Abstract
Spider males have evolved a remarkable way of transferring sperm by using a modified part of their pedipalps, the so-called palpal organ. The palpal organ is ontogenetically derived from tarsal claws; however, no nerves, sensory organs or muscles have been detected in the palpal bulb so far, suggesting that the spider male copulatory organ is numb and sensorily blind. Here, we document the presence of neurons and a nerve inside the male palpal organ of a spider for the first time. Several neurons that are located in the embolus are attached to the surrounding cuticle where stresses and strains lead to a deformation (stretching) of the palpal cuticle on a local scale, suggesting a putative proprioreceptive function. Consequently, the male copulatory organ of this species is not just a numb structure but likely able to directly perceive sensory input during sperm transfer. In addition, we identified two glands in the palpal organ, one of which is located in the embolus (embolus gland). The embolus gland appears to be directly innervated, which could allow for rapid modulation of secretory activity. Thus, we hypothesize that the transferred seminal fluid can be modulated to influence female processes.
Keywords: reproductive biology, finite-element modelling, arthropods, proprioreception, ejaculate plasticity
1. Introduction
Male fertilizing ability is strongly influenced by postcopulatory sexual selection, i.e. sperm competition and cryptic female choice [1]. However, whenever females mate with multiple males the ability of males to detect females as potential mating partners with respect to e.g. age or mating status is of particular relevance [2]. Consequently, if resources are limited, or the mating effort is high, mate choice and mating investment play a crucial role and males not only discriminate between potential mates, but also are able to adjust their investment, e.g. quality of ejaculates [3].
Males, however, initially need to identify and distinguish the quality of a female, at the latest while mating. During copulation, spider males use a modified part of their pedipalps, the so-called palpal organ, to transfer seminal fluid (sperm and secretions). One major finding from several histological studies of the last decades—including a considerable number of spider taxa—is the lack of nervous tissue in the male palpal organ [4,5]. For this reason, it has been assumed that spider males are not able to receive sensory input through their copulatory organs while mating, despite the fact that males need to manoeuvre these ‘numb’ structures precisely into the female genitalia [4]. Although many spiders, for example the Entelegynae, have evolved highly complex male pedipalps that are equipped with prelocking structures that may aid in mating, many other spiders are equipped only with simple pedipalps that lack such structures and could certainly profit from sensory input. Here, we demonstrate the presence of neurons in the simple male palpal organ of such a spider, the Tasmanian cave spider Hickmania troglodytes (Higgins & Petterd, 1883), for the first time (we believe). We discuss the potential role of these neurons in processing sensory information during copulation and in mediating the interaction between male and female genitalia. In addition, we provide evidence for a directly innervated gland in the furthermost part of the palpal organ, the embolus, which releases its secretions into a distinct glandular duct. This configuration might enable the male to directly adjust the transferred ejaculate during copulation.
2. Material and methods
We investigated adult males of the Tasmanian cave spider by means of histological and ultrastructural analyses, as well as X-ray micro-computed tomography. Furthermore, we provide a detailed three-dimensional reconstruction using Amira v. 5.6.0. (FEI, Visualization Science Group) and performed a finite-element modelling (FEM) analysis of the male palpal organ. For details of our methods and collection sites, see the electronic supplementary material (S1). Voucher specimens are deposited in the Zoological Museum of the University of Greifswald (ZIMG).
3. Results
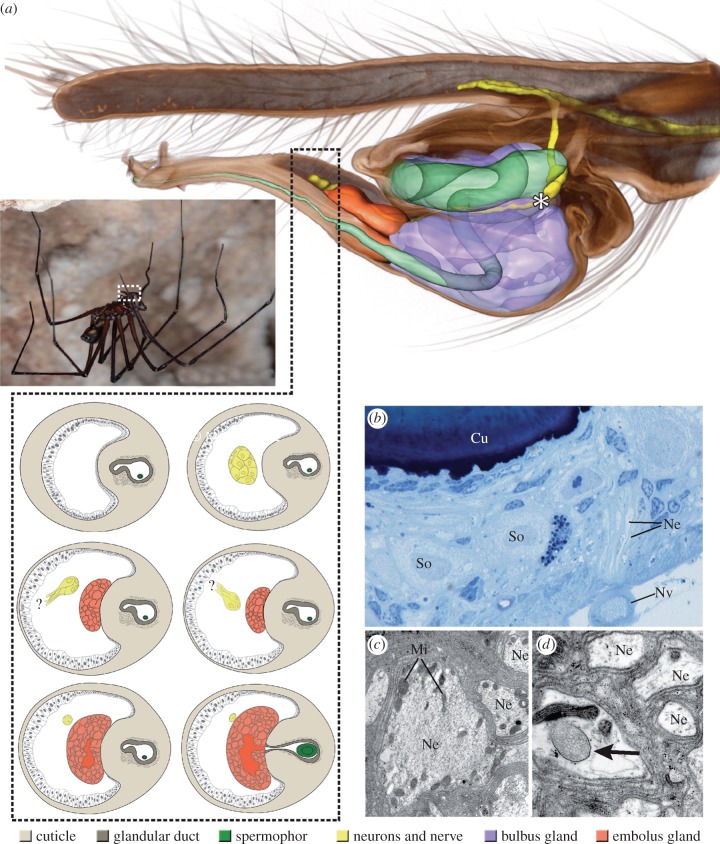
The major findings of this study are (i) the presence of a nerve, projecting as far as the embolus and (ii) the presence of two clusters of neurons within the male palpal bulb (figure 1). A first cluster of neurons is located near the blind end of the internal cuticular tube containing the seminal fluid, the spermophor (figure 1a, asterisk, b), while a second one is situated in the intromitting part of the organ, the embolus (figure 1a). In addition, transmission electron microscopy analysis revealed the presence of large neurites, forming a small nerve that projects through the palpal organ (figure 1b,c), as well as putative neurotransmitter (clear vesicles) inside some of these neurites (figure 1d).
Figure 1.
Male of the Tasmanian cave spider, Hickmania troglodytes, in its natural habitat (photograph courtesy of M. J. Ramírez, © M. J. Ramírez), three-dimensional model representation of the male copulatory organ and fine structural details of the neurons and the nerve. (a) The tube-like spermophor extends into the palpal organ. In addition to two distinct glands, two distinct clusters of neurons, one of which is located near the blind end of the spermophor (asterisk) can be distinguished. A second cluster of neurons is located in the furthermost part of the palpal organ in front of the embolus gland. Schematic drawings—cellular extensions that arise from some of these neurons are likely attached to the cuticle. Consecutive sections reveal a very thin cuticle in the region of these extensions when compared with adjacent sections. The properties of the thin cuticle with respect to stress and strain further support the presumed proprioreceptive function of the associated neurons, respectively. (b) Numerous neurites join to form a small nerve that projects through the palpal organ. (c,d) Some of these neurites bear a distinct amount of clear vesicles (arrow), in addition to mitochondria. Cu, part of the cuticular lining of the spermophor; Mi, mitochondrion; Ne, neurite; Nv, nerve; So, soma of neuron. (Online version in colour.)
We furthermore revealed the presence of two glands inside the male palpal organ. A prominent gland, which occupies the main part of the bulb, is associated and likely connected to the spermophor, through a porous portion at the base of the latter (data not shown). A second, smaller gland is located in the embolus and is provided with its own glandular duct. The spermophor runs through this duct, which opens in combination with the spermophor at the tip of the embolus (figure 1a).
Although only the tip of the embolus is inserted in the female genitalia during mating, the stresses and strains in the embolus expanding from the contact area of the tip and the female genitalia are distributed along the entire structure. As a result of the geometry, materials properties and wall thickness of the cuticle the embolus gets stretched or compressed in certain areas depending on the acting forces (figure 2). We found a pronounced stretching in the cuticle wall in the area next to the tip of one of the neuron branches (figure 2). Thus, neurons located in the embolus might have a proprioceptive function, enabling males of H. troglodytes to perceive sensory input with respect to stress and strain.
Figure 2.
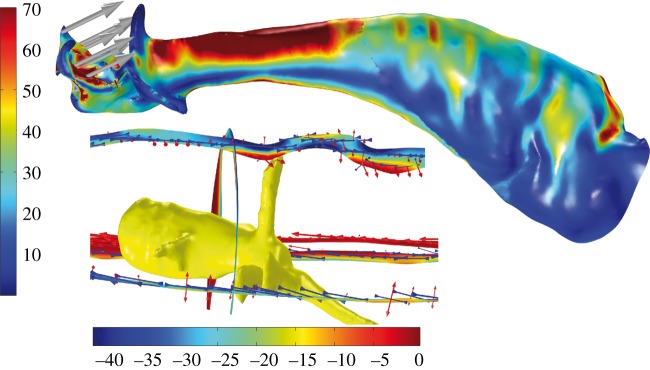
Surface model analysed with FEM displaying von Mises stress (N m–2) and load (grey arrows). Cut planes of the copulatory organ near the tip of the nerve are colour-coded according to the third principal stress (N m–2). Arrows indicate the principal stress and strain directions. (Online version in colour.)
4. Discussion
Our data provide the first evidence of neuronal tissue in the spider male copulatory organ. Thus, this spider's male genitalia are likely not numb and sensory input could play an important role in copulation and consequently in male mating investment and/or securing paternity. The majority of comparative studies suggest that female spiders are in control of processes regarding sperm utilization [6]. A putative sensitive palpal organ might be an advantage as it could be used to alter the male's copulatory behaviour in order to stimulate the female for the male's own benefit (e.g. [7]). Alternatively, a sensory copulatory organ might enable the male to assess female parameters and adjust his investment accordingly. However, this remains speculative at present, as there are no data relating male copulatory behaviour to paternity success in H. troglodytes.
Our study revealed the presence of two glands inside the palpal organ, the spermophor gland and a gland that we called the embolus gland. Based on the presence of neurotransmitter our data suggest that at least the embolus gland is likely directly innervated. The spermophor gland of H. troglodytes is associated with the base of the spermophor and therefore similar to the organization described for other spider taxa. Here, the glandular tissue is expected to play an important role during sperm uptake and transfer into the female during copulation [4].
Glands accessory to copulation, such as the embolus gland we have described here, are usually thought to play a role in assuring paternity [8–10]. For example, secretions from such glands are often associated with the production of mating plugs [11]. However, there is no evidence for mating plugs in H. troglodytes [12] and thus the gland present in the embolus would appear to serve other function(s). In theory, males should invest more sperm in high-quality females, but take advantage of any further mating. Thus, males of H. troglodytes might be able to attenuate the seminal fluid with additional secretions as shown for the accessory glands of other animal taxa. Such secretions can have a variety of functions influencing female processes to secure paternity. For example, male accessory gland secretions in insects are known to affect the receptivity to remate, to modulate oviposition behaviour and to influence egg development [13]. So far, in spiders, only indirect evidence from the wolf spider Schizocosa malitiosa suggests a positive correlation of male transferred substances and female reluctance to remate after a successful mating [14].
In conclusion, the possibility for sensory reception in males of H. troglodytes, together with the directly innervated embolus gland, suggests the presence of alternative mechanisms for securing paternity unknown for spiders. Future studies will reveal if similar mechanisms are also present in other spider species.
Supplementary Material
Acknowledgements
We are grateful to Dave Lee for his help to collect a male in the Marakoopa Cave and Arthur Clarke for providing access to and help in collecting on his private land in Francistown. We thank Martín J. Ramírez, Christian Wirkner and Stefan Richter for their help with the collecting and the logistics in Tasmania. In addition, we are indebted to Michael Gebhardt, Roland Melzer, as well as Steffen Harzsch, Andy Sombke and Gabriele Uhl for inspiring discussions. Martín J. Ramírez, as well as two anonymous reviewers provided helpful suggestions on the manuscript. We thank Martín J. Ramírez for allowing us to use his photograph of a male Tasmanian cave spider.
Ethics
All specimens were collected in Tasmania under permit no. FA 12287.
Data accessibility
The images stacks obtained by X-ray micro-computed tomography is stored in MorphDBase (www.morphdbase.de/?E_Lipke_20150323-M-30.1; www.morphdbase.de/?E_Lipke_20150323-M-31.1). A detailed description of collection sites and methods and an additional figure supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
E.L. and P.M. designed the study. E.L. performed the histological and X-Ray micro-CT studies, analysed the data, compiled the three-dimensional reconstructions and schematic drawings and wrote the manuscript. J.U.H. performed the FEM analysis, P.M. collected and dissected the specimens. J.U.H. and P.M. contributed to the writing of the manuscript. All authors gave final approval for publication.
Competing interests
The authors have no competing interests.
Funding
Funding for this research was provided by the German Research Foundation to P.M. (DFG Mi 1255/5-1). The micro-computed tomography was funded by the state of Mecklenburg-Vorpommern and the German Research Foundation (DFG INST 292/119-1 FUGG and DFG INST 292/120-1 FUGG), which is gratefully acknowledged.
References
- 1.Birkhead TR, Pizzari T. 2002. Postcopulatory sexual selection. Nat. Rev. Genet. 3, 262–273. ( 10.1038/nrg774) [DOI] [PubMed] [Google Scholar]
- 2.Wedell N, Gage MJG, Parker GA. 2002. Sperm competition, male prudence and sperm-limited females. Trends Ecol. Evol. 17, 313–320. ( 10.1016/S0169-5347(02)02533-8) [DOI] [Google Scholar]
- 3.Simmons LW. 2001. Sperm competition and its evolutionary consequences in the insects. Princeton, NJ: Princeton University Press. [Google Scholar]
- 4.Eberhard WG, Huber BA. 2010. Spider genitalia: precise maneuvers with a numb structure in a complex lock. In Evolution of primary sexual characters in animals (eds Leonard JL, Córdoba-Aguilar A.), pp. 249–284. Oxford, UK: Oxford University Press. [Google Scholar]
- 5.Eberhard WG, Huber BA. 1998. Possible links between embryology, lack of innervation, and the evolution of male genitalia in spiders. Bull. Br. Arachnol. Soc. 11, 73–80. [Google Scholar]
- 6.Schneider JM, Andrade MCB. 2011. Mating behaviour and sexual selection. In Spider behaviour (ed. Herberstein ME.), pp. 215–274. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 7.Córdoba-Aguilar A. 1999. Male copulatory sensory stimulation induces female ejection of rival sperm in a damselfly. Proc. R. Soc. Lond. B 266, 779–784. ( 10.1098/rspb.1999.0705) [DOI] [Google Scholar]
- 8.Sentenská L, Pékar S, Lipke E, Michalik P, Uhl G. 2015. Female control of a mate plugging in a female-cannibalistic spider (Micaria sociabilis). BMC Evol. Biol. 15, 18 ( 10.1186/s12862-014-0278-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suhm M, Thaler K, Alberti G. 1996. Glands in the male palpal organ and the origin of the mating plug in Amaurobius species (Araneae: Amaurobiidae). Zool. Anz. 234, 191–199. [Google Scholar]
- 10.Uhl G, Kunz K, Vöcking O, Lipke E. 2014. A spider mating plug: origin and constraints of production. Biol. J. Linn. Soc. 113, 345–354. ( 10.1111/bij.12359) [DOI] [Google Scholar]
- 11.Uhl G, Nessler SH, Schneider JM. 2010. Securing paternity in spiders? A review on occurrence and effects of mating plugs and male genital mutilation. Genetica 138, 75–104. ( 10.1007/s10709-009-9388-5) [DOI] [PubMed] [Google Scholar]
- 12.Doran NE, Richardson AMM, Swain R. 2001. The reproductive behaviour of the Tasmanian cave spider Hickmania troglodytes (Araneae: Austrochilidae). J. Zool. 253, 405–418. ( 10.1017/S0952836901000371) [DOI] [Google Scholar]
- 13.Gillott C. 2003. Male accessory gland secretions: modulators of female reproductive physiology and behavior. Annu. Rev. Entomol. 48, 163–184. ( 10.1146/annurev.ento.48.091801.11265) [DOI] [PubMed] [Google Scholar]
- 14.Aisenberg A, Costa FG. 2005. Females mated without sperm transfer maintain high sexual receptivity in the wolf spider Schizocosa malitiosa. Ethology 111, 545–558. ( 10.1111/j.1439-0310.2005.01077.x) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The images stacks obtained by X-ray micro-computed tomography is stored in MorphDBase (www.morphdbase.de/?E_Lipke_20150323-M-30.1; www.morphdbase.de/?E_Lipke_20150323-M-31.1). A detailed description of collection sites and methods and an additional figure supporting this article have been uploaded as part of the electronic supplementary material.