Abstract
Arthropod-borne viruses (arboviruses) are maintained in a cycle of alternating transmission between vertebrate hosts and arthropod vectors. Arboviruses possess RNA genomes capable of rapid diversification and adaptation, and the between-host trade-offs inherent to host alternation impose well-documented constraints on arbovirus evolution. Here, we investigate the less well-studied within-host trade-offs that shape arbovirus replication dynamics and transmission. Arboviruses generally establish lifelong infection in vectors but transient infection of variable magnitude (i.e. peak virus concentration) and duration in vertebrate hosts. In the majority of experimental infections of vertebrate hosts, both the magnitude and duration of arbovirus replication depended upon the dose of virus administered, with increasing dose resulting in greater magnitude but shorter duration of viraemia. This pattern suggests that the vertebrate immune response imposes a trade-off between the height and breadth of the virus replication curve. To investigate the impact of this trade-off on transmission, we used a simple modelling approach to contrast the effect of ‘tortoise’ (low magnitude, long duration viraemia) and ‘hare’ (high magnitude, short duration viraemia) arbovirus replication strategies on transmission. This model revealed that, counter to previous theory, arboviruses that adopt a tortoise strategy have higher rates of persistence in both host and vector populations.
Keywords: arthropod-borne virus, transmission, dynamics, host, vector, trade-off
1. The double life of arboviruses
The term arthropod-borne virus (arbovirus) groups taxonomically disparate viruses that share a key aspect of their life history: a horizontal transmission cycle that alternates between vertebrate hosts and haematophagous arthropod vectors (box 1). This cycle of alternation imposes unique constraints on arbovirus evolution, as these viruses are forced to simultaneously adapt to hosts from different phyla. The trade-off hypothesis proposes that host alternation shapes the evolution of arboviruses, preventing adaptation of optimal replication in either vertebrate host or arthropod vector and retarding rates of genetic change. This hypothesis has been the subject of a number of excellent reviews [19,20,23] and will not be addressed further here. Instead, in keeping with the theme of the issue, we will focus on the less-studied trade-offs that shape the dynamics of arbovirus replication within vertebrate hosts, and how such dynamics influence transmission to vectors.
Box 1. There and back again: the arbovirus life cycle.
Vertebrate host and vector use by individual arboviruses varies considerably. In aggregate, arboviruses are transmitted in nature by three orders of haematophagous insects (Diptera, Anoplura and Hemiptera) and two families of ticks (Argasidae and Ixodidae) to all four classes of terrestrial or semi-terrestrial vertebrates on all seven continents. The range of vertebrate host and vector species used also varies widely among individual arboviruses. Some are narrowly specialized in both vertebrate and vector use; for example, dengue virus in its human-endemic cycle uses one species of primate host and a small number of Aedes mosquito vectors. Others are rather promiscuous; in the USA alone West Nile virus is known to infect 65 species of mosquitoes in 10 different genera (http://www.cdc.gov/westnile/vectorControl/) and hundreds of species of birds [1,2]. Additionally, West Nile virus infects many mammalian species, some of which may support forward transmission but many of which represent dead-end hosts [1].
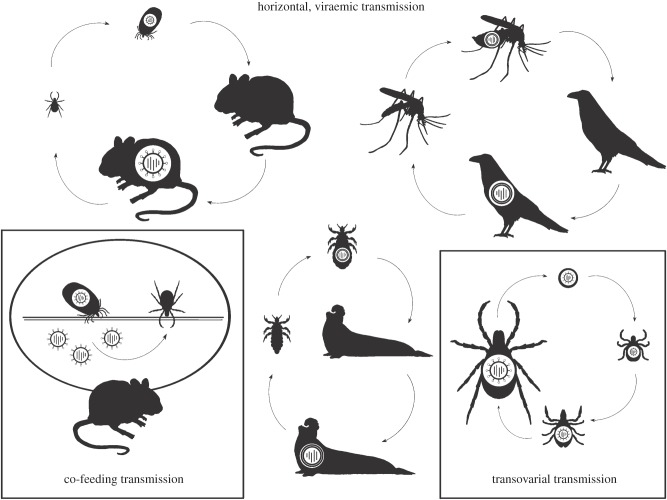
Horizontal, viraemic transmission. As illustrated in figure 1, a competent vector may become infected with an arbovirus when it imbibes viraemic blood from an infected vertebrate host. Vector competence, the ability of an arthropod to become infected with a pathogen and transmit it to a susceptible vertebrate host, depends upon many factors, including: (i) the genotype by genotype (G × G) interaction between vector and virus [3–5]; (ii) the dose of virus ingested (discussed in detail below); (iii) basal immune activation, which is a product of, among other factors, the arthropod's genotype and the composition of its microbiome [6]; and (iv) environmental conditions such as temperature, temperature fluctuation and humidity [3]. Prior or concurrent infections with other viruses may also modulate vector competence [7,8]. When taken up by a competent vector species, an arbovirus infects and disseminates across the midgut to circulate, via the haemolymph, to the salivary glands, where it replicates to sufficient concentrations to enable transmission to a susceptible vertebrate host. The period of time required to complete this process is termed the extrinsic incubation period (EIP). Once infection is established it generally persists for the life of the vector, although virus titre in tissues other than the salivary gland may wane [9].
Figure 1.
Figure produced by Sean Vidal Edgerton Science Illustration (http://www.theillustration.co/).
At the conclusion of the EIP, an infectious vector deposits viraemic saliva during bloodfeeding. Most of this saliva is actually released into the skin during probing, and components of the saliva substantially modify the immunological milieu at the probing site, often to the benefit of the virus [10]. Vertebrate host susceptibility depends upon numerous factors, including (i) the G × G interaction between host and virus genotype [11,12], (ii) physiological condition, (iii) immune status, e.g. [13–15], and (iv) dose of virus delivered. In a susceptible vertebrate host, arboviruses are transported from the bite site, usually by cells of the immune system, to lymph nodes and thence to other tissues that support replication and seed virus into the bloodstream, generating viraemia. The time lag between the infectious bite and viraemia is termed the intrinsic incubation period.
Alternative routes of transmission. In addition to horizontal, viraemic transmission described above, which is sometimes termed systemic transmission, arboviruses are also transmitted, to greater or lesser degree, by other routes as well. In vertebrates, these routes include vertical transplacental transmission and horizontal transmission via aerosols, direct contact, sexual contact, or consumption of vectors or other infected prey. In arthropods, they include vertical transovarial transmission (figure 1, lower right panel) and horizontal venereal transmission [9,16]. Additionally, co-feeding transmission (figure 1, lower left panel) occurs when virus is passed from an infected vector to an uninfected vector feeding simultaneously and in close physical proximity on the vertebrate host [9,17].
Co-feeding transmission is particularly important for maintenance of tick-borne viruses, as ticks tend to feed in aggregated groups and to occur in temperate regions where their activity is highly seasonal. Nonaka et al. [18] used a discrete time multiple matrix model to demonstrate that co-feeding was sufficient to maintain Powassan virus, a North American tick-transmitted virus, in the absence of horizontal, viraemic or vertical, transovarial transmission, whereas the virus did not persist in the absence of co-feeding. When it occurred in combination with co-feeding, vertical transmission substantially enhanced virus prevalence, while viraemic transmission had little effect. Although not addressed further in this review, co-feeding is particularly intriguing when considering the impact of within-host dynamics on transmission and evolution, because transmission success during co-feeding depends upon replication in the skin at the bite site rather than dissemination to other tissues in the vertebrate host, and may therefore select for lower systemic replication and lower virulence.
Within-host evolution. Arboviruses occur in nine genera of RNA viruses [9,19,20], whereas there is only a single known DNA arbovirus—African swine fever virus. Because RNA-dependent RNA polymerases lack proofreading, RNA virus genomes experience approximately one mutation per 10 000 nucleotides at each replication, a mutation rate that is orders of magnitude greater than those of comparable DNA genomes ([19–21], but see [22] for a more nuanced comparison of mutation rates). In a recent review, Coffey et al. [20, p. 11] pointed out that arboviruses achieve tremendous genetic diversity within their arthropod vectors, yet to date ‘no studies have examined how variant composition and diversity relate to transmitted doses'. As we describe in this review, transmitted dose is a key determinant of arbovirus within-host replication dynamics in the vertebrate host. Linking diversity to transmitted dose would greatly advance efforts to integrate within-host evolutionary and ecological dynamics.
We have chosen to concentrate on vertebrate hosts because, unlike the lifelong infections established in arthropods, arbovirus infections in vertebrates are usually acute, and the virus is cleared after a period of days (figure 2). Arbovirus infections in vertebrates are initially controlled by the innate immune response; most also stimulate an adaptive immune response that prevents homologous re-infection [20,26–29]. Some arboviruses can produce chronic infections that recrudesce at intervals, enabling survival over seasons, such as winters, that are inimical to vector activity [30–32], but these are the exception to the rule of transient infection. The limited time window for arbovirus replication in vertebrate hosts must impose strong selection for arbovirus replication dynamics that maximize forward transmission. Three major parameters of within-host viral dynamics determine the likelihood of such transmission: (i) the lag between an infectious vector bite and the presence of detectable virus in host blood, also known as the intrinsic incubation period, (ii) the magnitude of virus replication, which we here define as peak titre achieved by the virus in the blood and (iii) the duration of virus replication, which we here define as the number of days in which virus is detectable in host blood.
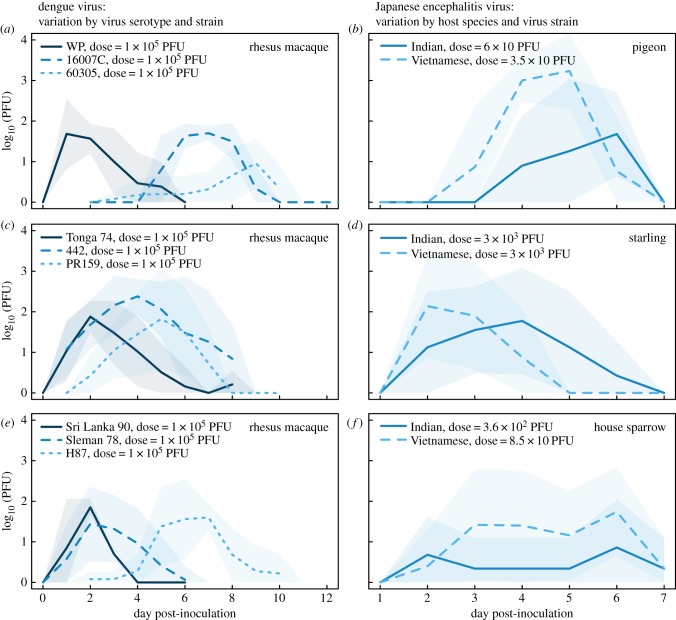
Figure 2.
Variation in viral replication curves across viruses and hosts. Figure shows viral replication (log10 PFU by day) of different strains of dengue 1, 2 and 3 in rhesus macaques ((a), (c) and (e), respectively; data from Althouse et al. [24]), and of two strains of Japanese encephalitis virus (Indian and Vietnamese) in three species of bird ((b,d,f), data from Nemeth et al. [25]). Lines are mean log10 PFU and shaded regions are mean ±1 s.d. Clear differences in the lag to viraemia, magnitude of viraemia and duration of viraemia are evident. (Online version in colour.)
Viruses may fine-tune their replication dynamics and thereby shape interactions with the immune system and virulence to their advantage. Mutations in the virus genome can modulate the rapidity of replication within the vertebrate host itself or within the invertebrate vector, which will in turn determine the dose of virus delivered to the vertebrate host. While we do not have space here to review the mechanisms by which viruses up- and downregulate replication, we note that several studies have now shown that some RNA viruses possess sequences within untranslated regions of their genomes that retard viral replication and in so doing increase viral fitness [33–35].
Experimental infections of vertebrates with arboviruses, reviewed in the section below, have generally shown that both the magnitude and duration of arbovirus replication depend upon the dose of virus administered, with increasing dose of a given strain of virus resulting in greater magnitude but shorter duration of viraemia. Based on these data, we propose that there is a trade-off between the magnitude and duration of arbovirus replication in the vertebrate host. Such a trade-off is widely accepted in evolutionary biology and is one of the foundations of theory on the evolution of virulence. However, as noted by Bull & Lauring [36, p. 1] ‘a large body of theoretical work on the evolution of virulence has yet to gain traction in the virology community’. Moreover, work on the evolution of virulence has largely focused on the trade-off between magnitude of replication and mortality, which limits duration of infection [36]. Arthropod vectors suffer slight but significant decreases in fitness due to arbovirus infections, and the strength of these effects depend upon the primary mode of transmission (horizontal versus vertical, box 1) [37]. Arboviruses have a much wider range of fitness effects in their vertebrate hosts [38]. Some arboviruses, such as West Nile virus, are highly lethal even in reservoir hosts [39], while others, such as dengue virus and yellow fever virus, have little or no detectable effect on vertebrate host fitness in their ancestral non-human primate hosts [40,41]. As mortality and even morbidity are unlikely to be a universal selective force on arbovirus dynamics, in this review we focus instead on the impact of immune responses on such dynamics.
Given a trade-off between magnitude and duration of replication, what pattern of arbovirus dynamics in vertebrate hosts maximizes forward transmission? To try to answer this question, we searched for empirical studies on the effect of dose of virus ingested on infection of arthropods. Results of such studies are also presented below, but we found that, to date, they fail to provide the data needed to adequately characterize the impact of within-host dynamics on transmission. In particular, transmission to mosquitoes has occurred when no virus was detected in host blood using standard in vitro and in culture methods, indicating the inadequacy of such assays. We therefore present a simple modelling approach to contrast the effect of ‘tortoise’ (low magnitude, long duration viraemia) versus ‘hare’ (high magnitude, short duration viraemia) arbovirus replication strategies on transmission from vertebrate hosts to arthropod vectors. Our hope is that this work will serve to motivate other investigators to coordinate and conduct the empirical and modelling studies needed to fully plumb the nature and consequences of this intrinsic trade-off.
2. Trade-offs in within-host replication dynamics of arboviruses
(a). Replication dynamics within the vertebrate host
We have recently completed a systematic review and individual pooled analysis of the replication kinetics of dengue virus following experimental infections of non-human primates, as reported in 51 published and three unpublished studies [42]. Dengue virus comprises four genetically distinct serotypes (dengue virus types 1–4), and the within-host dynamics of these serotypes were tested in 11 different species of non-human primates. With the majority of the data, it was possible to analyse lag to detectable viraemia (intrinsic incubation period) and duration of viraemia, but not the magnitude of viraemia. Both lag to and duration of viraemia were significantly different among serotypes (some examples are shown in figure 2) but not among primate species. Importantly, dose of virus inoculated was a major determinant of duration of viraemia, which decreased with increasing dose. By contrast, in dose de-escalation studies of dengue virus in non-human primates, peak viraemia generally increased with increasing dose (data reviewed in [41]). A 2013 study of dengue virus replication in baboons [43] that was not included in these aforementioned reviews, also found that non-human primates receiving lower doses of dengue virus challenge experienced longer durations of viraemia.
These findings suggest an inherent trade-off between duration and magnitude of viraemia. Moreover, we favour the interpretation, initially suggested by some of the authors of these studies, that this trade-off is mediated by the immune response to infection, and that a higher infecting dose enables more rapid virus replication but a concomitantly rapid and strong immune response [44,45]. An alternative explanation for this trade-off is resource limitation—i.e. that the high magnitude of replication following a high dose of infection exhausts key resources. However, it is well known that antibody raised during a previous infection by a different serotype can enhance dengue virus replication by 10-fold or more in humans and in non-human primates [46,47], indicating that dengue replication during primary infection is probably not limited by resource availability.
Evidence for a trade-off between magnitude and duration of virus replication in studies of dengue virus infection of the human host is equivocal. Both Vaughn et al. [48] and Tricou et al. [49] found that secondary dengue virus infection in humans (i.e. infection with a given serotype occurring after clearance of infection of a different serotype) was associated with higher levels of virus replication and also more rapid viral clearance, whereas Murgue et al. [50] reported the opposite. However, human studies must be interpreted with caution, as they often fail to capture peak virus titre and rarely encompass the entire duration of viraemia.
Data from experimental infections with several other arboviruses also support a trade-off between magnitude and duration of replication. When rufous night herons were injected with varying doses of the mosquito-borne viruses Murray Valley encephalitis virus (MVEV), Kunjin virus and Japanese encephalitis virus (JEV), there was an evident trend of increasing infection duration and decreasing peak titre with declining virus dose in the inoculum, although this pattern was not specifically analysed in the paper [51]. Similarly, Baylis et al. [52] fed varying numbers of midges (one, five or 20) infected with bluetongue virus on sheep and found that sheep that were fed upon by larger numbers of midges (and therefore inoculated with a larger virus dose) developed viraemia more rapidly, developed a higher magnitude of viraemia and also marshalled a stronger antibody response.
By contrast, studies in which cliff swallows [53] and robins [54] were experimentally infected with West Nile virus did not find a correlation between virus dose and either duration or peak titre. Additionally, Weingartl et al. [55] found that the cultured cells in which an arbovirus stock are produced affect replication dynamics in vivo: sheep inoculated with a lower dose of Rift Valley fever virus (RVFV) raised in mammalian cells produced a longer duration of viraemia but not a higher peak titre than sheep infected with a higher dose, whereas sheep inoculated with a lower dose of RVFV raised in mosquito cells produced a shorter duration of viraemia and a similar peak titre to sheep inoculated with a higher dose of the virus. While we do not claim to have identified all studies in which the impact of arbovirus dose on infection dynamics has been measured, we believe that the studies presented offer an unbiased view of general trends in the literature.
An important caveat to the interpretation of these studies is that some of them were conducted using natural hosts of the target virus, such as studies of MVEV, Kunjin virus and JEV infection of herons and dengue virus infection of African green monkeys. However others, such as studies of dengue virus infection of rhesus macaques, were not [41]. Arbovirus replication dynamics, fitness effects and immune response can be substantially different in natural compared with novel vertebrate hosts [56,57]. For example, mosquito-borne JEV circulates in Asia in a number of avian host species, among them the Japanese tree sparrow. When Japanese tree sparrows were experimentally infected with a high dose of JEV, viraemia was brief, with no birds producing detectable virus after day 3 post-infection [58]. By contrast, when Nemeth et al. [25] infected a variety of North American bird species with JEV, these novel vertebrate hosts produced a wide range in magnitude and duration of viraemia (figure 2), with some species sustaining high virus titres beyond 7 days post-infection. In another example, tick-borne encephalitis virus can be highly virulent (up to 50% mortality) in laboratory mice [59] but rarely causes symptoms in the bank vole, a reservoir host of this virus [60]. Finally, yellow fever, which evolved in circulation between non-human primates and sylvatic mosquito vectors in Africa, is well known to be avirulent in its African reservoir hosts but highly virulent in many New World monkeys (reviewed in [41]). Mandl et al. [61] experimentally infected both sooty mangabeys, an African reservoir host of this virus, and rhesus macaques, a novel host, with an attenuated strain of yellow fever virus. They found that the virus replicated to a lower titre and for a shorter duration in sooty mangabeys. They also found that yellow fever virus neutralizing antibody levels were lower overall and waned to undetectable levels more quickly in sooty mangabeys than rhesus macaques. While studies in novel vertebrate host-virus combinations can reveal important aspects of immune control, they are best considered in combination with data from natural host-virus associations.
(b). Replication dynamics within the arthropod vector
As described below, numerous studies have demonstrated that increasing the arbovirus dose in a bloodmeal results in higher levels of vector infection. Moreover, once arbovirus infection is established in a vector, it generally persists for life, although Slovak et al. [62] have recently shown that infection prevalence of tick-borne encephalitis virus in Ixodes ricinus ticks declined substantially as these vectors moulted from larvae to nymphs and nymphs to adults. However, as noted by Christofferson & Mores [63], relatively few studies have investigated the dynamics of viral replication within the vector, i.e. the change in the magnitude of virus replication, particularly in the saliva, over the duration of the extrinsic incubation period (EIP, box 1) and beyond. Salazar et al. [64] monitored the progress of dengue virus infection in Aedes aegypti mosquitoes that had ingested a high titre (approx. 7 log10 plaque-forming units (PFU)/ml). They found that virus titre in the mosquito as a whole increased from 2.2 to 5.5 log10 PFU between days 2 and 14 post-infection, after which time the titre levelled off. However, there was considerable variation in individual tissues: virus titre peaked in the midgut by day 10 post-infection and then dropped, whereas titre in the salivary glands increased steadily over the course of infection. The impact of dose on virus replication has been investigated in Culex tarsalis mosquitoes fed an artificial bloodmeal containing either a high (6 log10 PFU/ml) or low (4 log10 PFU/ml) virus dose. Mosquitoes that ingested the high dose showed a shorter EIP [65,66] and were more likely to transmit virus [66] than mosquitoes that fed on a low titre of the virus. These latter findings indicate that the magnitude of arbovirus titre in the vertebrate host may affect not only the likelihood of infecting vectors, but also the subsequent dynamics of virus replication within those vectors, particularly the EIP, an effect that has not been addressed in any mathematical models of transmission of which we are aware.
3. Effect of within-host arbovirus replication dynamics on transmission: empirical studies
In order to understand how the variation in arbovirus replication dynamics described above impacts transmission, it is first necessary to characterize the effect of virus titre, in vertebrate and vector, on transmission to the next host. Below we summarize what is known about infectious dose for arboviruses and why, to date, this knowledge is inadequate to infer the impact of within-host dynamics on transmission.
(a). Vertebrate host to vector transmission
In their review of arbovirus transmission, Kuno & Chang have noted that ‘Theoretically, the higher the concentration of virus in blood and the longer the duration of viremia, the greater is the probability of a vector acquiring virus from infected vertebrates' [9, p. 618]. This is clearly true, but, from the perspective of within-host dynamics, it is crucial to know how much transmission is gained with an increase in virus concentration, and to define the minimum concentration that enables meaningful transmission. The ability of an arbovirus to infect a particular vector species is usually measured by feeding groups of vectors on artificial bloodmeals spiked with a range of known concentrations of the virus and measuring infection at the conclusion of a standard EIP. These studies have demonstrated that transmission of arboviruses is dose-dependent and that transmission only occurs at a rather high dose threshold [3,9,67]. For example, Ponsiri et al. found the 10% oral infectious dose (OID10) of dengue virus for Ae. aegypti to range from approximately 1.5 to approximately 5.0 log10 PFU ml−1 bloodmeal. Tsetsarkin et al. showed that infection of Ae. aegypti with chikungunya virus declined from approximately 90% to less than 15% as virus titre dropped from 7.0 to 4.0 log10 tissue culture infectious dose 50 ml−1 bloodmeal. Of course, mosquitoes imbibe only a small fraction of the bloodmeal, but measuring in virus units per millilitre allows comparison to studies of within-host replication (such as the examples in figure 2).
Together, data from artificial bloodmeals suggest that arboviruses would have to reach quite high titres in vertebrate hosts before significant transmission could occur. However, this inference may be misleading as the threshold for transmission of a given arbovirus is substantially lower when a vector feeds on a living vertebrate host rather than an artificial meal [68–70]. The interaction effect of bloodmeal type (live vertebrate host versus artificial bloodmeal) and virus titre on virus transmissibility has not been completely characterized for any arbovirus, suggesting that our current understanding of transmission at low virus titres is inadequate. Two studies of dengue virus emphasize this point. First, Scott et al. [71] fed Ae. aegypti mosquitoes, the major dengue vector, on rhesus macaques that had been experimentally infected with wild-type dengue virus. Although they did not report the percentage of mosquitoes infected for each day, they did note that ‘The days on which virus could be detected in mosquitoes were usually but not always the same as those on which viremia was detected’ (p. 182). Second Watts et al. [72] fed Ae. aegypti on a rhesus macaque that had been infected with dengue virus. Even though no virus was detectable in the monkey's blood at the time of feeding, up to 80% of mosquitoes developed a disseminated dengue virus infection. While methods of virus detection varied among studies, nonetheless it is clear that mosquitoes became infected from monkeys at a much lower viraemia than would be predicted based on studies employing artificial bloodmeals.
Virologists are becoming increasingly aware of the importance of quantifying arbovirus transmission from live vertebrate hosts across the window of infection [67,70,73]. In a landmark study, Nguyen et al. [74] obtained data on the transmissibility of dengue virus to Ae. aegypti from febrile dengue patients across a 4-day span following presentation at a clinic. Aedes aegypti were fed upon 208 febrile dengue patients on two randomly assigned days post-enrolment, with all feedings scheduled within 4 days post-enrolment. Blood was also drawn from each patient at the time of feeding to determine virus titre. Transmission increased in a sigmoidal fashion; both virus titre and increasing antibody titres were independently associated with decreasing infectiousness for vectors. Additionally, high virus titres early in infection were associated with increased duration of infectiousness, seemingly contradicting the trade-off posed above. However, because these data were obtained from natural infections, it is possible that duration of infectiousness was actually inversely associated with magnitude of infection, but patients with high titres had come in to the clinic at a disproportionately early point in infection.
(b). Vector to vertebrate host transmission
The most common approach used to determine the infectious dose of an arbovirus for its vertebrate host or to characterize within-host dynamics is to inject vertebrate hosts with a serially diluted virus and monitor infection (e.g. [54,75]). Indeed, this is how most of the studies discussed in the section above were conducted. For many arboviruses, the infectious dose 50 for a vertebrate host is less than 10 PFU. However, as with vectors infected via artificial bloodmeals, host infections delivered by needle may not adequately recapitulate natural infections. There is a growing body of evidence that, owing to potentiation by vector saliva, an arbovirus delivered by vector bite is substantially more infectious, embarks on different replication dynamics and elicits a different immune response than the same virus delivered at the same dose by needle [76–81]. Although expensive and logistically difficult, it is possible to experimentally infect vertebrate hosts via vector bite. However when vectors are used to deliver virus, the dose of virus delivered cannot be controlled or measured. Measuring the amount of virus delivered by a vector requires that the body segment on which those vectors feed (a tail or a toe, for example) be amputated for viral assay (e.g. [82]). As a proxy measure, many investigators have quantified virus from fluid into which the vector has been induced to salivate. However, there is now clear evidence that mosquitoes deposit orders of magnitude more virus when probing on a live vertebrate host than when salivating into a capillary tube or a hanging drop of blood [82]. Thus, the virus dose required to infect a vertebrate host is not known for most arboviruses.
4. The tortoise or the hare: which pattern of arbovirus replication in the vertebrate host maximizes transmission to the vector?
Several investigators have recently attempted to explain among-strain variation in within-host arbovirus replication kinetics using models that link virus titre to immune response [83,84]. However, models of the effect of within-host replication on transmission to vectors have largely ignored the graded effect of titre on virus acquisition by vectors, and instead have assumed a threshold for transmission, wherein the vertebrate host is or is not infectious to the vector [70]. Several influential mathematical models of arbovirus transmission dynamics, (e.g. [24,85,86]) do not explicitly consider within-host viral titre, but they implicitly assume that total transmission is correlated with the peak titre, i.e. the magnitude of replication. More recently, Christofferson et al. [87] took a more nuanced approach with a stochastic, compartmental model of dengue virus introduction into a new population in which the infectivity of humans for mosquitoes varied with day post-infection. This model leveraged the data on human to mosquito transmission of dengue virus generated by Nguyen et al. [74]. However, this model did not address differences in replication among strains of dengue virus, though the authors noted that studies of such variation are needed.
In 2006, Lord et al. [70] contrasted the results of three models in which arbovirus transmission to vectors was determined by (i) a simple threshold, (ii) a probabilistic model in which virus titre in the blood determined the probability of acquisition of virus in the vector bloodmeal, and (iii) a probabilistic model in which high virus titre resulted in death of the vertebrate host, thus limiting duration of viraemia. The third model, which reflects high-virulence arboviruses such as West Nile virus, indicated that transmission was maximized when viruses replicated to just below the threshold for mortality. Lord et al. [70] discussed the possibility that the immune response might also impose a trade-off on the magnitude and duration of viraemia in vertebrate hosts, but concluded that there was inadequate understanding of these processes to draw conclusions. Ten years later, there are still extensive lacunae in our knowledge of the mechanisms of within-host trade-offs in replication, but we feel that there is adequate evidence of the existence of such trade-offs to begin to investigate their impact on transmission.
Experimental data do show variation in viral replication curves between arbovirus strains and vertebrate host species. Figure 2 shows the replication profile of three different strains each from dengue virus serotypes 1 through 3 in rhesus macaques (data from [42]), and the replication profile of two strains of JEV in three species of bird (data provided by Dr Nicole Nemeth; originally presented in a different form in [25]). Clear differences in lag to onset of viraemia, peak virus titre and duration of viraemia are evident. There are two points to be gleaned from these data: (i) in a single host species, different virus strains show dramatic differences in the magnitude and duration of viraemia, and (ii) strains of a given virus can undergo quite different dynamics in different vertebrate host species. Both of these factors may directly affect the efficiency of arbovirus transmission from vertebrate host to vector. We therefore use a mathematical model of arbovirus transmission to explore the effects of different within-host dynamics on transmission dynamics within a population of vertebrate hosts and vectors.
To do so, we extend a stochastic susceptible-infected-recovered (SIR) arbovirus transmission model previously presented in Althouse et al. [24]. Briefly, vectors and hosts are born susceptible to arbovirus infection and are infected at a rate proportional to the number of bites given or received per day and the probability of infection. These transmission probabilities vary seasonally to represent the fluctuation in per bite transmission probability due to seasonally varying processes. After infection, vertebrate hosts recover at a fixed rate and vectors are infected for the remainder of their life. We assume no disease-induced mortality.
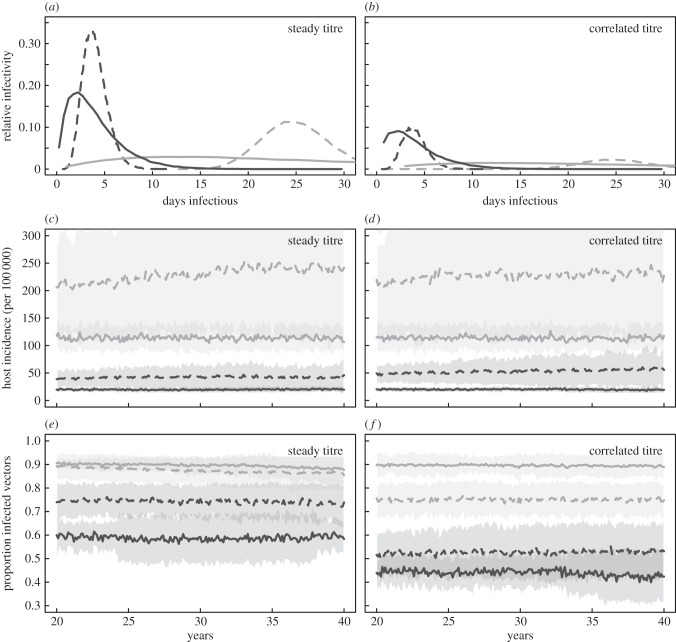
For simplicity, we restrict the model to a single vertebrate host and a single vector species. We also note that the model is disease-agnostic, and, with appropriate choice or fit of biological parameters, could be equally applied to any arbovirus, with the caveat that our model does not capture transmission via co-feeding or vertical passage and therefore may be less relevant for tick-borne viruses. Using this framework, we can explore what effects differences in viral replication dynamics in the vertebrate host have on transmission. First, we explore differences in duration of infectiousness in the vertebrate host using the method of stages [88] to expand the infected compartment into multiple infectious compartments. In a standard SIR model, the infectious period is exponentially distributed with mean equal to the mean duration of infectiousness (1/γ). In essence, the method of stages sums n independent exponentially distributed waiting times (time spent in each infectious class), resulting in a gamma distributed duration of infectiousness with mean equal to the mean duration of infectiousness (1/γ) and variance 1/(nγ2). Figure 3a,b shows the distributions of infectious periods used here. We explore four cases, chosen arbitrarily, but inspired by actual patterns in arbovirus replication kinetics (e.g. figure 2): mean duration 4 days with variance either 4 days (dark solid curve) or 1.6 days (dark dashed curve; similar to dengue virus in non-human primates [42]) and mean duration 25 days with variance either 25 days (light solid curve) or 12.5 days (light dashed curve; similar to bluetongue virus in cattle [89]). Note that we explore additional durations of viraemia in the electronic supplementary material. We account for correlation between duration of infectiousness and viral titre by allowing each of the n infectious compartments to contribute independently to transmission. If all compartments contribute equally, there is no correlation (figure 3a), if only the first or final few compartments contribute to transmission, there is correlation (figure 3b). This approximates the inverse relationship between magnitude of viraemia and transmissibility (i.e. tortoise versus hare strategies). Again, owing to the generality of the model, these distributions were chosen to illustrate the effects of correlated titres on transmission. All qualitative results reported here are insensitive to different values of the transmissibility of the arbovirus, though further sensitivity analyses should be conducted if the model is applied to a real-worldc system. We leave this to the interested reader.
Figure 3.
Results of the stochastic arbovirus transmission model. (a) The infectivity curves used in model runs for a steady viraemia titre over the course of infection (constant transmission probability across infection), and (b) the curves for viral titre correlated with peaks in the infectious duration (higher viraemia leading to higher transmission). Lines indicate a mean duration 4 days with variance either 4 days (dark solid curve) or 1.6 days (dark dashed curve), and mean duration 25 days with variance either 25 days (light solid curve) or 12.5 days (light dashed curve). (c,d) Virus incidence in vertebrate hosts for the steady and correlated titre scenarios, respectively, and (e,f) the proportion of infected vectors in each scenario. Colours and line type correspond to distributions in (a,b). Lines are means of 50 stochastic simulations and shaded regions are mean ±1 s.d. Model parameters: transmissibility (probability of infection per infectious bite) = 0.3, amplitude of seasonality = 0.1; 0.5 bites per vertebrate host per day, vector birthrate = 1/7 d−1, vertebrate host birthrate = 1/60 yr−1, initial number of vectors = 15 000, initial number of vertebrate hosts = 10 000.
There are a number of key results from this model. First, numbers of vertebrate host infections are larger for both longer durations of infection and more peaked durations of infection (figure 3c,d). The association between longer duration and greater incidence of vertebrate infection is a necessary finding for the case of steady titres—the longer the titre persists the more infections occur. However, in the case of correlated titres, this result is counter to previous theory indicating that rapid onset, extended periods of viraemia, as exemplified by the solid dark curve in figure 3, are beneficial to transmission. Second, with respect to incidence in vertebrate hosts, correlated infectious periods and virus titre do not appear different from uncorrelated, steady titre (figure 3c,d). This would indicate that the impact on between-host transmission of a sustained level of viraemia within vertebrate hosts and a viraemia that changes over the course of infection are equivalent. Third, despite no difference in incidence in vertebrate hosts between correlated and steady titre, there are differences in prevalence in the vector, with correlated titres showing lower prevalence than steady titres (figure 3e,f). This is especially true for earlier onset viraemias (dark lines). These last two results taken together suggest that arboviruses might benefit from a tortoise approach to within-host replication, as a constant viraemia over the entire course of infection maximizes viral persistence and thus transmission to both the vertebrate host and the vector.
5. Within-host dynamics and the epidemiology, emergence and evolution of arboviruses
The assumptions that (i) arboviruses are transmitted from vertebrate hosts to vectors only when viraemia exceeds a relatively high transmission ‘threshold’ [70] and (ii) arbovirus transmission is correlated to peak virus titre shape current thinking about adaptation of these viruses and are implicit in many mathematical models of arbovirus epidemiology [24,85,86]. These assumptions are also fundamental to the design of safe, live-attenuated arbovirus vaccines, in that it is generally agreed that constraining peak vaccine virus replication to levels below this putative transmission threshold acts as a safeguard against transmission (e.g. [90–92]). Finally, studies seeking to identify reservoir and amplification hosts for arboviruses, both in their existing ranges and in regions where they could be introduced in the future, generally test whether potential hosts support viraemias that exceed the putative transmission threshold (e.g. [70,93–95]). Here, however, we present empirical evidence that such thresholds, which are derived primarily from studies in which vectors feed on artificial bloodmeals, are likely to be inflated and therefore misleading. In particular, we have pointed out several studies in which arbovirus transmission to vectors from vertebrate hosts was detected even when virus in vertebrate host blood samples drawn at the same time was not.
Our model for the effect of within-host arbovirus dynamics on transmission to vectors relaxes the simplifying assumption of a threshold viraemia for transmission in favour of the more realistic view that transmission from vertebrate host to vector is proportional to virus titre in the vertebrate host, and that meaningful transmission may occur, at least in some arboviruses, at low virus titre (see the electronic supplementary material for model details and sensitivity analyses). The viral replication curves that underpin this model are shaped by evidence from the empirical literature for an immune-driven trade-off between the magnitude and duration of viraemia. This model reveals that arboviruses may maximize transmission by keeping a ‘low profile’ in the vertebrate host and thereby maintaining viraemia for a longer period of time than would be possible if viraemia spiked to high levels but was more rapidly cleared. This result may help to explain the otherwise paradoxical finding that many arboviruses produce an extremely low magnitude of viraemia in their reservoir hosts (e.g. [56–58,61]). Such viraemias often seem to be of brief duration as well, but this conclusion is subject to the caveat, discussed above, regarding the inadequacy of current techniques to directly detect infectious virus. Additionally, a growing body of evidence suggests that at least some arboviruses may establish persistent infection in their reservoir hosts [96–99]. This may only be possible if initial infections are restrained in the magnitude of replication and therefore elicit a muted immune response. Future experiments are needed in which vector infection, rather than genome detection or culture, is used as the gold standard assay for presence of transmissible virus.
The magnitude and duration of viral replication will also shape the quasi-species diversity of arboviral infections: smaller population sizes would allow more limited sampling of potential genome variants and longer duration of replication would offer greater scope for within-host selection. To date, there is little data on the impact of arboviral variation on transmission from vertebrate host to vector or vector to vertebrate host [20]. With the advent of technological advances in ultra-deep sequencing, it should be increasingly tractable to couple studies of within-host evolutionary dynamics of arboviruses to their within-host replication kinetics and between-host transmission. Such studies will greatly advance our current understanding of the epidemiology, emergence and evolution of these viruses and are likely to have important implications for the control of these emerging pathogens.
Supplementary Material
Acknowledgements
We are grateful to Nicole Nemeth (Ontario Veterinary College, University of Guelph) for graciously sharing her Japanese encephalitis virus data for inclusion in figure 2 and to three anonymous reviewers for substantially improving the manuscript. A NESCent working group and an NSF-RCN on Infectious Disease Evolution Across Scales provided the impetus for this article.
Authors' contributions
Both authors conceived of the review, drafted sections of the manuscript and approved of the final manuscript. B.M.A. generated and implemented the mathematical model.
Competing interests
We declare we have no competing interests.
Funding
K.A.H. was supported by NIH 1R15AI113628–01 during preparation of this manuscript; B.M.A. acknowledges support from the Santa Fe Institute and the Omidyar Group.
References
- 1.Brault AC. 2009. Changing patterns of West Nile virus transmission: altered vector competence and host susceptibility. Vet. Res. 40, 43 ( 10.1051/vetres/2009026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray KO, Mertens E, Despres P. 2010. West Nile virus and its emergence in the United States of America. Vet. Res. 41, 67 ( 10.1051/vetres/2010039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tabachnick WJ. 2013. Nature, nurture and evolution of intra-species variation in mosquito arbovirus transmission competence. Int. J. Environ. Res. Public Health 10, 249–277. ( 10.3390/ijerph10010249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lambrechts L, Chevillon C, Albright RG, Thaisomboonsuk B, Richardson JH, Jarman RG, Scott TW. 2009. Genetic specificity and potential for local adaptation between dengue viruses and mosquito vectors. BMC Evol. Biol. 9, 160 ( 10.1186/1471-2148-9-160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lambrechts L, Quillery E, Noel V, Richardson JH, Jarman RG, Scott TW, Chevillon C. 2013. Specificity of resistance to dengue virus isolates is associated with genotypes of the mosquito antiviral gene Dicer-2. Proc. R. Soc. B 280, 20122437 ( 10.1098/rspb.2012.2437) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jupatanakul N, Sim S, Dimopoulos G. 2014. The insect microbiome modulates vector competence for arboviruses. Viruses 6, 4294–4313. ( 10.3390/v6114294) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bell-Sakyi L, Attoui H. 2013. Endogenous tick viruses and modulation of tick-borne pathogen growth. Front. Cell Infect. Microbiol. 3, 25 ( 10.3389/fcimb.2013.00025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kent RJ, Crabtree MB, Miller BR. 2010. Transmission of West Nile virus by Culex quinquefasciatus Say infected with Culex Flavivirus Izabal. PLoS Negl. Trop. Dis. 4, e671 ( 10.1371/journal.pntd.0000671) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuno G, Chang GJ. 2005. Biological transmission of arboviruses: reexamination of and new insights into components, mechanisms, and unique traits as well as their evolutionary trends. Clin. Microbiol. Rev. 18, 608–637. ( 10.1128/CMR.18.4.608-637.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Briant L, Despres P, Choumet V, Misse D. 2014. Role of skin immune cells on the host susceptibility to mosquito-borne viruses. Virology 464–465, 26–32. ( 10.1016/j.virol.2014.06.023) [DOI] [PubMed] [Google Scholar]
- 11.Han N, et al. 2014. Comparison of genotypes I and III in Japanese encephalitis virus reveals distinct differences in their genetic and host diversity. J. Virol. 88, 11 469–11 479. ( 10.1128/JVI.02050-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weaver SC, et al. 2004. Genetic determinants of Venezuelan equine encephalitis emergence. Arch. Virol. Suppl. 18, 43–64. ( 10.1007/978-3-7091-0572-6_5) [DOI] [PubMed] [Google Scholar]
- 13.Carver S, Bestall A, Jardine A, Ostfeld RS. 2009. Influence of hosts on the ecology of arboviral transmission: potential mechanisms influencing dengue, Murray Valley encephalitis, and Ross River virus in Australia. Vector Borne Zoonotic Dis. 9, 51–64. ( 10.1089/vbz.2008.0040) [DOI] [PubMed] [Google Scholar]
- 14.Murphy BR, Whitehead SS. 2011. Immune response to dengue virus and prospects for a vaccine. Annu. Rev. Immunol. 29, 587–619. ( 10.1146/annurev-immunol-031210-101315) [DOI] [PubMed] [Google Scholar]
- 15.Powers AM. 2010. Chikungunya. Clin. Lab. Med. 30, 209–219. ( 10.1016/j.cll.2009.10.003) [DOI] [PubMed] [Google Scholar]
- 16.Lequime S, Lambrechts L. 2014. Vertical transmission of arboviruses in mosquitoes: a historical perspective. Infect. Genet. Evol. 28, 681–690. ( 10.1016/j.meegid.2014.07.025) [DOI] [PubMed] [Google Scholar]
- 17.Havlikova S, Lickova M, Klempa B. 2013. Non-viraemic transmission of tick-borne viruses. Acta Virol. 57, 123–129. ( 10.4149/av_2013_02_123) [DOI] [PubMed] [Google Scholar]
- 18.Nonaka E, Ebel GD, Wearing HJ. 2010. Persistence of pathogens with short infectious periods in seasonal tick populations: the relative importance of three transmission routes. PLoS ONE 5, e11745 ( 10.1371/journal.pone.0011745) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanley KA, Weaver SC. 2008. Arbovirus evolution. In Origin and evolution of viruses (eds Domingo E, Parrish C, Holland JF.), pp. 351–392, 2nd edn St Louis, MO: Elsevier. [Google Scholar]
- 20.Coffey LL, Forrester N, Tsetsarkin K, Vasilakis N, Weaver SC. 2013. Factors shaping the adaptive landscape for arboviruses: implications for the emergence of disease. Future Microbiol. 8, 155–176. ( 10.2217/fmb.12.139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Domingo E, Sheldon J, Perales C. 2012. Viral quasispecies evolution. Microbiol. Mol. Biol. Rev. 76, 159–216. ( 10.1128/MMBR.05023-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duffy S, Shackelton LA, Holmes EC. 2008. Rates of evolutionary change in viruses: patterns and determinants. Nat. Rev. Genet. 9, 267–276. ( 10.1038/nrg2323) [DOI] [PubMed] [Google Scholar]
- 23.Ciota AT, Kramer LD. 2010. Insights into arbovirus evolution and adaptation from experimental studies. Viruses 2, 2594–2617. ( 10.3390/v2122594) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Althouse BM, Lessler J, Sall AA, Diallo M, Hanley KA, Watts DM, Weaver SC, Cummings DAT. 2012. Synchrony of sylvatic dengue isolations: a multi-host, multi-vector SIR model of dengue virus transmission in Senegal. PLoS Negl. Trop. Dis. 6, e1928 ( 10.1371/journal.pntd.0001928) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nemeth N, Bosco-Lauth A, Oesterle P, Kohler D, Bowen R. 2012. North American birds as potential amplifying hosts of Japanese encephalitis virus. Am. J. Trop. Med. Hyg. 87, 760–767. ( 10.4269/ajtmh.2012.12-0141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arjona A, Wang P, Montgomery RR, Fikrig E. 2011. Innate immune control of West Nile virus infection. Cell Microbiol. 13, 1648–1658. ( 10.1111/j.1462-5822.2011.01649.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Augustine AD, Cassetti MC, Ennis FA, Harris E, Hildebrand WH, Repik PM. 2010. NIAID workshop on Flavivirus immunity. Viral Immunol. 23, 235–240. ( 10.1089/vim.2009.0114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quicke KM, Suthar MS. 2013. The innate immune playbook for restricting West Nile virus infection. Viruses 5, 2643–2658. ( 10.3390/v5112643) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weaver SC, Osorio JE, Livengood JA, Chen R, Stinchcomb DT. 2012. Chikungunya virus and prospects for a vaccine. Expert Rev. Vaccines 11, 1087–1101. ( 10.1586/erv.12.84) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wheeler SS, Vineyard MP, Barker CM, Reisen WK. 2012. Importance of recrudescent avian infection in West Nile virus overwintering: incomplete antibody neutralization of virus allows infrequent vector infection. J. Med. Entomol. 49, 895–902. ( 10.1603/ME11286) [DOI] [PubMed] [Google Scholar]
- 31.Charron MV, Balenghien T, Seegers H, Langlais M, Ezanno P. 2013. How much can diptera-borne viruses persist over unfavourable seasons? PLoS ONE 8, e74213 ( 10.1371/journal.pone.0074213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson A, Darpel K, Mellor PS. 2008. Where does bluetongue virus sleep in the winter? PLoS Biol. 6, e210 ( 10.1371/journal.pbio.0060210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson DE, Castan A, Bisaillon M, von Messling V. 2012. Elements in the canine distemper virus M 3′ UTR contribute to control of replication efficiency and virulence. PLoS ONE 7, e31561 ( 10.1371/journal.pone.0031561) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siemetzki U, Ashok MS, Briese T, Lipkin WI. 2009. Identification of RNA instability elements in Borna disease virus. Virus Res. 144, 27–34. ( 10.1016/j.virusres.2009.03.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trobaugh DW, et al. 2014. RNA viruses can hijack vertebrate microRNAs to suppress innate immunity. Nature 506, 245–248. ( 10.1038/nature12869) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bull JJ, Lauring AS. 2014. Theory and empiricism in virulence evolution. PLoS Pathog. 10, e1004387 ( 10.1371/journal.ppat.1004387) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lambrechts L, Scott TW. 2009. Mode of transmission and the evolution of arbovirus virulence in mosquito vectors. Proc. R. Soc. B 276, 1369–1378. ( 10.1098/rspb.2008.1709) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hubalek Z, Rudolf I, Nowotny N. 2014. Arboviruses pathogenic for domestic and wild animals. Adv. Virus Res. 89, 201–275. ( 10.1016/b978-0-12-800172-1.00005-7) [DOI] [PubMed] [Google Scholar]
- 39.Kramer LD, Styer LM, Ebel GD. 2008. A global perspective on the epidemiology of West Nile virus. Annu. Rev. Entomol. 53, 61–81. ( 10.1146/annurev.ento.53.103106.093258) [DOI] [PubMed] [Google Scholar]
- 40.Hanley KA, Guerbois M, Kautz TF, Brown M, Whitehead SS, Weaver SC, Vasilakis N, Marx PA. 2014. Infection dynamics of sylvatic dengue virus in a natural primate host, the African green monkey. Am. J. Trop. Med. Hyg. 91, 672–676. ( 10.4269/ajtmh.13-0492) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanley KA, Monath TP, Weaver SC, Rossi SL, Richman RL, Vasilakis N. 2013. Fever versus fever: the role of host and vector susceptibility and interspecific competition in shaping the current and future distributions of the sylvatic cycles of dengue virus and yellow fever virus. Infect. Genet. Evol. 19, 292–311. ( 10.1016/j.meegid.2013.03.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Althouse BM, Durbin AP, Hanley KA, Halstead SB, Weaver SC, Cummings DA. 2014. Viral kinetics of primary dengue virus infection in non-human primates: a systematic review and individual pooled analysis. Virology 452–453, 237–246. ( 10.1016/j.virol.2014.01.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valdes I, et al. 2013. Olive baboons: a non-human primate model for testing dengue virus type 2 replication. Int. J. Infect. Dis. 17, e1176–e1181. ( 10.1016/j.ijid.2013.08.007) [DOI] [PubMed] [Google Scholar]
- 44.Martin J, et al. 2009. Viremia and antibody response in green monkeys (Chlorocebus aethiops sabaeus) infected with dengue virus type 2: a potential model for vaccine testing. Microbiol. Immunol. 53, 216–223. ( 10.1111/j.1348-0421.2009.00112.x) [DOI] [PubMed] [Google Scholar]
- 45.Martin J, Hermida L, Castro J, Romero Y, Cardosa J, Guillen G. 2009. Viremia and the magnitude of the immune response upon infection of green monkeys with dengue virus type 2 are strain-dependent. Curr. Microbiol. 59, 579–583. ( 10.1007/s00284-009-9488-6) [DOI] [PubMed] [Google Scholar]
- 46.Goncalvez AP, Engle RE, St Claire M, Purcell RH, Lai CJ. 2007. Monoclonal antibody-mediated enhancement of dengue virus infection in vitro and in vivo and strategies for prevention. Proc. Natl Acad. Sci. USA 104, 9422–9427. ( 10.1073/pnas.0703498104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guzman MG, Alvarez M, Halstead SB. 2013. Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: an historical perspective and role of antibody-dependent enhancement of infection. Arch. Virol. 158, 1445–1459. ( 10.1007/s00705-013-1645-3) [DOI] [PubMed] [Google Scholar]
- 48.Vaughn DW, et al. 2000. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J. Infect. Dis. 181, 2–9. ( 10.1086/315215) [DOI] [PubMed] [Google Scholar]
- 49.Tricou V, Minh NN, Farrar J, Tran HT, Simmons CP. 2011. Kinetics of viremia and NS1 antigenemia are shaped by immune status and virus serotype in adults with dengue. PLoS Negl. Trop. Dis. 5, e1309 ( 10.1371/journal.pntd.0001309) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murgue B, Roche C, Chungue E, Deparis X. 2000. Prospective study of the duration and magnitude of viraemia in children hospitalised during the 1996–1997 dengue-2 outbreak in French Polynesia. J. Med. Virol. 60, 432–438. () [DOI] [PubMed] [Google Scholar]
- 51.Boyle DB, Dickerman RW, Marshall ID. 1983. Primary viraemia responses of herons to experimental infection with Murray Valley encephalitis, Kunjin and Japanese encephalitis viruses. Aust. J. Exp. Biol. Med. Sci. 61, 655–664. ( 10.1038/icb.1983.62) [DOI] [PubMed] [Google Scholar]
- 52.Baylis M, O'Connell L, Mellor PS. 2008. Rates of bluetongue virus transmission between Culicoides sonorensis and sheep. Med. Vet. Entomol. 22, 228–237. ( 10.1111/j.1365-2915.2008.00732.x) [DOI] [PubMed] [Google Scholar]
- 53.Oesterle PT, et al. 2009. Experimental infection of cliff swallows (Petrochelidon pyrrhonota) with varying doses of West Nile virus. Am. J. Trop. Med. Hyg. 81, 1159–1164. ( 10.4269/ajtmh.2009.09-0136) [DOI] [PubMed] [Google Scholar]
- 54.VanDalen KK, Hall JS, Clark L, McLean RG, Smeraski C. 2013. West Nile virus infection in American robins: new insights on dose response. PLoS ONE 8, e68537 ( 10.1371/journal.pone.0068537) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weingartl HM, Miller M, Nfon C, Wilson WC. 2014. Development of a Rift Valley fever virus viremia challenge model in sheep and goats. Vaccine 32, 2337–2344. ( 10.1016/j.vaccine.2014.02.066) [DOI] [PubMed] [Google Scholar]
- 56.Bean AG, Baker ML, Stewart CR, Cowled C, Deffrasnes C, Wang LF, Lowenthal JW. 2013. Studying immunity to zoonotic diseases in the natural host—keeping it real. Nat. Rev. Immunol. 13, 851–861. ( 10.1038/nri3551) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mandl JN, Ahmed R, Barreiro LB, Daszak P, Epstein JH, Virgin HW, Feinberg MB. 2014. Reservoir host immune responses to emerging zoonotic viruses. Cell 160, 20–35. ( 10.1016/j.cell.2014.12.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hasegawa T, Takehara Y, Takahashi K. 1975. Natural and experimental infections of Japanese tree sparrows with Japanese encephalitis virus. Arch. Virol. 49, 373–376. ( 10.1007/BF01318247) [DOI] [PubMed] [Google Scholar]
- 59.Gaumann R, Ruzek D, Muhlemann K, Strasser M, Beuret CM. 2011. Phylogenetic and virulence analysis of tick-borne encephalitis virus field isolates from Switzerland. J. Med. Virol. 83, 853–863. ( 10.1002/jmv.21993) [DOI] [PubMed] [Google Scholar]
- 60.Tonteri E, Kipar A, Voutilainen L, Vene S, Vaheri A, Vapalahti O, Lundkvist Å. 2013. The three subtypes of tick-borne encephalitis virus induce encephalitis in a natural host, the bank vole (Myodes glareolus). PLoS ONE 8, e81214 ( 10.1371/journal.pone.0081214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mandl JN, Akondy R, Lawson B, Kozyr N, Staprans SI, Ahmed R, Feinberg MB. 2011. Distinctive TLR7 signaling, type I IFN production, and attenuated innate and adaptive immune responses to yellow fever virus in a primate reservoir host. J. Immunol. 186, 6406–6416. ( 10.4049/jimmunol.1001191) [DOI] [PubMed] [Google Scholar]
- 62.Slovak M, Kazimirova M, Siebenstichova M, Ustanikova K, Klempa B, Gritsun T, Gould EA, Nuttall PA. 2014. Survival dynamics of tick-borne encephalitis virus in Ixodes ricinus ticks. Ticks Tick Borne Dis. 5, 962–969. ( 10.1016/j.ttbdis.2014.07.019) [DOI] [PubMed] [Google Scholar]
- 63.Christofferson RC, Mores CN. 2011. Estimating the magnitude and direction of altered arbovirus transmission due to viral phenotype. PLoS ONE 6, e16298 ( 10.1371/journal.pone.0016298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salazar MI, Richardson JH, Sanchez-Vargas I, Olson KE, Beaty BJ. 2007. Dengue virus type 2: replication and tropisms in orally infected Aedes aegypti mosquitoes. BMC Microbiol. 7, 9 ( 10.1186/1471-2180-7-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oviedo MV, Romoser WS, James CB, Mahmood F, Reisen WK. 2011. Infection dynamics of western equine encephalomyelitis virus (Togaviridae: Alphavirus) in four strains of Culex tarsalis (Diptera: Culicidae): an immunocytochemical study. Res. Rep. Trop. Med. 2011, 65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mahmood F, Chiles RE, Fang Y, Green EN, Reisen WK. 2006. Effects of time after infection, mosquito genotype, and infectious viral dose on the dynamics of Culex tarsalis vector competence for western equine encephalomyelitis virus. J. Am. Mosq. Control Assoc. 22, 272–281. ( 10.2987/8756-971X(2006)22[272:EOTAIM]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 67.Pongsiri A, Ponlawat A, Thaisomboonsuk B, Jarman RG, Scott TW, Lambrechts L. 2014. Differential susceptibility of two field Aedes aegypti populations to a low infectious dose of dengue virus. PLoS ONE 9, e92971 ( 10.1371/journal.pone.0092971) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hardy JL, Reeves WC. 1990. Experimental studies on infection in vectors. In Epidemiology and control of mosquito-borne arboviruses in California (ed. Reeves WC.), pp. 66–127. Sacramento, CA: California Mosquito Vector Control Association. [Google Scholar]
- 69.Kramer LD, Hardy JL, Presser SB, Houk EJ. 1981. Dissemination barriers for western equine encephalomyelitis virus in Culex tarsalis infected after ingestion of low viral doses. Am. J. Trop. Med. Hyg. 30, 190–197. [DOI] [PubMed] [Google Scholar]
- 70.Lord CC, Rutledge CR, Tabachnick WJ. 2006. Relationships between host viremia and vector susceptibility for arboviruses. J. Med. Entomol. 43, 623–630. ( 10.1093/jmedent/43.3.623) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Scott RM, et al. 1980. Dengue-2 vaccine: viremia and immune responses in rhesus monkeys. Infect. Immun. 27, 181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Watts DM, Burke DS, Harrison BA, Whitmire RE, Nisalak A. 1987. Effect of temperature on the vector efficiency of Aedes aegypti for dengue 2 virus. Am. J. Trop. Med. Hyg. 36, 143–152. [DOI] [PubMed] [Google Scholar]
- 73.Carrington LB, Simmons CP. 2014. Human to mosquito transmission of dengue viruses. Front. Immunol. 5, 290 ( 10.3389/fimmu.2014.00290) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nguyet MN, et al. 2013. Host and viral features of human dengue cases shape the population of infected and infectious Aedes aegypti mosquitoes. Proc. Natl Acad. Sci. USA 110, 9072–9077. ( 10.1073/pnas.1303395110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kraiselburd E, Gubler DJ, Kessler MJ. 1985. Quantity of dengue virus required to infect rhesus monkeys. Trans. R. Soc. Trop. Med. Hyg. 79, 248–251. ( 10.1016/0035-9203(85)90348-7) [DOI] [PubMed] [Google Scholar]
- 76.Styer LM, Bernard KA, Kramer LD. 2006. Enhanced early West Nile virus infection in young chickens infected by mosquito bite: effect of viral dose. Am. J. Trop. Med. Hyg. 75, 337–345. [PubMed] [Google Scholar]
- 77.Thangamani S, Higgs S, Ziegler S, Vanlandingham D, Tesh R, Wikel S. 2010. Host immune response to mosquito-transmitted chikungunya virus differs from that elicited by needle inoculated virus. PLoS ONE 5, e12137 ( 10.1371/journal.pone.0012137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Edwards JF, Higgs S, Beaty BJ. 1998. Mosquito feeding-induced enhancement of Cache Valley Virus (Bunyaviridae) infection in mice. J. Med. Entomol. 35, 261–265. ( 10.1093/jmedent/35.3.261) [DOI] [PubMed] [Google Scholar]
- 79.Schneider BS, Higgs S. 2008. The enhancement of arbovirus transmission and disease by mosquito saliva is associated with modulation of the host immune response. Trans. R. Soc. Trop. Med. Hyg. 102, 400–408. ( 10.1016/j.trstmh.2008.01.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cox J, Mota J, Sukupolvi-Petty S, Diamond MS, Rico-Hesse R. 2012. Mosquito bite delivery of dengue virus enhances immunogenicity and pathogenesis in humanized mice. J. Virol. 86, 7637–7649. ( 10.1128/JVI.00534-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Styer LM, Lim PY, Louie KL, Albright RG, Kramer LD, Bernard KA. 2011. Mosquito saliva causes enhancement of West Nile virus infection in mice. J. Virol. 85, 1517–1527. ( 10.1128/JVI.01112-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Styer LM, Kent KA, Albright RG, Bennett CJ, Kramer LD, Bernard KA. 2007. Mosquitoes inoculate high doses of West Nile virus as they probe and feed on live hosts. PLoS Pathog. 3, 1262–1270. ( 10.1371/journal.ppat.0030132) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ben-Shachar R, Koelle K. 2015. Minimal within-host dengue models highlight the specific roles of the immune response in primary and secondary dengue infections. J. R. Soc. Interface 12, 20140886 ( 10.1098/rsif.2014.0886) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Clapham HE, Tricou V, Van Vinh Chau N, Simmons CP, Ferguson NM. 2014. Within-host viral dynamics of dengue serotype 1 infection. J. R. Soc. Interface 11, 20140094 ( 10.1098/rsif.2014.0094) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ferguson N, Anderson R, Gupta S. 1999. The effect of antibody-dependent enhancement on the transmission dynamics and persistence of multiple-strain pathogens. Proc. Natl Acad. Sci. USA 96, 790–794. ( 10.1073/pnas.96.2.790) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cummings DA, Schwartz IB, Billings L, Shaw LB, Burke DS. 2005. Dynamic effects of antibody-dependent enhancement on the fitness of viruses. Proc. Natl Acad. Sci. USA 102, 15 259–15 264. ( 10.1073/pnas.0507320102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Christofferson RC, Chisenhall DM, Wearing HJ, Mores CN. 2014. Chikungunya viral fitness measures within the vector and subsequent transmission potential. PLoS ONE 9, e110538 ( 10.1371/journal.pone.0110538) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lloyd AL. 2001. Destabilization of epidemic models with the inclusion of realistic distributions of infectious periods. Proc. R. Soc. Lond. B 268, 985–993. ( 10.1098/rspb.2001.1599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Singer RS, MacLachlan NJ, Carpenter TE. 2001. Maximal predicted duration of viremia in bluetongue virus-infected cattle. J. Vet. Diagn. Invest. 13, 43–49. ( 10.1177/104063870101300109) [DOI] [PubMed] [Google Scholar]
- 90.Troyer JM, Hanley KA, Whitehead SS, Strickman D, Karron RA, Durbin AP, Murphy BR. 2001. A live attenuated recombinant dengue-4 virus vaccine candidate with restricted capacity for dissemination in mosquitoes and lack of transmission from vaccinees to mosquitoes. Am. J. Trop. Med. Hyg. 65, 414–419. [DOI] [PubMed] [Google Scholar]
- 91.Whitehead SS, Blaney JE, Durbin AP, Murphy BR. 2007. Prospects for a dengue virus vaccine. Nat. Rev. Microbiol. 5, 518–528. ( 10.1038/nrmicro1690) [DOI] [PubMed] [Google Scholar]
- 92.Murphy BR, Blaney JE, Jr, Whitehead SS. 2004. Arguments for live flavivirus vaccines. Lancet 364, 499–500. ( 10.1016/S0140-6736(04)16801-3) [DOI] [PubMed] [Google Scholar]
- 93.Arrigo NC, Adams AP, Watts DM, Newman PC, Weaver SC. 2010. Cotton rats and house sparrows as hosts for North and South American strains of eastern equine encephalitis virus. Emerg. Infect. Dis. 16, 1373–1380. ( 10.3201/eid1609.100459) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Golnar AJ, Turell MJ, LaBeaud AD, Kading RC, Hamer GL. 2014. Predicting the mosquito species and vertebrate species involved in the theoretical transmission of Rift Valley fever virus in the United States. PLoS Negl. Trop. Dis. 8, e3163 ( 10.1371/journal.pntd.0003163) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Panella NA, Young G, Komar N. 2013. Experimental infection of Eurasian collared-dove (Streptopelia decaocto) with West Nile virus. J. Vector Ecol. 38, 210–214. ( 10.1111/j.1948-7134.2013.12032.x) [DOI] [PubMed] [Google Scholar]
- 96.Nemeth N, Young G, Ndaluka C, Bielefeldt-Ohmann H, Komar N, Bowen R. 2009. Persistent West Nile virus infection in the house sparrow (Passer domesticus). Arch. Virol. 154, 783–789. ( 10.1007/s00705-009-0369-x) [DOI] [PubMed] [Google Scholar]
- 97.Cowled C, Melville L, Weir R, Walsh S, Gubala A, Davis S, Boyle D. 2012. Persistent and recrudescent infection in cattle following natural infection with Middle Point orbivirus. Arch. Virol. 157, 1161–1165. ( 10.1007/s00705-012-1277-z) [DOI] [PubMed] [Google Scholar]
- 98.Wheeler SS, Vineyard MP, Woods LW, Reisen WK. 2012. Dynamics of West Nile virus persistence in house sparrows (Passer domesticus). PLoS Negl. Trop. Dis. 6, e1860 ( 10.1371/journal.pntd.0001860) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mlera L, Melik W, Bloom ME. 2014. The role of viral persistence in flavivirus biology. Pathog. Dis. 71, 137–163. ( 10.1111/2049-632X.12178) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.