Abstract
Wildfires have increased in frequency and intensity worldwide with climate change as a main driving factor. While a number of studies have focused on population changes in regard to fires, there are essentially no quantitative data on behavioural and physiological adjustments that are vital for the persistence of individuals during and after fires. Here we show that brown antechinus, a small insectivorous marsupial mammal, (i) endured a prescribed fire in situ, (ii) remained in their scorched home range despite unburned areas nearby, and (iii) substantially increased post-fire torpor use and thus reduced foraging requirements and exposure to predators. Hence, torpor is a physiological adaptation that, although not quantified in this context previously, appears to play a key role in post-fire survival for this and other heterothermic species.
Keywords: antechinus, climate change, fire, marsupial, predation, torpor
1. Introduction
Fuelled by droughts, reduced soil moisture and elevated ambient temperatures (Ta), an increase in the frequency and severity of wildfires is one aspect of climate change that will have irreversible effects on many ecosystems [1]. The effects are already evident in Australia, the Mediterranean Basin, the west coast of the United States and many other parts of the world that feature fire-prone vegetation communities [1]. Arguably, fires are most detrimental for small terrestrial animals as they cannot evade them. Although many can shelter from the fire front in refuges, they subsequently have to cope with an environment that deprives them abruptly of vegetation cover and food. The consequences are twofold: (i) increased exposure to predators while foraging in the open [2,3] and (ii) for most species a decrease in food availability [4]. To compensate for the latter, small mammals with their inherently high energy requirements and limited body fat stores [5,6] will have to either intensify foraging or reduce energy expenditure.
A potential and highly effective energy saving mechanism is torpor, a controlled reduction in metabolic rate and body temperature (Tb) [5–7]. While torpor in heterothermic mammals has been traditionally associated with low winter Ta and low food availability, recent work has revealed that torpor is used by diverse species in all climate zones including during reproduction and growth [6–10]. As torpor decreases energy expenditure, it can in turn shorten foraging times and thereby also reduce exposure to predators [11,12], which is likely important during the aftermath of a fire.
Prescribed fires to reduce fuel load are employed in many parts of the world to limit the frequency and severity of potential wildfires and may even promote biodiversity if a mosaic of habitats is created [13]. Nevertheless, the localized detrimental impact of hazard-reduction burns on plant and animal communities can still be substantial. While it is known that small mammals can survive such fires, it is the obliteration of ground cover and the resulting reduction in food and increase in exposure to predation that often leads to a decrease in small mammal populations [14].
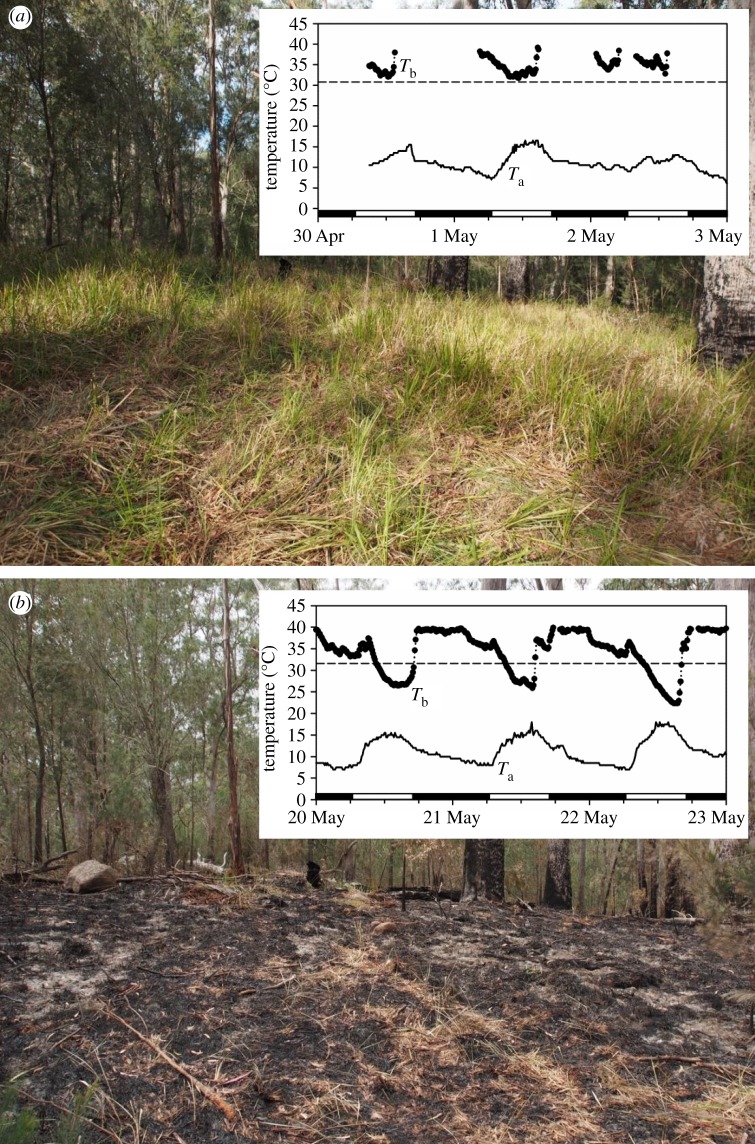
In May 2014 (austral autumn), a prescribed fire was implemented at Guy Fawkes River National Park (New South Wales, Australia). Before the fire the ground was densely covered with grass and shrubs (figure 1a). After the fire only charcoal and ash remained of this understorey, whereas the higher vegetation layers remained largely intact (figure 1b). As data on the physiological and behavioural mechanisms used by animals to survive changes in climate and fires are scant [13,15], we used this opportunity to test the hypothesis that the use of torpor for energy conservation is an important post-fire survival strategy for the most common terrestrial mammal in the study area, the brown antechinus (Antechinus stuartii; body mass 18–60 g). Antechinus are daily heterotherms and because use of torpor is more pronounced in females than males [16], we focused on females and predicted that they would increase torpor use and decrease activity after the fire.
Figure 1.
Photos of the study site and body temperature (dotted line) and ambient temperature (solid line) traces over 3 days: (a) the same area before the fire (pre-burn group) and (b) after the fire (post-burn group). The dashed line represents the torpor threshold. Note the time-series gaps in (a) show nocturnal activity away from the nest; torpor was expressed during the daytime (b).
2. Material and methods
The area of the hazard-reduction burn (prescribed site; 30°04′58.6″ S, 152°20′0.9″ E) was 379 ha, confined by an escarpment and dirt roads. The unburned forest beyond these roads encompassed our control site. Ambient temperatures (Ta) were measured at both sites using temperature data loggers (± 0.5°C, iButton thermochron DS1921G, Maxim Integrated Products, Inc., Sunnyvale, CA, USA).
Physiological and behavioural variables were investigated in three groups of antechinus: (i) pre-fire: prescribed site before the fire (25 days of data from four animals, one was killed by a predator before the fire and one was killed during the fire; an additional two animals were captured and released just before the fire); (ii) post-fire: prescribed site after the fire (56 days of data from four animals); (iii) control: control site after the fire (17 days of data from two animals). All individuals were implanted intraperitoneally with temperature-sensitive radio-transmitters (2 g, Sirtrack, Havelock North, New Zealand) [17]. Each individual was radio-tracked daily to its nest and a receiver/logger [18] was placed in range of the individual's transmitter signal, recording Tb once every 10 min. Four remote cameras (HC600 Hyperfire, Reconyx Inc., Holmen, WI, USA) recorded the movement of animals along the road bordering the fire site for 3 days before and for 8 days after the fire.
The torpor threshold (Tb-onset) for antechinus was calculated as 31.5°C using eqn (3) from [19], for bouts longer than 30 min. Activity periods were calculated from the time the individual's transmitter signal was absent from the receiver/logger and thus from the nest where torpor was expressed. Statistical tests were undertaken in R (R v. 3.0.1, R Core Team, 2013). Differences among the treatment groups for torpor use, Tb, activity and Ta were tested using an ANOVA followed by a post hoc Tukey test. Frequency numbers were arcsine transformed. Data are presented as the mean of the mean of each individual ±1 standard deviation. Significant differences when p < 0.05.
3. Results and discussion
Four of five female antechinus survived the fire and remained in the same area they occupied before the fire, although unburned vegetation was in close vicinity. One individual was hiding under shallow rocks close to the surface and died during the fire. The four survivors preferred nesting in tall trees that were not touched by the ground fire. This highlights the importance of refuge selection for survival of the fire event itself [14].
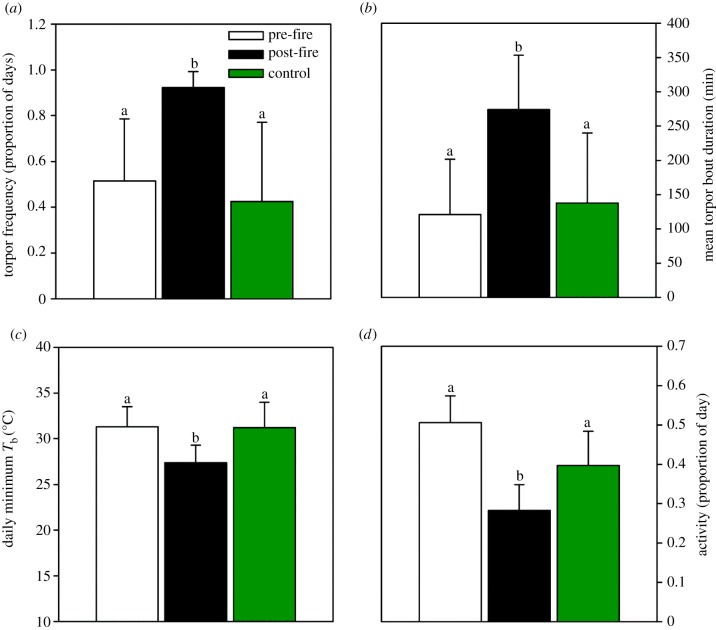
Interestingly, in the post-fire group, torpor use was approximately twofold of that in the pre-fire and control groups (figure 2a) and so were mean torpor bout duration and the longest torpor bout recorded (figure 2b; table 1; see figure 1a,b for an example of a Tb trace). Furthermore, in the post-fire group mean daily minimum Tb was 3.9°C lower (figure 2c) and the absolute lowest Tb recorded during the entire study period was more than 4.5°C below that of any other groups (table 1). This substantially enhanced torpor expression in the post-fire group was observed despite an unseasonal increase in post-fire Ta (table 1). Evidently, the effect of fire of increasing torpor expression was stronger than the known effect of elevated Ta of decreasing torpor expression [6,7].
Figure 2.
Summary of physiological and behavioural variables. (a) Torpor frequency (number of days with torpor/days measured), (b) mean torpor bout duration (min), (c) daily minimum Tb (°C) and (d) duration of activity as the proportion of the day (24 h) individuals were not detected by the data loggers. Means are shown with ±standard deviation. Significant differences are identified by letters.
Table 1.
Summary of torpor variables, activity values and ambient temperatures (Ta). If means (±s.d.) differed significantly between the groups (*) they are identified by different letters.
| pre-fire | post-fire | control | ANOVA p-value | |
|---|---|---|---|---|
| torpor frequency (proportion of days) | 0.52 ± 0.27a | 0.92 ± 0.07b | 0.43 ± 0.35a | 0.022* |
| torpor bout duration (min) | 120.8 ± 80.8a | 274.2 ± 79.2b | 137.5 ± 102.5a | 0.008* |
| longest torpor bout of individuals (min) | 187.5 ± 133.8a | 562.5 ± 75.4b | 175.0 ± 190.9a | 0.006* |
| absolute longest torpor bout recorded (min) | 330 | 650 | 310 | n/a |
| minimum Tb (°C) | 31.3 ± 2.2a | 27.4 ± 1.9b | 31.2 ± 2.8a | <0.0001* |
| lowest Tb of individuals (°C) | 28.5 ± 3.1a | 21.9 ± 1.8b | 29.4 ± 2.4a | 0.011* |
| absolute lowest Tb recorded (°C) | 24.3 | 19.8 | 27.6 | n/a |
| activity (proportion of day) | 0.51 ± 0.07a | 0.28 ± 0.07b | 0.40 ± 0.09a | <0.0001* |
| daily Ta (°C) | 8.3 ± 2.8a | 11.1 ± 1.2b | 10.9 ± 1.1b | 0.002* |
| maximum Ta (°C) | 13.5 ± 2.6a | 16.5 ± 1.7b | 16.3 ± 2.0b | 0.006* |
| absolute highest maximum Ta (°C) | 17.5 | 20.0 | 19.5 | n/a |
| minimum Ta (°C) | 4.8 ± 2.8a | 7.4 ± 1.4b | 7.0 ± 1.4b | 0.008* |
| absolute lowest minimum Ta (°C) | 2.0 | 5.5 | 5.0 | n/a |
One of the implicit functions of torpor is to reduce energy expenditure [7] and therefore torpor should lower foraging requirements. Indeed, a significant reduction in daily activity in the post-fire group in comparison with both the pre-fire and control groups was observed (figure 2d; table 1). As no predators were captured on camera in the area before the fire, but were recorded after the fire (fox, feral cat and wild dog), torpor may have also moderated predation risk in the post-fire landscape with almost no ground cover.
For the short- to medium-term our data on antechinus suggest that some terrestrial mammals do not seek refuge in unburned habitat after fire, not even if they are near. If this applies to other small mammals, the changes in physiology and behaviour afforded by torpor will give heterothermic small mammals a competitive edge over homeothermic species that continue to have high energy and foraging requirements. This interpretation is supported by the findings that the catastrophic Black Saturday fire of February 2009 in Australia was more detrimental to populations of the homeothermic bush rat (Rattus fuscipes) than to those of the heterothermic agile antechinus (Antechinus agilis), a close relative to our study species [20].
In the long-term habitats will recover after fires—to a degree. Scorched trees often fall and leaf litter and grass accumulate relatively quickly offering at least some cover for terrestrial small mammals. However, other vital habitat characteristics, such as tree hollows, require years to develop and not all plant species survive or recolonize burned areas. In northern Australia, the habitat altering effect of frequent and severe wildfires appears to facilitate predation by introduced predators and has been linked to the recent demise of the once abundant small mammal fauna [3]. Nevertheless, heterotherms appear to be resilient to habitat changes as shown in our study, and indeed there is strong evidence that most recent mammalian extinctions were in fact homeothermic species [11] and fewer heterotherms are currently at risk [12]. It also has been suggested that torpor may have played a role in the survival of small mammals during the mass extinction event at the Cretaceous–Palaeogene boundary as a result of a meteorite impact [21,22]. As torpor is an important survival tool for many species, more studies such as ours are needed to gain a better understanding of the behavioural and physiological aspects of torpor use and the habitat characteristics that aid the survival of heterotherms in a changing world, an urgent area of research that is lacking data [15].
Acknowledgements
We thank NSW NPWS staff, Artiom Bondarenco, Stuart Cairns, Anna Doty, Geoffrey James, Arne Müller, Margaret Stawski and Lihong Yuan for contributions to field work and the manuscript.
Ethics
Approval for this study was granted by the University of New England Animal Ethics Committee (AEC13-088) and the New South Wales National Parks and Wildlife Service (SL100791).
Data accessibility
Data can be accessed from Dryad: http://dx.doi.org/10.5061/dryad.5h5pf.
Authors' contributions
C.S. and F.G. designed the study, collected and analysed the data, and wrote the manuscript. G.K. and J.N. helped to collect data and draft the manuscript. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This project was supported by the University of New England Deputy Vice-Chancellor (Research) to C.S.; the German Academic Exchange Service (DAAD) and the A.F.W. Schimper Stiftung für ökologische Forschung to J.N. and the Australian Research Council to F.G.
References
- 1.Moritz MA, et al. 2014. Learning to coexist with wildfire. Nature 515, 58–66. ( 10.1038/nature13946) [DOI] [PubMed] [Google Scholar]
- 2.Körtner G, Pavey CR, Geiser F. 2007. Spatial ecology of the mulgara in arid Australia: impact of fire history on home range size and burrow use. J. Zool. 273, 350–357. ( 10.1111/j.1469-7998.2007.00334.x) [DOI] [Google Scholar]
- 3.McGregor HW, Legge S, Jones ME, Johnson CN. 2014. Landscape management of fire and grazing regimes alters the fine-scale habitat utilisation by feral cats. PLoS ONE 9, e109097 ( 10.1371/journal.pone.0109097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sutherland EF, Dickman CR. 1999. Mechanisms of recovery after fire by rodents in the Australian environment: a review. Wildlife Res. 26, 405–419. ( 10.1071/WR97045) [DOI] [Google Scholar]
- 5.Boyer BB, Barnes BM. 1999. Molecular and metabolic aspects of mammalian hibernation. BioScience 49, 713–724. ( 10.2307/1313595) [DOI] [Google Scholar]
- 6.Geiser F. 2013. Hibernation. Curr. Biol. 23, R188–R193. ( 10.1016/j.cub.2013.01.062) [DOI] [PubMed] [Google Scholar]
- 7.Ruf T, Geiser F. 2014. Daily torpor and hibernation in birds and mammals. Biol. Rev. ( 10.1111/brv.12137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giroud S, Zahn S, Criscuolo F, Chery I, Blanc S, Turbill C, Ruf T. 2014. Late-born intermittently fasted juvenile garden dormice use torpor to grow and fatten prior to hibernation: consequences for ageing processes. Proc. R. Soc. B 281, 20141131 ( 10.1098/rspb.2014.1131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson ML, Mzilikazi N, Bennett NC, McKechnie AE. 2015. Solar radiation during rewarming from torpor in elephant shrews: supplementation or substitution of endogenous heat production? PLoS ONE 10, e0120442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stawski C. 2010. Torpor during the reproductive season in a free-ranging subtropical bat, Nyctophilus bifax. J. Therm. Biol. 35, 245–249. ( 10.1016/j.jtherbio.2010.05.009) [DOI] [Google Scholar]
- 11.Geiser F, Turbill C. 2009. Hibernation and daily torpor minimize mammalian extinctions. Naturwissenschaften 96, 1235–1240. ( 10.1007/s00114-009-0583-0) [DOI] [PubMed] [Google Scholar]
- 12.Liow LH, Fortelius M, Lintulaakso K, Mannila H, Stenseth NC. 2009. Lower extinction risk in sleep-or-hide mammals. Am. Nat. 173, 264–272. ( 10.1086/595756) [DOI] [PubMed] [Google Scholar]
- 13.Fontaine JB, Kennedy PL. 2012. Meta-analysis of avian and small-mammal response to fire severity and fire surrogate treatments in U.S. fire-prone forests. Ecol. Appl. 22, 1547–1561. ( 10.1890/12-0009.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawrence GE. 1966. Ecology of vertebrate animals in relation to chaparral fire in the Sierra Nevada foothills. Ecology 47, 278–291. ( 10.2307/1933775) [DOI] [Google Scholar]
- 15.McCain CM, King SRB. 2014. Body size and activity times mediate mammalian responses to climate change. Glob. Change Biol. 20, 1760–1769. ( 10.1111/gcb.12499) [DOI] [PubMed] [Google Scholar]
- 16.Rojas AD, Körtner G, Geiser F. 2014. Torpor in free-ranging antechinus: does it increase fitness? Naturwissenschaften 101, 105–114. ( 10.1007/s00114-013-1136-0) [DOI] [PubMed] [Google Scholar]
- 17.Rojas AD, Körtner G, Geiser F. 2010. Do implanted transmitters affect maximum running speed of two small marsupials? J. Mammal. 91, 1360–1364. ( 10.1644/10-MAMM-A-052.1) [DOI] [Google Scholar]
- 18.Körtner G, Geiser F. 2000. Torpor and activity patterns in free-ranging sugar gliders Petaurus breviceps (Marsupialia). Oecologia 123, 350–357. ( 10.1007/s004420051021) [DOI] [PubMed] [Google Scholar]
- 19.Willis CKR. 2007. An energy-based body temperature threshold between torpor and normothermia for small mammals. Physiol. Biochem. Zool. 80, 643–651. ( 10.1086/521085) [DOI] [PubMed] [Google Scholar]
- 20.Banks SC, Dujardin M, McBurney L, Blair D, Barker M, Lindenmayer DB. 2011. Starting points for small mammal population recovery after wildfire: recolonisation or residual populations? Oikos 120, 26–37. ( 10.1111/j.1600-0706.2010.18765.x) [DOI] [Google Scholar]
- 21.Lovegrove BG, Lobban KD, Levesque DL. 2014. Mammal survival at the Cretaceous–Palaeogene boundary: metabolic homeostasis in prolonged tropical hibernation in tenrecs. Proc. R. Soc. B 281, 20141304 ( 10.1098/rspb.2014.1304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robertson DS, McKenna MC, Toon OB, Hope S, Lillegraven JA. 2004. Survival in the first hours of the Cenozoic. Geol. Soc. Am. Bull. 116, 760–768. ( 10.1130/B25402.1) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data can be accessed from Dryad: http://dx.doi.org/10.5061/dryad.5h5pf.