed analyses of fossil cocoon inclusions, perhaps using nanotomographic methods (e.g. [16]), might reveal preservation of ultrastructural features in embedded spermatozoa, which would provide a great leap forward for precisely identifying cocoon-producing annelids in the fossil record.
Despite these constraints, we contend that several features of the fossil spermatozoa point to probable affinities of the cocoon producer with the peculiar ‘crayfish worms' (Branchiobdellida). The long, conspicuous ‘drill-bit’ structures (figure 1d–f) are very similar to the characteristic, greatly elongate acrosomes of Branchiobdella (figure 1k–m; [20,22]), which in extreme cases can reach 90 µm in length, forming the longest acrosomes in nature [18]. Moreover, the beaded appearance of some flagella (figure 1d,i) may be due to the presence of a helical marginal fibre (figure 1k), a feature unique to the tails of branchiobdellid spermatozoa [19,20]. Finally, the granular surface texture of some elements anterior to the tail (figure 1g,h) matches that of the nuclear regions of branchiobdellid sperm as seen in freeze-dried material studied via SEM (figure 1m,n). Extant branchiobdellids are obligate symbionts of freshwater crayfish of the Northern Hemisphere, and their evolutionary and phylogeographic history is supposed to be closely linked to that of their host taxa [22]. Hence, their possible fossil occurrence in Antarctic freshwater ecoystems of the early Eocene greenhouse world would markedly extend their palaeobiogeographic distribution, indicating that their evolutionary history may be more complex than currently recognized.
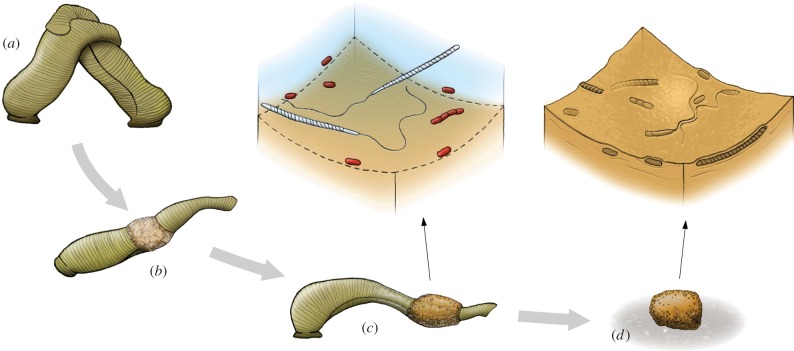
Clitellate annelids secrete their cocoons from the clitellum—a series of specialized body segments—initially in the form of a mucous substance onto which proteinaceous material is deposited in successive layers and in characteristic arrangements (figure 2; [12]). In most cases, eggs and sperm are then released into the cocoon by the hermaphroditic adult as the animal withdraws (figure 2c). The cocoon is finally sealed and deposited, and then cures over several hours to days to form a resistant egg case for the developing embryos [12]. Spermatozoa appear to commonly become entrapped together with other microorganisms inside the proteinaceous cocoon wall before the material is completely solidified (figure 2c,d). Two previous studies have identified microscopic soft-bodied organisms encased in the walls of fossil clitellate cocoons: a nematode in an Early Cretaceous cocoon from Svalbard [24] and a Vorticella-like ciliate protozoan in a Triassic cocoon from Antarctica [9]. This unusual fossilization process appears to be analogous to entombment in amber—both processes permitting three-dimensional preservation of hard- or soft-bodied microorganisms with very fine morphological and structural details [25]. Thus, clitellate annelid cocoons offer an outstanding and little-studied preservational medium for a potentially broad range of microscopic soft-bodied organisms of various freshwater, soil and leaf-litter habitats. We anticipate that systematic surveys of ancient cocoons may open a unique window into the evolutionary history of a range of soft-bodied microorganisms that otherwise lack a fossil record.
Figure 2.
Diagram illustrating the inferred mode of fossilization of microorganisms in clitellate cocoons, exemplified by a common medicinal leech (reproductive stages modified from Sims [23]). (a) Two leeches mate; (b) a cocoon is secreted from the clitellum; (c) eggs and sperm are released into the cocoon before the animal retracts and eventually deposits the sealed cocoon on a suitable substrate (d). Insets depict enlargements of the inner cocoon-wall surface showing how spermatozoa and microbes become encased in the solidifying inner cocoon wall.
Supplementary Material
Acknowledgements
We gratefully acknowledge logistic support for fieldwork on Seymour Island by the Argentine Antarctic Institute (IAA-DNA), the Argentine Air Force and the Swedish Polar Research Secretariat (SPFS). We thank Johannes Bouchal (Stockholm) for assistance with SEM analyses; Else Marie Friis (Stockholm) and Federica Marone and Marco Stampanoni (Villigen) for assistance in SRXTM analysis; Lena Gustavsson (Stockholm) and Roberto Marotta (Genoa) for helpful discussion; and Renate Matzke-Karasz (Munich) and an anonymous referee for constructive criticism on earlier manuscript versions.
Authors' contributions
T.M. and M.A.R. collected and processed the material. B.B. and S.M. analysed and photographed the material. M.F. provided illustrations of extant material. All authors discussed the results and wrote the manuscript.
Competing interests
We have no competing interests.
Funding
Financial support by the Swedish Research Council (VR grants 2014-5232 to B.B., 2009-4447 to T.M. and 2014-5234 to S.M.), the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET grant PIP 0462 to M.A.R.), and the Argentine National Agency for Promotion of Science and Technology (ANPCyT grant PICTO-2010–0093 to M.A.R.) is gratefully acknowledged.
References
- 1.Erséus C, Källersjö M. 2004. 18S rDNA phylogeny of Clitellata (Annelida). Zool. Scr. 33, 187–196. ( 10.1111/j.1463-6409.2004.00146.x) [DOI] [Google Scholar]
- 2.Marotta R, Ferraguti M, Erséus C, Gustavsson LM. 2008. Combined-data phylogenetics and character evolution of Clitellata (Annelida) using 18S rDNA and morphology. Zool. J. Linn. Soc. 154, 1–26. ( 10.1111/j.1096-3642.2008.00408.x) [DOI] [Google Scholar]
- 3.Rousset V, Pleijel F, Rouse GW, Erséus C, Siddall ME. 2007. A molecular phylogeny of annelids. Cladistics 23, 41–63. ( 10.1111/j.1096-0031.2006.00128.x) [DOI] [PubMed] [Google Scholar]
- 4.Struck TH, et al. 2011. Phylogenomic analyses unravel annelid evolution. Nature 471, 95–98. ( 10.1038/nature09864) [DOI] [PubMed] [Google Scholar]
- 5.Weigert A, et al. 2014. Illuminating the base of the annelid tree using transcriptomics. Mol. Biol. Evol. 31, 1391–1401. ( 10.1093/molbev/msu080) [DOI] [PubMed] [Google Scholar]
- 6.Manum SB, Bose MN, Sawyer RT. 1991. Clitellate cocoons in freshwater deposits since the Triassic. Zool. Scr. 20, 347–366. ( 10.1111/j.1463-6409.1991.tb00300.x) [DOI] [Google Scholar]
- 7.McLoughlin S, Tosolini A-MP, Nagalingum NS, Drinnan AN. 2002. Early Cretaceous (Neocomian) flora and fauna of the Lower Strzelecki Group, Gippsland Basin, Victoria. Assoc. Australas. Palaeont. Mem. 26, 1–144. ( 10.1080/03115510208619239) [DOI] [Google Scholar]
- 8.Jansson I-M, McLoughlin S, Vajda V. 2008. Early Jurassic leech cocoons from eastern Australia. Alcheringa 32, 285–296. ( 10.1080/03115510802096226) [DOI] [Google Scholar]
- 9.Bomfleur B, Kerp H, Taylor TN, Moestrup Ø, Taylor EL. 2012. Triassic leech cocoon from Antarctica contains fossil bell animal. Proc. Natl Acad. Sci. USA 109, 20 971–20 974. ( 10.1073/pnas.1218879109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vigran JO, Mangerud G, Mørk A, Worsley D, Hochuli PA. 2014. Palynology and geology of the Triassic succession of Svalbard and the Barents Sea. Geol. Surv. Norw. Spec. Pub. 14, 1–269. ( 10.5167/uzh-99116) [DOI] [Google Scholar]
- 11.Ivany LC, Lohmann KC, Hasiuk F, Blake DB, Glass A, Aronson RB, Moody RM. 2008. Eocene climate record of a high southern latitude continental shelf: Seymour Island, Antarctica. Geol. Soc. Am. Bull. 120, 659–678. ( 10.1130/B26269.1) [DOI] [Google Scholar]
- 12.Coleman J, Shain DH. 2009. Clitellate cocoons and their secretion. In Annelids in modern biology (ed. Shain DH.), pp. 328–346. Hoboken, NJ: John Wiley and Sons. [Google Scholar]
- 13.Kerp H, Trewin NH, Hass H. 2003. New gametophytes from the Early Devonian Rhynie chert. Trans. R. Soc. Edinb. Earth Sci. 94, 411–428. ( 10.1017/S026359330000078X) [DOI] [Google Scholar]
- 14.Nishida H, Pigg KB, Rigby JF. 2003. Swimming sperm in an extinct Gondwanan plant. Nature 422, 396–397. ( 10.1038/422396a) [DOI] [PubMed] [Google Scholar]
- 15.Poinar G. 2000. First fossil record of stalked spermatophores with sperm (Collembola: Hexapoda). Hist. Biol. 14, 229–234. ( 10.1080/10292380009380570) [DOI] [Google Scholar]
- 16.Matzke-Karasz R, Neil JV, Smith RJ, Symonová R, Mořkovský L, Archer M, Hand SJ, Cloetens P, Tafforeau P. 2014. Subcellular preservation in giant ostracod sperm from an early Miocene cave deposit in Australia. Proc. R. Soc. B 281, 20140394 ( 10.1098/rspb.2014.0394) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jamieson BGM, Rouse GW. 1989. The spermatozoa of the Polychaeta (Annelida): an ultrastructural review. Biol. Rev. 64, 93–157. ( 10.1111/j.1469-185X.1989.tb00673.x) [DOI] [PubMed] [Google Scholar]
- 18.Ferraguti M, Erséus C. 1999. Sperm types and their use for a phylogenetic analysis of aquatic clitellates. Hydrobiologia 402, 225–237. ( 10.1023/A:1003752811830) [DOI] [Google Scholar]
- 19.Marotta R, Ferraguti M. 2009. Sperm ultrastructure in assessing phylogenetic relationships among clitellate annelids. In Annelids in modern biology (ed. Shain DH.), pp. 314–327. Hoboken, NJ: John Wiley and Sons. [Google Scholar]
- 20.Cardini A, Ferraguti M. 2004. The phylogeny of Branchiobdellida (Annelida, Clitellata) assessed by sperm characters. Zool. Anz. 243, 37–46. ( 10.1016/j.jcz.2004.06.001) [DOI] [Google Scholar]
- 21.Ben Ahmed R, Bacchetta R, Boesi R, Froman N, Marotta R, Ferraguti M. 2015. The spermatozoa of Hirudinea with examples from three different taxa. Zool. Anz. 255, 54–61. ( 10.1016/j.jcz.2015.02.001) [DOI] [Google Scholar]
- 22.Cardini A, Ferraguti M, Gelder SR. 2000. A phylogenetic assessment of the branchiobdellidan family Branchiobdellidae (Annelida, Clitellata) using spermatological and somatic characters. Zool. Scr. 29, 347–366. ( 10.1046/j.1463-6409.2000.00043.x) [DOI] [Google Scholar]
- 23.Sims G. 1989. Leech mania. Aust. Geogr. 14, 35–48. [Google Scholar]
- 24.Manum SB, Bose MN, Sawyer RT, Boström S. 1994. A nematode (Captivonema cretacea gen. et sp. n.) preserved in a clitellate cocoon wall from the Early Cretaceous. Zool. Scr. 23, 27–31. ( 10.1111/j.1463-6409.1994.tb00370.x) [DOI] [Google Scholar]
- 25.Schmidt AR, Ragazzi E, Coppellotti O, Roghi G. 2006. A microworld in Triassic amber. Nature 444, 835 ( 10.1038/444835a) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.