Abstract
Oceanic islands host a disproportionately high fraction of endangered or recently extinct endemic species. We report on species extinctions among endemic Azorean beetles following 97% habitat loss since AD 1440. We infer extinctions from historical and contemporary records and examine the influence of three predictors: geographical range, habitat specialization and body size. Of 55 endemic beetle species investigated (out of 63), seven can be considered extinct. Single-island endemics (SIEs) were more prone to extinction than multi-island endemics. Within SIEs restricted to native habitat, larger species were more extinction-prone. We thus show a hierarchical path to extinction in Azorean beetles: species with small geographical range face extinction first, with the larger bodied ones being the most threatened. Our study provides a clear warning of the impact of habitat loss on island endemic biotas.
Keywords: Azores, body size, endemic beetles, extinction, habitat destruction, oceanic islands
1. Introduction
The destruction and fragmentation of natural habitats have raised species extinctions significantly above background rates [1]. Extinction is non-random: differences in biology influence species' susceptibility to extinction [2–4]. Geographical range size, habitat specialization and body size are traits commonly identified to correlate with extinction risk, through their relationship with demographic parameters, abundance and population fluctuations [3–5].
Island—and especially oceanic island—biotas have provided the majority of recorded global species extinctions since AD 1600, as a result of extensive deforestation, transformation of natural habitats and introduction of non-natives [6]. Having evolved in isolation, oceanic island endemics occur in small geographical ranges, often have small overall population sizes, tend to be habitat specialists, and are more vulnerable to the introduction of non-native predators or competitors [1,7,8].
Insects represent nearly two-thirds of described species of animals and plants, but only 0.1% of the almost 1 million known insect species have been classified as globally extinct or threatened (i.e. Critically Endangered, Endangered or Vulnerable) ([9], cf. [10]). However, a significant number of insect species have most probably gone extinct unnoted and, taking knowledge bias into account, invertebrates overall show much higher extinction rates and proportions of threatened species compared with better-known vertebrate taxa [11]. Beetles (Coleoptera) represent the largest order and about 40% of all described insect species, but just 1.6% of recorded extinct animal species [9].
Thus, there is an urgent need to focus on poorly studied taxa to address the extinction shortfall and quantify the extinction debt [12,13]. Based on the most extensive survey of arthropods in an oceanic archipelago, we use historical and contemporary records to infer extinctions of endemic Azorean beetles and examine the influence of: (i) the number of islands (single or multiple) where species occur as an estimate of geographical range size, (ii) the species dependence on native vegetation as an estimate of habitat specialization, and (iii) body size. Since human settlement of the Azores in AD 1440, more than 97% of the natural forest has been destroyed: as a result, it has been estimated that more than 50% of extant forest-dependent Azorean beetle species may already be committed to extinction [12].
2. Material and methods
(a). Study area
The Azores is one of the world's most isolated oceanic archipelagos (1580 km west of Iberia, 2150 km east of North America (electronic supplementary material, figure S1)). Prior to human habitation, the islands were almost entirely clothed with Laurisilva, a type of humid, evergreen, broadleaf forest (dominated by Laurus azorica). Today, the remaining patches of natural forest (3%) are found only in isolated high-elevation areas, surrounded by pasture and plantations. Parallel to the deforestation, the introduction of non-native species has radically altered the composition of the biota: almost 70% of vascular plant and 58% of arthropod species (60% of beetles) are exotic [14].
(b). Data
We used the checklist of Borges et al. [14] to determine the beetles of the archipelago. This source includes historical records dating back to the nineteenth century alongside extensive recent survey data (details in the electronic supplementary material). Two long-term arthropod sampling projects, BALA in 1999–2000 and BALA2 in 2010, sampled the same 30 sites, covering all remnants of native forest on each island. Additional field surveys were conducted in all other major habitat types, both natural (e.g. caves and coastal areas) and anthropogenic (e.g. exotic forest, pastures and urban areas, see [15] and the electronic supplementary material)—a total of 383 sites. The intensive fieldwork and exhaustive taxonomic work involved have generated a comprehensive presence/absence dataset of species in each island and their distribution across habitat types: the most extensive oceanic island arthropod dataset yet compiled (see the electronic supplementary material for species distributions and species extinctions). Of the 63 endemic coleopteran species, eight cave-adapted, troglodyte species were excluded from analysis, owing to their non-dependence on the native forest.
(c). Collection of traits
For geographical range and habitat specialization, we classified each species into single-island endemics (SIE) and multi-island endemics (MIE; present in two or more islands); and into species strictly confined to native forest (SCL) and those also found in other habitat types (OHT), respectively. We calculated body size as the sum of the length of the thorax and the length of the abdomen. We measured 10 individuals per species per island, using digital photography via a stereoscopic microscope; in the absence of specimens, we used original descriptions and other literature references (see the electronic supplementary material for data and reliability of approach).
(d). Statistical analysis
Initially, we considered all endemic species together (n = 55). We fitted the three predictors (geographical range, habitat specialization and body size) independently to species extinction status (0 = extant, 1 = extinct) using a binomial generalized linear mixed model [16] including family and genus as nested random effects to account for phylogenetic relatedness between species. The statistical significance of each predictor was determined by likelihood ratio tests (LRT). Next, we included the three predictors in a global model and then simplified this model using backward elimination based on LRT [17]. Nagelkerke's R2 was computed for both independent effect and multi-predictor models.
We also computed the same analyses as above but restricting analysis to SIE species (n = 32). Consequently, only habitat specialization and body size were included as predictors. Next, we restricted analysis to SIE + SCL species (n = 18) and tested the independent effect of body size. For comparison, the analyses were re-run with phylogenetic generalized mixed models using a reconstructed calibrated phylogenetic tree at sub-family level (see the electronic supplementary material). While it would have been desirable to also examine interactions between predictors, the data structure limited our analysis to additive effects.
3. Results
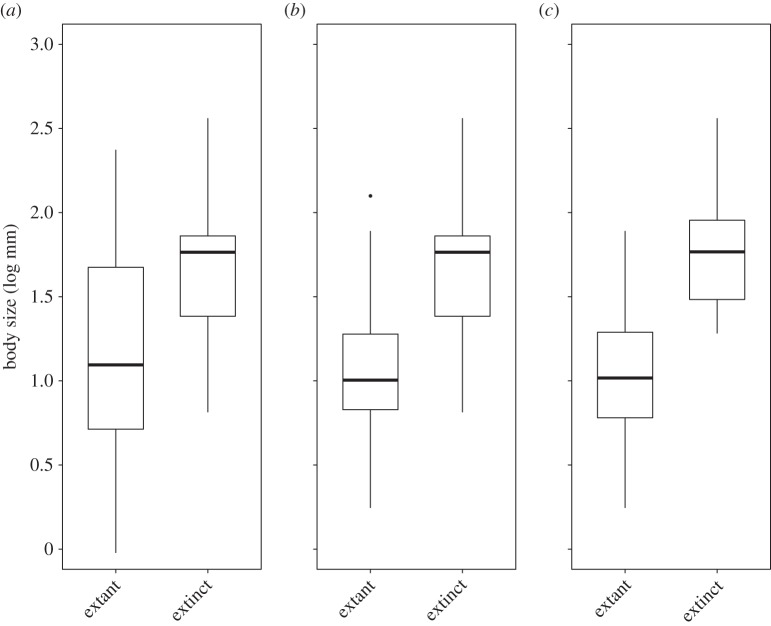
Of the 55 endemic species, 32 were SIE, 54 occur in the native forest and 22 are restricted to this habitat type (SCL). Seven species, all SIE, were classified as Extinct in the Wild according to the IUCN criteria (table 1; electronic supplementary material, table S1, for further details), hence geographical range significantly explained species status when all species were analysed together (LRT: χ2 = 6.32, p = 0.01, R2 = 0.22). SCL were more prone to extinction than OHT species (χ2 = 4.94, p = 0.03, R2 = 0.18; table 1; and electronic supplementary material, table S1), but there was no body size effect (χ2 = 0.64, p = 0.42, R2 = 0.02). The best multi-predictor model included geographical range and body size, with the extinct species being larger (electronic supplementary material, table S2). When restricting our analysis to SIE species, body size became the most important predictor of species extinction status when tested independently and when included in a model with habitat specialization (χ2 = 4.79, p = 0.03, R2 = 0.23; electronic supplementary material, table S2; figure 1), again with extinct species being larger. Considering SIE species occurring strictly in native forest (SIE + SCL), body size was again related to species status (χ2 = 8.25, p = 0.004, R2 = 0.52; figure 1). Results remained identical when using a phylogenetic generalized mixed model (electronic supplementary material, tables S3 and S4).
Table 1.
Number of endemic Azorean beetle species (extinct in parenthesis) by geographical range and habitat specialization (species confined to native forest (SCL) and those also found in other habitat types (OHT)).
| predictors |
habitat specialization |
total | |
|---|---|---|---|
| geographical range | specialists (SCL) | generalists (OHT) | |
| SIE | 18 (6) | 14 (1) | 32 (7) |
| MIE | 4 | 19 | 23 |
| total | 22 | 33 | 55 (7) |
Figure 1.
Body size distribution for extant and extinct Azorean endemic beetle species: (a) all species (n = 55), (b) single-island endemic species (SIE; n = 32) and (c) SIE native-forest specialist species (SIE + SCL; n = 18). Box plots indicate the median, upper and lower quartiles and the confidence intervals. Outlying points are indicated by black dots.
4. Discussion
Extinction in Azorean beetles is linked to a confined geographical range (all extinct species being SIE), habitat specialization and body size (electronic supplementary material, table S2). Species with large ranges also tend to have greater local abundances and larger numbers of spatially separated populations [1,18]. Larger populations are more resistant to stochastic fluctuations in demography compared with smaller ones, while metapopulation restocking effects can reduce the effect of local habitat loss [3,19]. Species with specialized resource requirements tend to be more vulnerable to threats such as habitat loss than are generalists: indeed, it has been shown that extinction of narrow-habitat specialists is more common in insects than in other taxa [20]. All but one of the species we considered extinct were forest-dependent. Of the extant endemic beetles of the Azores, 16 are forest-dependent and thus in urgent need of protection measures [12].
The link between larger body size and extinction risk is widely documented for several vertebrate taxa, e.g. birds, mammals [21], and in some cases for arthropods [22]. Larger species tend to occur in lower population densities, have slower reproductive rates and longer lifespans [23] and to recover less rapidly from population declines than do rapidly growing populations of smaller species [3,7]. These tendencies are enhanced in arthropods due their more frequent fluctuations in population size compared with vertebrates. Although studies on correlates of extinction risk for insects are scarce, positive relationships between body size and extinction risk have been documented, either directly or as an interaction with other biological traits ([22,24], but see [21]). We found that the role of large body size as a predictor of extinction became most evident when restricting the analysis to SIE species. This result was even stronger among the SIEs strictly dependent on native forest. This implies that while large body size might increase extinction risk, this threat can be counterbalanced by a wider distribution. However, an ongoing research need is to determine whether records of endemic species found outside native forest might represent transient individuals (sink-populations) rather than self-sustaining populations.
Assuming these seven species of beetles really have gone extinct, they have done so within the last 141 years: an extinction rate of 4.96 species per century. As a large fraction of Azorean forest was cleared before the first reliable species records in the 1850s, the extinction of other disturbance-sensitive species probably went unrecorded [12]; for example, the epigean relatives of seven of the eight Trechus cave beetle species are unknown [25]. Given that previous work has suggested that more than 50% of the endemic, forest-dependent beetles of the archipelago may be considered committed to extinction [12], the emerging picture presents a clear warning of the impact of habitat loss on the native Azorean biota.
Supplementary Material
Acknowledgements
We thank the numerous colleagues that have contributed to BALA field and laboratory work since 1999.
Data accessibility
See the electronic supplementary material.
Authors' contributions
K.A.T., S.T. and F.R. designed the research; F.R. and S.T. undertook the statistical analyses; S.T., K.A.T., F.R., P.A.V.B. and R.J.W. wrote the paper; all authors approved the final version of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This work was funded by Direcção Regional dos Recursos Florestais (PROJ.17.01–080203), Fundação para a Cie̊ncia e a Tecnologia FCT-PTDC/BIA-BEC/100182/2008, FCT-PTDC/BIA-BIC/119255/2010.
References
- 1.Ladle RJ, Whittaker RJ. (eds). 2011. Conservation biogeography. Chichester, UK: John Wiley and Sons. [Google Scholar]
- 2.Purvis A, Agapow PM, Gittleman JL, Mace GM. 2000. Nonrandom extinction and the loss of evolutionary history. Science 288, 328–330. ( 10.1126/science.288.5464.328) [DOI] [PubMed] [Google Scholar]
- 3.Lawton JH, May RM. (eds). 1995. Extinction rates. Oxford, UK: Oxford University Press. [Google Scholar]
- 4.Hambler C, Henderson PA, Speight MR. 2011. Extinction rates, extinction-prone habitats, and indicator groups in Britain and at larger scales. Biol. Conserv. 144, 713–721. ( 10.1016/j.biocon.2010.09.004) [DOI] [Google Scholar]
- 5.Fisher DO, Owens IP. 2004. The comparative method in conservation biology. Trends Ecol. Evol. 19, 391–398. ( 10.1016/S0169-5347(04)00140-5) [DOI] [PubMed] [Google Scholar]
- 6.Whittaker RJ, Fernández-Palacios JM. 2007. Island biogeography: ecology, evolution, and conservation. Oxford, UK: Oxford University Press. [Google Scholar]
- 7.Gillespie RG. 1999. Naiveté and novel perturbations: conservation of native spiders on an oceanic island system. J. Insect Conserv. 3, 263–272. ( 10.1023/A:1009654820519) [DOI] [Google Scholar]
- 8.Purvis A, Gittleman JL, Cowlishaw G, Mace GM. 2000. Predicting extinction risk in declining species. Proc. R. Soc. Lond. B 267, 1947–1952. ( 10.1098/rspb.2000.1234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.IUCN. 2014. The IUCN Red List of Threatened Species, v. 2014.3 See http://www.iucnredlist.org (accessed 20 February 2015).
- 10.Cardoso P, Borges PAV, Triantis KA, Ferrández MA, Martín JL. 2011. Adapting the IUCN red listing criteria for invertebrates. Biol. Conserv. 144, 2432–2440. ( 10.1016/j.biocon.2011.06.020) [DOI] [Google Scholar]
- 11.McKinney ML. 1999. High rates of extinction and threat in poorly studied taxa. Conserv. Biol. 13, 1273–1281. ( 10.1046/j.1523-1739.1999.97393.x) [DOI] [Google Scholar]
- 12.Triantis KA, et al. 2010. Extinction debt on oceanic islands. Ecography 33, 285–294. ( 10.1111/j.1600-0587.2010.06203.x) [DOI] [Google Scholar]
- 13.Tedesco PA, Bigorne R, Bogan AE, Giam X, Jézéquel C, Hugueny B. 2014. Estimating how many undescribed species have gone extinct. Conserv. Biol. 28, 1360–1370. ( 10.1111/cobi.12285) [DOI] [PubMed] [Google Scholar]
- 14.Borges PAV, et al. 2010. List of the terrestrial and marine biota from the Azores. Cascais, Portugal: Princípia. [Google Scholar]
- 15.Borges PAV, et al. 2005. Ranking protected areas in the Azores using standardised sampling of soil epigean arthropods. Biodivers. Conserv. 14, 2029–2060. ( 10.1007/s10531-004-4283-y) [DOI] [Google Scholar]
- 16.Bates D, Maechler M, Bolker B, Walker S. 2014. lme4: linear mixed-effects models using Eigen and S4. R package v. 1.1–6 See http://CRAN.R-project.org/package=lme4.
- 17.Crawley MJ. 2012. The R book. New York, NY: John Wiley and Sons. [Google Scholar]
- 18.Lawton JH. 1993. Range, population abundance and conservation. Trends Ecol. Evol. 8, 409–413. ( 10.1016/0169-5347(93)90043-O) [DOI] [PubMed] [Google Scholar]
- 19.Hanski I. 1999. Habitat connectivity, habitat continuity, and metapopulations in dynamic landscapes. Oikos 87, 209–219. ( 10.2307/3546736) [DOI] [Google Scholar]
- 20.Dunn RR. 2005. Modern insect extinctions, the neglected majority. Conserv. Biol. 19, 1030–1036. ( 10.1111/j.1523-1739.2005.00078.x) [DOI] [Google Scholar]
- 21.Gaston KJ, Blackburn TM. 1996. Conservation implications of geographic range size–body size relationships. Conserv. Biol. 10, 638–646. ( 10.1046/j.1523-1739.1996.10020638.x) [DOI] [Google Scholar]
- 22.Seibold S, Brandl R, Buse J, Hothorn T, Schmidl J, Thorn S, Müller J. 2015. Association of extinction risk of saproxylic beetles with ecological degradation of forests in Europe. Conserv. Biol. 29, 382–390. ( 10.1111/cobi.12427) [DOI] [PubMed] [Google Scholar]
- 23.McKinney ML. 1997. Extinction vulnerability and selectivity: combining ecological and paleontological views. Annu. Rev. Ecol. Syst. 28, 495–516. ( 10.1146/annurev.ecolsys.28.1.495) [DOI] [Google Scholar]
- 24.Mattila N, Kaitala V, Komonen A, Kotiaho JS, Päeivinen J. 2006. Ecological determinants of distribution decline and risk of extinction in moths. Conserv. Biol. 20, 1161–1168. ( 10.1111/j.1523-1739.2006.00404.x) [DOI] [PubMed] [Google Scholar]
- 25.Borges PAV, Oromí P, Serrano ARM, Amorim IR, Pereira F. 2007. Biodiversity patterns of cavernicolous ground-beetles and their conservation status in the Azores, with the description of a new species: Trechus isabelae n. sp. (Coleoptera, Carabidae, Trechinae). Zootaxa 1478, 21–31. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
See the electronic supplementary material.