Abstract
Oceans, or other wide expanses of inhospitable environment, interrupt present day distributions of many plant groups. Using molecular dating techniques, generally incorporating fossil evidence, we can estimate when such distributions originated. Numerous dating analyses have recently precipitated a paradigm shift in the general explanations for the phenomenon, away from older geological causes, such as continental drift, in favour of more recent, long-distance dispersal (LDD). For example, the ‘Gondwanan vicariance’ scenario has been dismissed in various studies of Indian Ocean disjunct distributions. We used the gentian tribe Exaceae to reassess this scenario using molecular dating with minimum (fossil), maximum (geological), secondary (from wider analyses) and hypothesis-driven age constraints. Our results indicate that ancient vicariance cannot be ruled out as an explanation for the early origins of Exaceae across Africa, Madagascar and the Indian subcontinent unless a strong assumption is made about the maximum age of Gentianales. However, both the Gondwanan scenario and the available evidence suggest that there were also several, more recent, intercontinental dispersals during the diversification of the group.
Keywords: calibration, dating, Exaceae, long-distance dispersal, vicariance
1. Introduction
Estimation of divergence times from phylogenetic trees is a key component of any study investigating the biogeographic patterns underlying speciation. Based on numerous studies in the last decade employing molecular dating techniques (e.g. [1–3]), the general paradigm for intercontinental plant distribution patterns has substantially shifted from older vicariance to more recent long-distance dispersal (LDD) scenarios.
However, the critical issue for accurately dating phylogenetic trees remains age calibration, which is mostly achieved using fossil information as a form of (minimum) age constraint (e.g. [4,5]) or more rarely using geological evidence, particularly the known ages of volcanic islands, as maximum age constraints for endemic clades (e.g. [6]). This is a challenging process subject to various sources of uncertainty [4,5,7,8].
The historical biogeographic connection around the Indian Ocean Basin is a key topic in the biogeography of plants and animals [2,9,10] and exemplifies the paradigm shift from vicariance to dispersal. The current position of continental landmasses is the result of sequential break-up of the Gondwanan supercontinent and subsequent continental drift [11]. The Madagascar–Seychelles–India block separated from the Africa–South America block between ca 165 Myr ago and 130–118 Myr ago [12], after which Madagascar remained in its position with respect to Africa, whereas Australia and Antarctica separated from the Madagascar–Seychelles–India block ca 132 Myr ago [12] and India separated from Madagascar ca 88 Myr ago [11,12]. Various studies have concluded that more recent LDD can explain current disjunct distributions in the region (e.g. [1,3,13]). However, to our knowledge, none have been able to reject conclusively a vicariance scenario for taxa distributed around the Indian Ocean Basin based on maximum, as well as minimum, age constraints.
The gentian tribe Exaceae (specifically genus Exacum) is an often-cited example of an Indian Ocean Basin distribution [2,14]. Exaceae comprises ca 170 spp. assigned to eight monophyletic genera [15] showing major centres of endemism in continental Africa (ca 78 endemic species and two endemic genera), Madagascar (55 endemic species and four endemic genera) and the southern tip of India and Sri Lanka (14 endemic species; see the electronic supplementary material). The dispersal mechanism of the majority of Exaceae species is currently unknown. However, the seeds are small (less than 0.5 mm across) and discharged from dry capsules, therefore occasional LDD cannot be ruled out.
Here, we use a well-sampled phylogeny of Exaceae and different calibration strategies to test the past impact of vicariance on current day species distributions.
2. Material and methods
We applied a widely used relaxed-clock approach implemented in BEAST [16] to infer minimum, maximum and hypothetical clade ages for Gentianaceae using a phylogeny based on our previous work [15,17] (TreeBase ID: 17666) and the following calibration strategies (see the electronic supplementary material).
(a). Fossil evidence
Three fossils were used for calibration: stem nodes of Emmenopterys at 45 Myr ago (infructescence and fruit [18]), Lisianthius at 40 Myr ago (fossil pollen [19]) and Gentiana sect. Cruciata at 5 Myr ago (fossil seeds [20]). These fossils have been used to date the Gentianaceae (e.g. [2,21,22]) and were chosen because they can be assigned unequivocally to nodes within the phylogeny. We used lognormal and exponential priors with arbitrary bounds (mean/s.d. = 1) to represent minimum constraints.
(b). Geological evidence
The age of the volcanic Canary Islands, 21 Myr ago [23] as a maximum constraint for the crown node of Ixanthus (represented by two samples of its single species), which is endemic to the Canary Islands [24]. We set this calibration to 21 Myr ago, which represents an estimate of the age of the current terrestrial habitat [23]. However, the Canary Island Seamount Province dates as far back as 142 Myr ago [25] and the degree of emergence during this time is uncertain. Using 21 Myr ago therefore represents a bias against inferring vicariance, with older maximum constraints less likely to reject the hypothesis.
(c). Hypothesis-driven calibration
Here we set the separation of Africa and the Madagascar–Seychelle–India block to 118 Myr ago and the separation of India from Madagascar to 88 Myr ago. However, these ages themselves are subject to a high degree of uncertainty, and it is not obvious when the landmasses might be judged to have been sufficiently far apart to obviate gene flow. Madagascar and India rafted laterally, presenting an extended ‘dispersal window’, and the protracted separation of Madagascar from East Africa is still incompletely understood [12]. These ages can therefore also be regarded as presenting a bias against inferring vicariance.
(d). Secondary calibration
Here we used the age estimate for Gentianales from Magallón et al. [26] as a fixed constraint (normal prior, mean 100 Myr ago with minimal bounds) for the root node.
3. Results and discussion
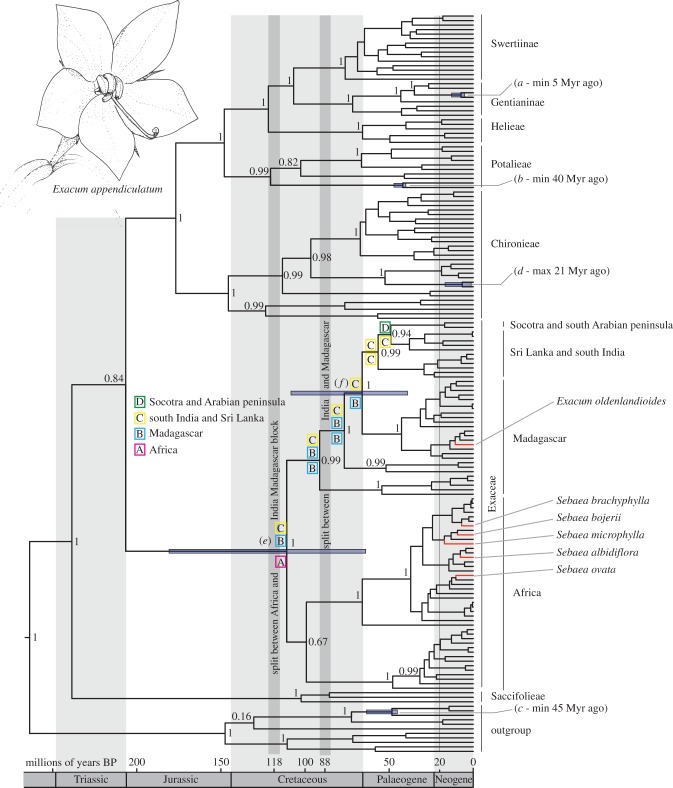
Our test of the Gondwanan vicariance hypothesis consists of comparing age estimates given minimum and maximum age constraints with those obtained by assuming that the hypothesis is true. The ages inferred using fossils within Gentianales as internal age constraints are consistent with those inferred assuming the vicariance hypothesis and compatible with the maximum age constraint implied by Ixanthus (21 Myr ago maximum compared to 6.5 Myr ago (1.7–17.8) given vicariance and 7.5 Myr ago (1.2–16.7) with fossil calibration; see figure 1 and the electronic supplementary material, figure S1). This could be interpreted to suggest that, despite the deliberate bias in our analyses in favour of rejecting the hypothesis, at least part of the widespread distribution of the tribe Exaceae could indeed be explained by the break-up of Gondwana as concluded by Klackenberg [14] on the basis of phylogeny alone.
Figure 1.
Chronogram based on BEAST analyses using fossil calibration. The scale shows ages in million years. The 95% highest posterior density bars are indicated for the nodes of interest, labelled as follows: (a) the Gentiana fossil, (b) the Lisianthius fossil, (c) the Emmenopterys fossil, (d) crown node of Ixanthus, (e) and (f) nodes corresponding, respectively, to the potential vicariance events due to the separation of the Madagascar–India block from Africa and later of India from Madagascar. Coloured letters above selected branches indicate ancestral areas (see the electronic supplementary material) and numbers above the branches are posterior probabilities derived from the Bayesian inferences analysis. (Online version in colour.)
However, calibration points placed closer to the tips of the tree can result in wide age ranges, and even erroneously old age estimates for deeper (older) nodes [5,27,28]. Swenson et al. [29] presented a similar test of a vicariance scenario, using the age of the putative vicariance event as calibration and rejecting it based on the resulting unrealistically old deeper node estimates. Our analyses in which the root node was unconstrained also returned ages that might be considered old, particularly compared to recent angiosperm-wide analyses (greater than 250 Myr ago for Gentianales given the fossil and vicariance strategy, compared with ca 100 Myr ago in Magallón et al. [26]).
We therefore tested a fixed constraint to the root node, corresponding to the estimate of [26]. The resulting age estimates for Exaceae were recent enough to reject Gondwanan vicariance. However, they were too recent to explain Lisianthius (3.5–11.6, compared to 40 Myr ago) and Emmenopterys (12.9–37.0, compared to 45 Myr ago). They also arguably postdated another fossil, Pistillipollenites macgregorii (Palaeocene/Early Eocene, ca 50 Myr ago). Although the position of this fossil within Gentianaceae is still debated [30,31] its interpretation as a member of Gentianaceae–Helieae would be consistent with our fossil and vicariance-based ages (22.4–87.9) and not with the root constrained ones (17.3–30.9). Our root-unconstrained results therefore appear to fit the evidence within Gentianales better, although the ages of the deepest nodes should be interpreted with caution.
The evidence available to calibrate molecular dating analyses generally implies minimum or—more rarely—maximum constraints, and not absolute ages. We would therefore argue that a hypothesis testing approach such as ours is a more appropriate means to improve our understanding of biogeographic and other ancient processes using molecular dating techniques than any reconstruction of individual events per se.
Based on our results, Gondwanan vicariance could explain some disjunct distributions in Exaceae. However, in the context of the general vicariance versus LDD debate, it is worth noting that clade ages consistent with the Gondwanan vicariance scenario for Indian Ocean disjunctions in Exaceae would lead us to reject vicariance scenarios to explain at least five further disjunctions between different land masses (figure 1): (i) Sebaea albidiflora and Sebaea ovata in Australia and New Zealand, (ii) Sebaea microphylla in Asia and Africa, (iii) Sebaea bojerii and Sebaea brachyphylla in Madagascar and Africa, (iv) Exacum oldenlandioides in Africa and (v) four Exacum species in Socotra. In these cases, LDD must still be invoked.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Woody F. P. D. Cotterill and two anonymous reviewers for critical comments. The Vital-IT facilities from the Swiss Institute of Bioinformatics were used for the computational aspects of this study.
Data accessibility
Sequences are in GenBank (see the electronic supplementary material).
Authors' contributions
J.K. conceived the study, performed the laboratory work; G.L., J.K. and M.D.P. analysed the data; D.B. and N.S. contributed to the acquisition of data, provided ideas and critically assessed the results; J.K. and M.D.P. led the writing, to which all authors contributed.
Competing interests
We have no competing interests.
Funding
This study was funded through the Swiss National Science Foundation (PA00P3_129140), the Velux Stiftung (project no. 679), the Swiss Academy of Sciences to J.K. and the South African National Research Foundation to D.U.B. and M.D.P.
References
- 1.Clayton JW, Soltis PS, Soltis DE. 2009. Recent long-distance dispersal overshadows ancient biogeographical patterns in a pantropical angiosperm family (Simaroubaceae, Sapindales). Syst. Biol. 58, 1–16. ( 10.1093/sysbio/syp041) [DOI] [PubMed] [Google Scholar]
- 2.Yuan Y-M, Wohlhauser S, Moller M, Klackenberg J, Callmander M, Kupfer P. 2005. Phylogeny and biogeography of Exacum (Gentianaceae): a disjunctive distribution in the Indian Ocean Basin resulted from long distance dispersal and extensive radiation. Syst. Biol. 54, 21–34. ( 10.1080/10635150590905867) [DOI] [PubMed] [Google Scholar]
- 3.Zhou LL, Su YCF, Thomas DC, Saunders RMK. 2012. ‘Out-of-Africa’ dispersal of tropical floras during the Miocene climatic optimum: evidence from Uvaria (Annonaceae). J. Biogeogr. 39, 322–335. ( 10.1111/j.1365-2699.2011.02598.x) [DOI] [Google Scholar]
- 4.Ho SY, Phillips MJ. 2009. Accounting for calibration uncertainty in phylogenetic estimation of evolutionary divergence times. Syst. Biol. 58, 367–380. ( 10.1093/sysbio/syp035) [DOI] [PubMed] [Google Scholar]
- 5.Pirie MD, Doyle JA. 2012. Dating clades with fossils and molecules: the case of Annonaceae. Bot. J. Linn. Soc. 169, 84–116. ( 10.1111/j.1095-8339.2012.01234.x) [DOI] [Google Scholar]
- 6.Richardson JE, Weitz FM, Fay MF, Cronk QCB, Linder HP, Reeves G, Chase MW. 2001. Rapid and recent origin of species richness in the Cape flora of South Africa. Nature 412, 181–183. ( 10.1038/35084067) [DOI] [PubMed] [Google Scholar]
- 7.Yang Z, Rannala B. 2006. Bayesian estimation of species divergence times under a molecular clock using multiple fossil calibrations with soft bounds. Mol. Biol. Evol. 23, 212–226. ( 10.1093/molbev/msj024) [DOI] [PubMed] [Google Scholar]
- 8.Ho SY. 2014. The changing face of the molecular evolutionary clock. Trends Ecol. Evol. 29, 496–503. ( 10.1016/j.tree.2014.07.004) [DOI] [PubMed] [Google Scholar]
- 9.Raxworthy CJ, Forstner MRJ, Nussbaum RA. 2002. Chameleon radiation by oceanic dispersal. Nature 415, 784–787. ( 10.1038/415784a) [DOI] [PubMed] [Google Scholar]
- 10.Raven PH, Axelrod DI. 1974. Angiosperm biogeography and past continental movements. Ann. Mo. Bot. Gard. 61, 539–673. ( 10.2307/2395021) [DOI] [Google Scholar]
- 11.Storey BC. 1995. The role of mantle plumes in continental breakup: case-histories from Gondwanaland. Nature 377, 301–308. ( 10.1038/377301a0) [DOI] [Google Scholar]
- 12.Reeves C. 2014. The position of Madagascar within Gondwana and its movements during Gondwana dispersal. J. Afr. Earth Sci. 94, 45–57. ( 10.1016/j.jafrearsci.2013.07.011) [DOI] [Google Scholar]
- 13.Muellner AN, Savolainen V, Samuel R, Chase MW. 2006. The mahogany family ‘out-of-Africa’: divergence time estimation, global biogeographic patterns inferred from plastid rbcL DNA sequences, extant, and fossil distribution of diversity. Mol. Phylogenet. Evol. 40, 236–250. ( 10.1016/j.ympev.2006.03.001) [DOI] [PubMed] [Google Scholar]
- 14.Klackenberg J. 2002. Tribe Exaceae. In Gentianaceae: systematics and natural history (eds Struwe L, Albert VA.), pp. 66–108. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 15.Kissling J, Yuan Y-M, Kupfer P, Mansion G. 2009. The polyphyletic genus Sebaea (Gentianaceae): a step forward in understanding the morphological and karyological evolution of the Exaceae. Mol. Phylogenet. Evol. 53, 734–748. ( 10.1016/j.ympev.2009.07.025) [DOI] [PubMed] [Google Scholar]
- 16.Drummond AJ, Suchard MA, Xie D, Rambaut A. 2012. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 29, 1969–1973. ( 10.1093/molbev/mss075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kissling J, Endress PK, Bernasconi G. 2009. Ancestral and monophyletic presence of diplostigmaty in Sebaea (Gentianaceae) and its potential role as a morphological mixed mating strategy. New Phytol. 184, 303–310. ( 10.1111/j.1469-8137.2009.03000.x) [DOI] [PubMed] [Google Scholar]
- 18.Graham A. 2009. Fossil record of the Rubiaceae. Ann. Mo. Bot. Gard. 96, 90–108. ( 10.3417/2006165) [DOI] [Google Scholar]
- 19.Graham A. 1984. Lisianthius pollen from the Eocene of Panama. Ann. Mo. Bot. Gard. 71, 987–993. ( 10.2307/2399236) [DOI] [Google Scholar]
- 20.Mai DH, Walther H. 1988. Die pliozaenen Floren von Thüringen, Deutsche Demokratische Republik. Quartaerpalaeontologie 7, 55–297. [Google Scholar]
- 21.Merckx VS, Kissling J, Hentrich H, Janssens SB, Mennes CB, Specht CD, Smets EF. 2013. Phylogenetic relationships of the mycoheterotrophic genus Voyria and the implications for the biogeographic history of Gentianaceae. Am. J. Bot. 100, 712–721. ( 10.3732/ajb.1200330) [DOI] [PubMed] [Google Scholar]
- 22.Favre A, Yuan Y-M, Küpfer P, Alvarez N. 2010. Phylogeny of subtribe Gentianinae (Gentianaceae): biogeographic inferences despite limitations in temporal calibration points. Taxon 59, 1701–1711. [Google Scholar]
- 23.Coello J, et al. 1992. Evolution of the eastern volcanic ridge of the Canary Islands based on new K-Ar data. J. Volcanol. Geoth. Res. 53, 251–274. ( 10.1016/0377-0273(92)90085-R) [DOI] [Google Scholar]
- 24.Thiv M, Struwe L, Kadereit JW. 1999. The phylogenetic relationships and evolution of the Canarian laurel forest endemic Ixanthus viscosus (Aiton) Griseb. (Gentianaceae): evidence from matK and ITS sequences, and floral morphology and anatomy. Plant Syst. Evol. 218, 299–317. ( 10.1007/BF01089233) [DOI] [Google Scholar]
- 25.van den Bogaard P. 2013. The origin of the Canary Island Seamount Province: new ages of old seamounts. Sci. Rep. 3, 2107 ( 10.1038/srep02107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magallón S, Gómez-Acevedo S, Sánchez-Reyes LL, Hernández-Hernández T. In press. A metacalibrated time-tree documents the early rise of flowering plant phylogenetic diversity. New Phytol. ( 10.1111/nph.13264) [DOI] [PubMed] [Google Scholar]
- 27.Sanderson MJ. 2003. r8s: inferring absolute rates of molecular evolution and divergence times in the absence of a molecular clock. Bioinformatics 19, 301–302. ( 10.1093/bioinformatics/19.2.301) [DOI] [PubMed] [Google Scholar]
- 28.Duchene S, Lanfear R, Ho SY. 2014. The impact of calibration and clock-model choice on molecular estimates of divergence times. Mol. Phylogenet. Evol. 78, 277–289. ( 10.1016/j.ympev.2014.05.032) [DOI] [PubMed] [Google Scholar]
- 29.Swenson U, Nylander S, Wagstaff SJ. 2012. Are Asteraceae 1.5 billion years old? A reply to Heads. Syst. Biol. 61, 522–532. ( 10.1093/sysbio/syr121) [DOI] [PubMed] [Google Scholar]
- 30.Crepet WL, Daghlian CP. 1981. Lower Eocene and Paleocene Gentianaceae: floral and palynological evidence. Science 214, 75–77. ( 10.1126/science.214.4516.75) [DOI] [PubMed] [Google Scholar]
- 31.Stockey RA, Manchester SR. 1988. A fossil flower with in situ Pistillipollenites from the Eocene of British Columbia. Can. J. Bot. 66, 313–318. ( 10.1139/b88-051) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequences are in GenBank (see the electronic supplementary material).