Abstract
The major clades of vertebrates differ dramatically in their current species richness, from 2 to more than 32 000 species each, but the causes of this variation remain poorly understood. For example, a previous study noted that vertebrate clades differ in their diversification rates, but did not explain why they differ. Using a time-calibrated phylogeny and phylogenetic comparative methods, I show that most variation in diversification rates among 12 major vertebrate clades has a simple ecological explanation: predominantly terrestrial clades (i.e. birds, mammals, and lizards and snakes) have higher net diversification rates than predominantly aquatic clades (i.e. amphibians, crocodilians, turtles and all fish clades). These differences in diversification rates are then strongly related to patterns of species richness. Habitat may be more important than other potential explanations for richness patterns in vertebrates (such as climate and metabolic rates) and may also help explain patterns of species richness in many other groups of organisms.
Keywords: diversification, ecology, habitat, phylogeny, species richness, Vertebrata
1. Introduction
Vertebrates are the dominant group of animals on the Earth, given their abundance, large body sizes and presence at the top of both aquatic and terrestrial foodwebs [1]. They include humans and most animals they consume, and may be the most intensively studied organisms. Yet, we know little about what explains their patterns of species richness among clades. For example, some vertebrate clades contain only a handful of living species (e.g. coelacanth (Actinistia) with 2, lungfish (Dipnoi) with 6 and crocodilians with 25), whereas others contains thousands (for example, birds (Aves) with more than 10 000, ray-finned fish (Actinopterygia) with more than 32 000; electronic supplementary material, table S1). To clarify, numerous studies have tested the causes of richness patterns within major vertebrate clades, such as the latitudinal richnesss gradient within amphibians and mammals (e.g. [2,3]). However, few studies have addressed the dramatic variation in richness across these clades. For example, Alfaro et al. [4] described dramatic variation in diversification rates among vertebrate clades, but did not test whether any intrinsic traits or ecological correlates explained why these rates varied. Several authors have hypothesized that occurrence in terrestrial habitats might generally increase diversification and richness of clades (given that terrestrial habitats were colonized later but have higher richness today), but without explicit quantitative analyses [5–8]. Here, I show that much of the variation in diversification rates and richness among major vertebrate clades can be explained by a simple ecological variable (ignored in most vertebrate studies): whether the clade contains predominantly aquatic or terrestrial species.
2. Material and methods
Detailed methods are provided in the electronic supplementary material, appendix S1. The time-calibrated phylogeny of gnathostomes from Alfaro et al. [4] was used to obtain clade ages. These ages and relationships are very similar to those from phylogenomic analyses using species-tree methods [9], but the tree [4] differs in including all major gnathostome clades. This phylogeny was expanded to include cyclostomes (lamprey, hagfish), using two sets of divergence times (trees, hereafter). Current numbers of described species in each habitat and clade were summarized from literature sources and online databases. Species were considered aquatic if they spend a significant portion of their lives in water, including fish, amphibians with aquatic larval stages, and tetrapods occuring primarily in aquatic habitats. The net diversification rate of each clade was estimated using the method-of-moments estimator for stem ages [10], using three assumed ratios of extinction to speciation (relative extinction fraction: e = 0, 0.5 and 0.9). Analyses were repeated for all three relative extinction fractions and both trees. The net diversification rate incorporates the impacts of both speciation and extinction, but does not require that speciation, extinction or diversification be constant over time [8]. The stem age incorporates the entire history of the clade, and not simply the age of the clade of surviving species (which could be substantially younger). The relationship between the diversification rate of each clade and its proportion of terrestrial species was tested using phylogenetic generalized least-squares (PGLS) regression, which accounts for phylogenetic non-independence of clades [11]. Data on stem ages, richness, diversification rates and proportions of terrestrial species for each major clade are summarized in the electronic supplementary material, table S1. The phylogeny and richness patterns are also summarized in figure 1.
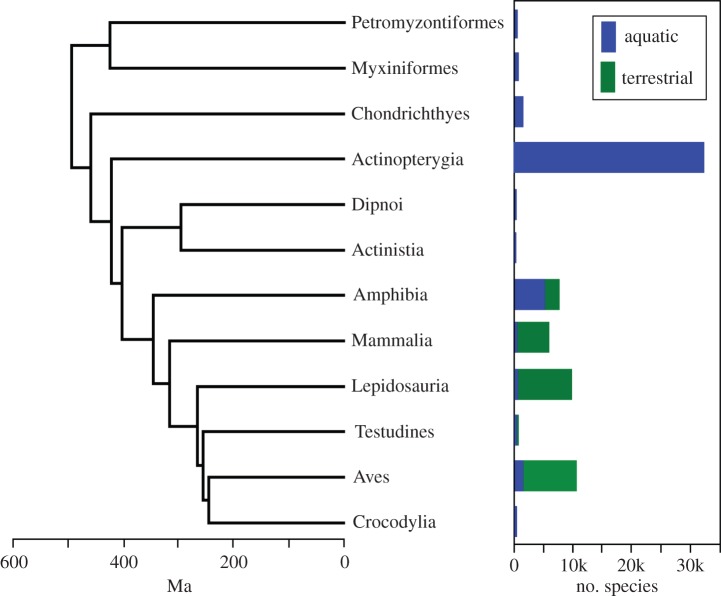
Figure 1.
Phylogenetic tree of vertebrates used in the analyses, and species richness of the 12 major clades in aquatic and terrestrial habitats. The tree shown used ages for cyclostomes and the vertebrate root from Erwin et al. [12]; an alternative tree used dates from Blair & Hedges [13]. Myxiniformes, hagfish; Petromyzontiformes, lamprey; Actinopterygia, ray-finned fish; Chondrichthyes, sharks, rays, and relatives; Actinistia, coelacanth; Dipnoi, lungfish; Lepidosauria, lizards, snakes and tuatara; Testudines, turtles; Aves, birds; alligators, crocodiles, and relatives.
3. Results
There is a significant, positive relationship between the proportion of terrestrial species in each clade and the clade's diversification rate (figure 2), explaining roughly two-thirds of the variation in diversification rates among clades (table 1). Moreover, there is a significant, positive relationship between diversification rates and richness among these clades (table 1). Although ray-finned fish (Actinopterygia) might appear to be a strong exception to the overall pattern (aquatic, with high richness; figure 1), they are relatively old, and therefore have lower diversification rates than predominantly terrestrial clades (birds, mammals and lepidosaurs; figure 2).
Figure 2.
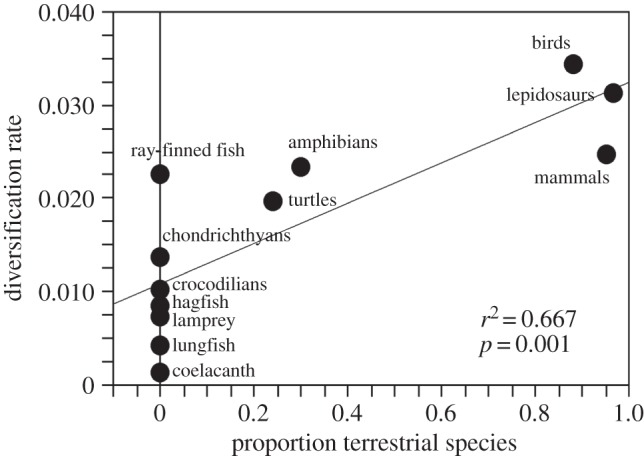
Relationship between habitat (proportion of terrestrial species) and net diversification rates among 12 major vertebrate clades. Results are based on ordinary least-squares regression (to illustrate the raw data), the root age of Erwin et al. [12], and an extinction fraction of 0.5 (full results in table 1).
Table 1.
Relationships between the proportion of terrestrial species in major vertebrate clades and their net diversification rates (estimated under three relative extinction fractions), and using two alternate ages for cyclostomes and the vertebrate root [12,13]. AIC, Akaike information criterion; div. rate, diversification rate; prop., proportion.
| variables | r2 | p-value | AIC |
|---|---|---|---|
| Erwin et al. [12] tree | |||
| div. rate (e = 0) ∼ prop. terrestrial | 0.6594 | 0.0008 | 75.4384 |
| div. rate (e = 0.5) ∼ prop. terrestrial | 0.6615 | 0.0008 | 74.8602 |
| adiv. rate (e = 0.9) ∼ prop. terrestrial | 0.6685 | 0.0011 | 79.8808 |
| ln-species ∼ div. rate (e = 0) | 0.8471 | <0.0001 | 40.5479 |
| ln-species ∼ div. rate (e = 0.5) | 0.8617 | <0.0001 | 39.4395 |
| ln-species ∼ div. rate (e = 0.9) | 0.8765 | <0.0001 | 38.2015 |
| Blair & Hedges [13] tree | |||
| div. rate (e = 0) ∼ prop. terrestrial | 0.6503 | 0.0020 | 70.3817 |
| div. rate (e = 0.5) ∼ prop. terrestrial | 0.6431 | 0.0022 | 69.7182 |
| div. rate (e = 0.9) ∼ prop. terrestrial | 0.6308 | 0.0026 | 67.1607 |
| ln-species ∼ div. rate (e = 0) | 0.7996 | 0.0002 | 39.3290 |
| ln-species ∼ div. rate (e = 0.5) | 0.8164 | 0.0001 | 38.4524 |
| ln-species ∼ div. rate (e = 0.9) | 0.8356 | <0.0001 | 37.3477 |
aOptimization for PGLS analysis failed, and ordinary least-squares regression was used instead.
4. Discussion
Vertebrates are one of the most well-studied groups of organisms, but few studies have addressed why their major clades differ so dramatically in their current richness. The results here show that habitat explains most variation in large-scale patterns of vertebrate diversification and richness. These results are surprising in showing that a relatively simple ecological variable explains variation in diversification rates over a timescale of more than 500 Myr (figure 1), and that variation in diversification rates in turn explains large-scale richness patterns among clades (table 1). Furthermore, this ecological variable is not often considered in explaining large-scale richness patterns among clades. Yet, these results suggest that habitat might be more important than other, more conventional explanations for vertebrate richness patterns.
For example, the results here show that climate may not always be the primary driver of large-scale richness patterns among clades. Many vertebrate clades do show a strong latitudinal diversity gradient (e.g. amphibians and mammals; [2,3]). However, the most species-poor of these 12 major vertebrate clades are aquatic clades that occur almost exclusively in tropical regions (e.g. actinistians, crocodilians and dipnoans; [1]). In these cases, the negative effects of habitat on diversification seem to overcome the potentially positive impacts associated with occurrence in tropical regions.
Similarly, some authors have suggested that higher metabolic rates drive higher rates of diversification (e.g. [14,15]). Birds and mammals are endothermic and thus have metabolic rates approximately 10 higher than those of most comparably sized ectothermic vertebrates [1]. However, treating endothermy as a binary variable shows no significant relationship with diversification rates (electronic supplementary material, table S2). Moreover, models including both habitat and endothermy explain negligible additional variation and have a higher Akaike information criterion (AIC), relative to models based on habitat alone (table 1 and electronic supplementary material, table S2).
Previous authors have suggested that clades occurring in marine habitats may have lower diversification rates than those on land, given higher species richness in terrestrial environments overall coupled with the more recent invasion of these habitats [5–8]. However, none has tested whether habitats explain large-scale patterns of diversification and species richness among clades. Further, the results here combine marine and freshwater habitats. Interestingly, marine habitats alone (i.e. proportion of primarily marine species in each clade) do not show a significant relationship with diversification rates (electronic supplementary material, table S3, appendix S1).
Several potential sources of error might change the detailed results of this study, but not the overall conclusions. For example, the tree used (figure 1) may be incorrect in showing Actinistia (coelacanth) and Dipnoi (lungfish) as sister taxa (instead of Dipnoi as sister to Tetrapoda; e.g. [16]). However, these alternative relationships would simply make these two clades older and would lower their diversification rates even further. The same reasoning applies to Mixiniformes (hagfish) and Petromyzontiformes (lamprey), whose relationships with each other and with other vertebrates have been controversial (see electronic supplementary material, appendix S1). Furthermore, there is a continuum in habitat usage between species that are fully aquatic and fully terrestrial. Here, species were considered aquatic if they were dependent on water, even if they spend considerable time on land. A different threshold might change the results somewhat. However, all fish clades, all crocodilians and most turtle species should be considered aquatic by most definitions. Categorizing some birds, lepidosaurs and mammals may be more challenging, but any changes would have little impact on the conclusions here, since these clades are mostly terrestrial anyways (electronic supplementary material, table S1). Considering most amphibians to be terrestrial rather than aquatic (70 versus 30%) yields similar results, with an even stronger relationship between terrestrial habitats and diversification (electronic supplementary material, table S4). There are also different possible ways of dividing vertebrates into clades, which might influence the results. However, the goal here was to explain patterns of richness among major vertebrate groups. Subdividing these clades further would presumably generate additional variation in diversification rates that would then require additional variables to explain. Finally, new species continue to be described in most clades. The analyses here focus on differences in diversification rates among clades, and should be robust to increasing species numbers (especially in larger clades).
Importantly, the results here are not inconsistent with very fast diversification rates in some aquatic subclades within these major groups (e.g. acanthomorphs and cichlids within actinopterygian fish [4]) nor with fluctuations in species richness within clades over time (for example, due to global mass extinction events). The rates estimated here are the outcome of both of these dynamics on net diversification rates and present-day richness of these major clades. The results show that habitat is an important predictor of these large-scale patterns of diversification and diversity, even if habitat alone does not explain every pattern in every subclade at every point in time.
A major topic for future research following from these results is to understand how occurrence in terrestrial habitats drives higher diversification rates, and how aquatic habitats lower diversification rates. Various hypotheses have been proposed to explain why marine habitats have lower diversification rates, such as more limited barriers to dispersal in marine habitats [5–7]. Interestingly, this explanation may not apply to freshwater habitats, which may instead promote geographical isolation. One possibility is that both marine and freshwater habitats lower diversification rates of aquatic clades by increasing their net extinction rates. Indeed, many of the species-poor, aquatic clades of vertebrates have extensive fossil records and appear to have had much higher diversity in the past (for example, crocodilians, coelacanth and lungfish; [1]). Addressing this topic quantitatively could be an intriguing area for further study.
In summary, the results here show that a simple ecological variable (habitat) explains much of the variation in diversification rates and species richness among major vertebrate clades. This variable has been largely ignored in explaining patterns of vertebrate diversity and may be relevant to many other major groups (e.g. plants, arthropods and molluscs). The results also raise the possibility that local-scale habitats may be more important than large-scale climates in explaining patterns of clade diversification and richness.
Supplementary Material
Acknowledgements
I thank E. Park for assistance compiling richness data and two reviewers for helpful comments.
Data accessibility
All data are accessible in the electronic supplementary material.
Author contributions
J.J.W. designed the study, performed the analyses and wrote the paper.
Competing interests
The author declares no competing interests.
Funding
I received no specific funding for this study.
References
- 1.Pough FH, Janis CM, Heiser JB. 2009. Vertebrate life, 8th edn San Francisco, CA: Pearson. [Google Scholar]
- 2.Pyron RA, Wiens JJ. 2013. Large-scale phylogenetic analyses reveal the causes of high tropical amphibian diversity. Proc. R. Soc. B 280, 20131622 ( 10.1098/rspb.2013.1622) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rolland J, Condamine FL, Jiguet F, Morlon H. 2014. Faster speciation and reduced extinction in the tropics contribute to the mammalian latitudinal diversity gradient. PLoS Biol. 12, e1001775 ( 10.1371/journal.pbio.1001775) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alfaro ME, et al. 2009. Nine exceptional radiations plus high turnover explain species diversity in jawed vertebrates. Proc. Natl Acad. Sci. USA 106, 13 410–13 414. ( 10.1073/pnas.0811087106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.May RM. 1994. Biological diversity: differences between land and sea. Phil. Trans. R. Soc. Lond. B 343, 105–111. ( 10.1098/rstb.1994.0014) [DOI] [Google Scholar]
- 6.Benton MJ. 2001. Biodiversity on land and in the sea. Geol. J. 36, 211–230. ( 10.1002/gj.877) [DOI] [Google Scholar]
- 7.Vermeij GJ, Grosberg RK. 2010. The great divergence: when did diversity on land exceed that in the sea? Int. Comp. Biol. 50, 675–682. ( 10.1093/icb/icq078) [DOI] [PubMed] [Google Scholar]
- 8.Wiens JJ. 2011. The causes of species richness patterns across space, time, and clades and the role of ‘ecological limits’. Q. Rev. Biol. 86, 75–96. ( 10.1086/659883) [DOI] [PubMed] [Google Scholar]
- 9.Chiari Y, Cahais V, Galtier N, Delsuc F. 2012. Phylogenomic analyses support the position of turtles as the sister group of birds and crocodiles (Archosauria). BMC Biol. 10, 65 ( 10.1186/1741-7007-10-65) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magallón S, Sanderson MJ. 2001. Absolute diversification rates in angiosperm clades. Evolution 55, 1762–1780. ( 10.1111/j.0014-3820.2001.tb00826.x) [DOI] [PubMed] [Google Scholar]
- 11.Martins EP, Hansen TF. 1997. Phylogenies and the comparative method: a general approach to incorporating phylogenetic information into the analysis of interspecific data. Am. Nat. 119, 646–667. ( 10.1086/286013) [DOI] [Google Scholar]
- 12.Erwin DH, Laflamme M, Tweedt SM, Sperling EA, Pisani D, Peterson KJ. 2011. The Cambrian conundrum: early divergence and later ecological success in the early history of animals. Science 334, 1091–1097. ( 10.1126/science.1206375) [DOI] [PubMed] [Google Scholar]
- 13.Blair JE, Hedges SB. 2005. Molecular phylogeny and divergence times of deuterostome animals. Mol. Biol. Evol. 22, 2275–2284. ( 10.1093/molbev/msi225) [DOI] [PubMed] [Google Scholar]
- 14.Allen AP, Gillooly JF, Savage VM, Brown JH. 2006. Kinetic effects of temperature on rates of genetic divergence and speciation. Proc. Natl Acad. Sci. USA 103, 9130–9135. ( 10.1073/pnas.0603587103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sibly RM, Brown JH, Kodric-Brown A. (eds). 2012. Metabolic ecology: a scaling approach. Chichester, UK: Wiley-Blackwell. [Google Scholar]
- 16.Amemiya MT, et al. 2013. The African coelacanth genome provides insights into tetrapod evolution. Nature 496, 311–316. ( 10.1038/nature12027) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are accessible in the electronic supplementary material.