Abstract
Avian malaria has historically played an important role as a model in the study of human malaria, being a stimulus for the development of medical parasitology. Avian malaria has recently come back to the research scene as a unique animal model to understand the ecology and evolution of the disease, both in the field and in the laboratory. Avian malaria is highly prevalent in birds and mosquitoes around the world and is amenable to laboratory experimentation at each stage of the parasite's life cycle. Here, we take stock of 5 years of experimental laboratory research carried out using Plasmodium relictum SGS1, the most prevalent avian malaria lineage in Europe, and its natural vector, the mosquito Culex pipiens. For this purpose, we compile and analyse data obtained in our laboratory in 14 different experiments. We provide statistical relationships between different infection-related parameters, including parasitaemia, gametocytaemia, host morbidity (anaemia) and transmission rates to mosquitoes. This analysis provides a wide-ranging picture of the within-host and between-host parameters that may bear on malaria transmission and epidemiology.
Keywords: Plasmodium relictum, transmission, virulence, within-host dynamics, serial passages
1. Introduction
Avian malaria is the oldest experimental system for investigating the biology and transmission of Plasmodium parasites. In 1898, Ronald Ross, an army surgeon working in India, carried out a series of carefully controlled experiments using Plasmodium-infected sparrows and Culex mosquitoes to demonstrate that the disease was transmitted through mosquito bites, thereby solving a centuries-old puzzle and preparing the ground for the first epidemiological models of malaria transmission and the first successful attempts at malaria control [1]. For the following 50 years, avian malaria became the experimental system of choice for malaria research. Avian malaria was used for elucidating key aspects of the biology and transmission of malaria parasites [2,3], as well as for the routine testing and development of the first antimalarial drugs [4]. The discovery of Plasmodium berghei in thicket rats in Central Africa in 1949, however, marked a switch to rodent malaria research and the decline of experimental studies on avian malaria.
In the past 15 years, however, work on avian malaria has seen a drastic surge, largely spurred on by the routine blood screening of wild-caught birds around the world (electronic supplementary material, figure S1). Molecular studies have revealed an unexpected level of diversity in avian malaria parasites, which rivals anything that has been found in other vertebrate hosts. There are currently around 600 mitochondrial cytochrome b-based lineages of avian Plasmodium available (MalAvi Database [5]), although disagreement continues about the relative relevance of morphological characteristics and sequence differences for establishing species boundaries in avian malaria [6]. Avian malaria is much more prevalent (in some areas up to 80% of birds are infected [7]) and widespread (it is present in all continents except Antarctica) than any other vertebrate malaria. Compared to the exponentially growing body of information on the interactions between Plasmodium and its bird hosts, however, we still know comparatively very little about avian malaria vectors in the wild [8,9]. Several dozen different vector species are currently listed on the MalAvi database, although for Plasmodium, Culex mosquitoes are by far the most common vectoring genus [9].
In addition to work carried out in the wild, avian malaria has recently come back to the research scene as an experimental laboratory model for investigating the evolutionary ecology of malaria transmission. For the past 5 years, we have been working in the laboratory on the interactions between the most common avian malaria lineage in Europe, Plasmodium relictum SGS1, and its natural vector, the mosquito Culex pipiens. This research has been spurred on by the attractive possibility of using an experimental model that bypasses both the ethical and technical constraints associated with experimentation on human malaria, and the two main drawbacks of using rodent malaria: the use of strains that have been kept in the laboratory since their isolation from the wild in the 1950s, and the fact that their natural vector is either unknown or cannot be kept in the laboratory, constraining transmission experiments to convenient, but unnatural, mosquito–parasite combinations.
SGS1 is the most generalist of all the currently described avian malaria lineages: it has thus far been found infecting 95 different species in 10 different orders (MalAvi database, [5]). Its prevalence depends on the host species and geographical region, but it can attain extremely high levels, particularly in passerine birds: in the South of France 70–80% of sparrows and up to 4% of Cx pipiens mosquitoes are infected with this lineage [7,8]. Our SGS1 strain was isolated from wild sparrows caught 5 years ago and has since been kept in the laboratory through serial intraperitoneal passages between domestic canaries (Serinus canaria) with the occasional passage via Cx pipiens mosquitoes (figure 1). Although we ignore whether wild canaries are infected with SGS1 in their natural range, in France domestic canaries kept outdoors become naturally infected by the parasite (R. Pigeault, A. Nicot, S. Gandon, A. Rivero 2012, personal observation), suggesting they are suitable hosts for this generalist Plasmodium lineage outside the laboratory context.
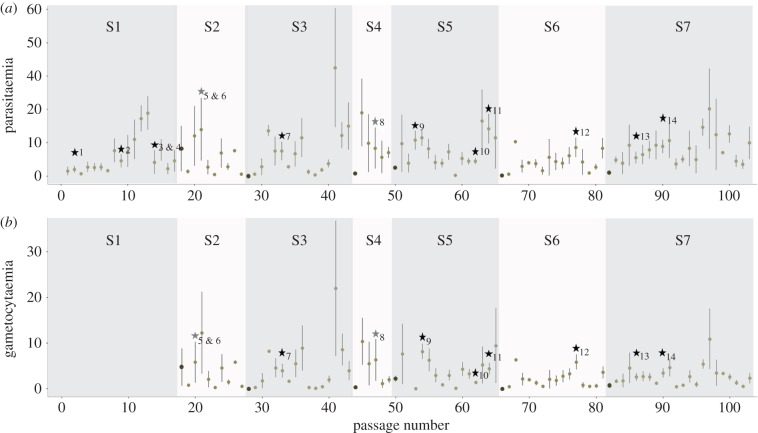
Figure 1.
Parasitaemias (a) and gametocytaemias (b) of P. relictum-infected birds across all the passages since the isolation of the parasite strain from wild sparrows in 2009. Parasitaemia is quantified as the total % of red blood cells infected; gametocytaemia as the % of red blood cells infected by gametocytes. Dots indicate the mean; bars on either side indicate the standard errors. The horizontal axis indicates the total passage number comprising both ‘standard’ (bird-to-bird) passages (pale grey dots) and ‘mosquito’ (bird-to-mosquito-to-bird) passages (dark grey dots). The graph is divided into seven different segments (labelled S1–S7). Each segment starts with a mosquito passage followed by a (varying) number of standard passages. Stars indicate the times at which the 14 different experiments analysed in this paper took place (black stars, acute infection experiments; grey stars, chronic infection experiments). In the passages that took place during the first segment (S1), the gametocytaemia was not quantified, hence the missing values. The number of birds that were used in each passage is given in the electronic supplementary material, figure S2. (Online version in colour.)
Our experiments on avian malaria have shed light on several aspects of mosquito–Plasmodium interaction, including: the role of mosquito genetic diversity [10–14] and bacterial co-infections [8,15] on the outcome of the infection, and the ability of Plasmodium both to manipulate mosquito behaviour [16] and adjust its within-bird transmission strategies in response to mosquito availability [17] to maximize its own transmission. These experiments have, however, also generated a great deal of ancillary data on the role of host variability in shaping Plasmodium transmission, a crucial piece of the transmission puzzle about which there is still insufficient information in the malaria literature. Indeed, the overwhelming majority of mosquito infection experiments are aimed at comparing the traits of infected and uninfected mosquitoes under different experimental conditions and therefore logically use mosquito as the replication unit. For practical as well as ethical reasons, most of these experiments typically involve up to five vertebrate hosts, and between-host variability is seen as a cumbersome source of statistical noise that needs to be controlled for.
Here we compile and analyse data obtained in our laboratory in 14 different experiments involving around 120 birds and over 5000 mosquito hosts. The aim is to take stock of 5 years of experimental avian malaria research and provide a wider picture of the within-host and between-host parameters that may bear on Plasmodium transmission and, ultimately, epidemiology [18]. For this purpose, we provide statistical relationships between different infection-related parameters, including parasite multiplication, gametocyte production, host morbidity (anaemia) and transmission rates to mosquitoes. We begin by analysing the data arising from the serial passage of our strain through both intraperitoneal injections and mosquito bites during the past 5 years. We then analyse data arising from the experiments to elucidate: (i) the relationships between different traits within the bird, such as parasite multiplication (parasitaemia), investment in transmission (gametocytaemia and gametocyte conversion ratio) and virulence (anaemia); (ii) which of the variables measured in the bird and in the mosquitoes are best at predicting the probability and intensity of Plasmodium infection in mosquitoes and, finally, (iii) which variables are best at predicting the fitness of infected mosquitoes. We discuss our results and the limitations of our system with respect, most notably, to the rodent malaria system, the laboratory model that has for many years been the reference for addressing evolutionary questions in malaria [19,20].
2. Material and methods
(a). Malaria parasite
The data originate from 14 different studies spanning 5 years (electronic supplementary material, table S1). Plasmodium relictum SGS1 is the aetiological agent of the most prevalent form of avian malaria in Europe [21]. The lineage used in our experiments was isolated by Gabriele Sorci (CNRS Dijon) from sparrows caught in the region of Dijon (France) in 2009. The lineage was passaged to naive canaries (Serinus canaria) by intraperitoneal injection. Since then it has been maintained by carrying out regular passages between our stock canaries through intraperitoneal injections (henceforth ‘standard passages’) around every three weeks. Roughly, every 30 weeks the line was passaged through a mosquito (‘mosquito passages’; figure 1).
Recipient canaries were infected by injecting them with ca 80–100 µl of blood from the infected canary stock. All canaries within an experiment were infected using the same pool of blood. For this purpose, blood from between two and five donor canaries was collected using heparinized tubes to avoid coagulation. The blood was then pooled in an Eppendorf tube and diluted in an equal volume of phosphate-buffered saline (PBS). This pool of blood was then injected intraperitoneally to several recipient birds (the number of birds used varied between experiments, see electronic supplementary material, table S1). In most of the experiments, mosquito feeding took place using recipient birds in the acute phase of the infection (days 10–12 post infection), except for three experiments which were carried out during the chronic phase of the infection (days 30–304 post infection, depending on the experiments, electronic supplementary material, table S1). In all experiments, parasite load was monitored at the putative peak of the acute infection (days 10–12 post infection, [17]). In chronic infections, the parasite load was also checked immediately before the mosquito feed. In both cases, blood was sampled by puncturing the wing vein. In acute infections, parasite load was quantified using blood smears as described by Valkiunas et al. [21] and two different parameters were measured: the parasitaemia (total proportion of red blood cells infected) and the gametocytaemia (proportion of red blood cells infected by gametocytes, the sexual stages of the parasite that are transmitted to mosquitoes). Packed cell volume, a standard proxy for anaemia [22], was calculated by taking 50 µl of blood from the brachial vein using a heparinized glass microcapillaries. Samples were immediately centrifuged (5 min, 8000 r.p.m.) and the proportion of red blood cells in the blood was estimated using a micro-haematocrit reader [23].
(b). Mosquito experimental infections and dissections
The large majority of our experiments were carried out using a laboratory strain of Cx pipiens (SLab). Three of the experiments were carried out with Cx pipiens collected from the field (see the electronic supplementary material, table S1 for details). The experimental protocol was identical irrespective of mosquito origin. Mosquito feeding took place either 10–12 days (acute phase) or 30–300 days (chronic phase) after the onset of the infection (electronic supplementary material, table S1). In those experiments in which we were able to quantify blood meal size we did so by placing engorged females individually in numbered plastic tubes until all haematin (a product of the degradation of haemoglobin) was excreted. When fecundity was studied 3–5 days later, females were transferred to a new tube containing 4–5 ml of mineral water to allow them to lay their eggs. The egg rafts were photographed and the eggs were counted using the Mesurim Pro freeware (http://svt.ac-amiens.fr/spip.php?article40&lang=fr). Female size was estimated by measuring the length of its wing along its longest axis. Seven to nine days after the infected blood meal, females were sampled and dissected to count the oocysts in their midguts [10]. This allowed us to estimate infection prevalence (oocyst presence/absence) and burden (number of oocysts per female). Measurement of lifespan was incompatible with the quantification of oocysts. In those experiments in which lifespan was measured, mosquitoes were transferred from the oviposition tube to a new tube which was checked daily to record mortality.
(c). Statistical analysis
Analyses were carried out using the R statistical package (v.3.1.0, http://www.cran.r-project.org/). The different statistical models built to analyse the data (numbered models 1–33) are described in the electronic supplementary material, table S2. Of the 14 experiments reported here, 10 were carried out using acute stage infections and SLab mosquitoes, two using chronic infection and wild mosquitoes and one using acute infections and wild mosquitoes (electronic supplementary material, table S1). As there were not enough data from chronic infections or from wild mosquito experiments to do any in-depth analyses, unless specifically stated the statistical analyses were restricted to the 11 experiments that used acute infections and SLab mosquitoes. The serial passage analyses were carried out using our full parasitaemia and gametocytaemia records since the isolation of the parasite strain 5 years ago. This dataset comprises 103 passages (including standard passages and mosquito passages) and over 415 birds. The general procedure for building the statistical models was as follows. Maximal models were built by including all biologically appropriate main effects and higher order interactions. In some of the models, we included random effects to correct for two potential sources of pseudoreplication: the experiment effect (experiments were separated by several months, or years, and it is inevitable that environmental conditions change slightly from one experiment to the next) and the bird effect (mosquitoes feeding on the same bird are not independent from each other). Whether the model contained random effects or not determined the type of R subroutine we used (electronic supplementary material, table S2). Models containing exclusively fixed effects were analysed using standard glm procedures and a normal error structure. Some of the response variables had to be transformed to correct for non-normal errors. Models containing both fixed and random effects, on the other hand, were analysed using mixed effects models with a normal distribution (lme, nlme package, http://www.cran.r-project.org/), except when the response variable was a proportion (e.g. oocyst prevalence), in which case the data were analysed using a binomial error distribution (lmer, lme4 package, http://www.cran.r-project.org/). The serial passage analyses were corrected for temporal autocorrelation by including a lag 1 term (i.e. parasitaemia or gametocytaemia at time t as a function of parasitaemia or gametocytaemia at t – 1). The maximal model was simplified by sequentially eliminating non-significant terms and interactions to establish a minimal model [24]. The significance of explanatory variables was established using a likelihood ratio test (LRT) which is approximately distributed as a χ2 distribution [25]. The significant χ2-values given in the text are for the minimal model, while non-significant values correspond to those obtained before deletion of the variable from the model.
3. Results
(a). Serial passage analyses
Since its isolation from the wild, our strain or P. relictum SGS1 was maintained through a combination of standard and mosquito passages. Figure 1a,b shows the variation in parasitaemia and gametocytaemia across the different passages. Parasitaemia increased slightly but significantly across the whole passage sequence (model 1: 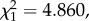 p = 0.0230). The mean parasitaemia over the first 10 passages was 3.5 ± 0.74% (±s.e.), while over the last 10 passages the mean parasitaemia had more than doubled to 8.21 ± 1.24%. To test whether this was a global trend or whether passage through mosquitoes bore any effect on the observed trend, we divided the dataset into seven consecutive segments, each starting with a mosquito passage and followed by a series of standard passages (figure 1a). This trend for an increase in parasitaemia was observed after each mosquito passage (model 2: slope = 0.032,
p = 0.0230). The mean parasitaemia over the first 10 passages was 3.5 ± 0.74% (±s.e.), while over the last 10 passages the mean parasitaemia had more than doubled to 8.21 ± 1.24%. To test whether this was a global trend or whether passage through mosquitoes bore any effect on the observed trend, we divided the dataset into seven consecutive segments, each starting with a mosquito passage and followed by a series of standard passages (figure 1a). This trend for an increase in parasitaemia was observed after each mosquito passage (model 2: slope = 0.032, 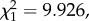 p = 0.001), irrespective of the segment (model 2:
p = 0.001), irrespective of the segment (model 2: 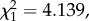 p = 0.0614). Interestingly, there was a significant decrease in parasitaemia immediately following a mosquito passage (electronic supplementary material, figure S3). Such a decrease could simply be due to differences in the inoculum size: there are likely to be significantly fewer parasites injected by the mosquito than by an intraperitoneal injection. If this is the case, we would expect parasitaemia to first decrease following a mosquito passage, then recover following the subsequent standard passage. When the first infection following a mosquito passage was removed from the analyses, the slope of the trend decreased slightly (slope = 0.022), but the trend remained significant, albeit marginally (model 3:
p = 0.0614). Interestingly, there was a significant decrease in parasitaemia immediately following a mosquito passage (electronic supplementary material, figure S3). Such a decrease could simply be due to differences in the inoculum size: there are likely to be significantly fewer parasites injected by the mosquito than by an intraperitoneal injection. If this is the case, we would expect parasitaemia to first decrease following a mosquito passage, then recover following the subsequent standard passage. When the first infection following a mosquito passage was removed from the analyses, the slope of the trend decreased slightly (slope = 0.022), but the trend remained significant, albeit marginally (model 3: 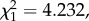 p = 0.031). However, when both passages were removed from the analyses, the resulting trend within each segment was lost (model 4: slope = 0.009,
p = 0.031). However, when both passages were removed from the analyses, the resulting trend within each segment was lost (model 4: slope = 0.009, 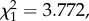 p = 0.4125), indicating that both points were influential in the observed increase in parasitaemia within each segment. This was confirmed with a second analysis, where the parasitaemia within each segment was fitted as a categorical variable with three values: first passage, second passage and the rest. As expected, this new variable was highly significant (model 5:
p = 0.4125), indicating that both points were influential in the observed increase in parasitaemia within each segment. This was confirmed with a second analysis, where the parasitaemia within each segment was fitted as a categorical variable with three values: first passage, second passage and the rest. As expected, this new variable was highly significant (model 5: 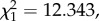 p = 0.0012). Contrast analyses revealed that the estimated average parasitaemia for the first (mosquito) passage was marginally but significantly lower than that of the second (standard) passage (model 5: t = 2.30, p = 0.021). These two parasitaemias were, however, much lower than the mean parasitaemia in the rest of the passages (model 5: t = 3.64, p = 0.0003).
p = 0.0012). Contrast analyses revealed that the estimated average parasitaemia for the first (mosquito) passage was marginally but significantly lower than that of the second (standard) passage (model 5: t = 2.30, p = 0.021). These two parasitaemias were, however, much lower than the mean parasitaemia in the rest of the passages (model 5: t = 3.64, p = 0.0003).
By contrast, gametocytaemia did not show any change across the whole passage sequence (model 6: 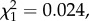 p = 0.8501). The mean gametocytaemia over the first 10 passages was 2.8 ± 0.75% (± s.e.), while over the last 10 passages the mean gametocytaemia had barely changed 2.3 ± 0.50%. To test whether passage through mosquitoes bore any effect on the observed trend, we analysed each segment separately, as above. No trend in gametocytaemia was observed in any of the segments (model 7:
p = 0.8501). The mean gametocytaemia over the first 10 passages was 2.8 ± 0.75% (± s.e.), while over the last 10 passages the mean gametocytaemia had barely changed 2.3 ± 0.50%. To test whether passage through mosquitoes bore any effect on the observed trend, we analysed each segment separately, as above. No trend in gametocytaemia was observed in any of the segments (model 7: 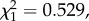 p = 0.3753).
p = 0.3753).
(b). Trait relationships within the bird
Each of the 11 acute stage infection experiments reported here is represented in figure 1a,b by a black star. These experiments showed a large variance in both the bird parasitaemias and gametocytaemias (electronic supplementary material, figure S4) both among experiments (parasitaemia, model 8: F10,61 = 3.03 p = 0.0044; gametocytaemia, model 9: F8,53 = 5.01 p = 0.0002) and within experiments, even though the birds were infected concomitantly with the same pool of blood (electronic supplementary material, figure S4).
Within each bird, there was a strong association between peak parasitaemia and gametocytaemia (model 10: 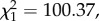 p < 0.0001; figure 2). This was partially expected, as our definition of total parasitaemia encompasses all Plasmodium stages, including gametocytes, so both axes were not independent. The results were, however, identical when we analysed separately asexual and sexual (gametocyte) stages of Plasmodium (model 11:
p < 0.0001; figure 2). This was partially expected, as our definition of total parasitaemia encompasses all Plasmodium stages, including gametocytes, so both axes were not independent. The results were, however, identical when we analysed separately asexual and sexual (gametocyte) stages of Plasmodium (model 11: 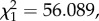 p < 0.0001). We calculated the reproductive effort of malaria parasites as the density of gametocytes relative to the total number of parasites at the peak of the infection. For simplicity, we term this ‘conversion ratio’, to distinguish it from ‘conversion rate’ which refers to the investment into gametocytes across parasite cohorts [26]. In our system, gametocytes constituted on average a third (mean ± s.e., 33 ± 2.4%) of the intracellular parasite population in the blood. There was, however, a great degree of variation around this mean, much of which was explained by differences between experiments (model 12: F8,53 = 9.88 p < 0.001).
p < 0.0001). We calculated the reproductive effort of malaria parasites as the density of gametocytes relative to the total number of parasites at the peak of the infection. For simplicity, we term this ‘conversion ratio’, to distinguish it from ‘conversion rate’ which refers to the investment into gametocytes across parasite cohorts [26]. In our system, gametocytes constituted on average a third (mean ± s.e., 33 ± 2.4%) of the intracellular parasite population in the blood. There was, however, a great degree of variation around this mean, much of which was explained by differences between experiments (model 12: F8,53 = 9.88 p < 0.001).
Figure 2.
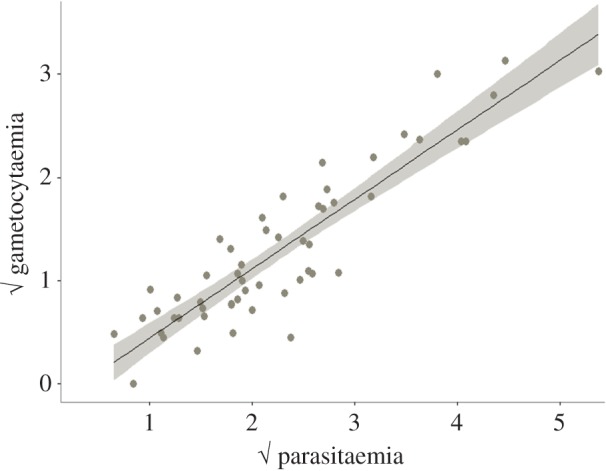
Regression between the parasitaemia and gametocytaemia (expressed as the square root of the proportion of red blood cells infected with a particular stage) in each bird infection. (Online version in colour.)
As expected, infection by P. relictum lead to a marked loss of red blood cells (anaemia). Infected birds showed a 28.5% decrease in the packed cell volume with respect to uninfected birds (model 13: 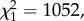 p < 0.0001). This decrease was, however, independent of the density of parasites: highly parasitaemic birds had similar anaemia to weakly parasitaemic birds (model 14:
p < 0.0001). This decrease was, however, independent of the density of parasites: highly parasitaemic birds had similar anaemia to weakly parasitaemic birds (model 14: 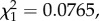 p = 0.782). We found no association between conversion ratio and anaemia (model 15:
p = 0.782). We found no association between conversion ratio and anaemia (model 15:  p = 0.165).
p = 0.165).
(c). Predictors of mosquito infection
(i). Bird-related factors
We analysed the effect of four bird-related factors (infection stage, parasitaemia, gametocytaemia, anaemia) on the probability and intensity of Plasmodium transmission to mosquitoes. One of the strongest predictors of whether mosquitoes became infected by Plasmodium (oocyst prevalence), and of the intensity of the subsequent infection (oocyst burden) was whether they fed on an acutely or chronically infected bird. Acute stage infections resulted in four times higher prevalences (model 16: 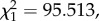 p < 0.0001) and more than 10 times higher oocyst burdens (model 20:
p < 0.0001) and more than 10 times higher oocyst burdens (model 20:  p < 0.0001) than chronic stage infections (figure 3).
p < 0.0001) than chronic stage infections (figure 3).
Figure 3.
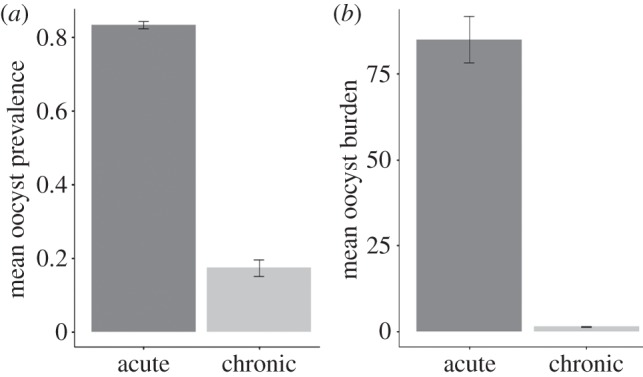
Mean infection prevalence (a) and oocyst burden (b) in mosquitoes fed on acute or chronic bird infections. Bars represent standard errors around the mean.
As there were not enough data from chronic infections to do any in-depth analyses (electronic supplementary material, table S1), all subsequent results come from acute infections. When mosquitoes fed on acutely infected birds, there was a very strong positive association between the probability that they would become infected and the intensity of the ensuing infection: birds that rendered higher oocyst prevalences also rendered higher oocyst burdens (model 23: 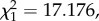 p < 0.0001; electronic supplementary material, figure S5). Despite this, however, oocyst prevalence and oocyst burden were not always explained by the same explanatory variables.
p < 0.0001; electronic supplementary material, figure S5). Despite this, however, oocyst prevalence and oocyst burden were not always explained by the same explanatory variables.
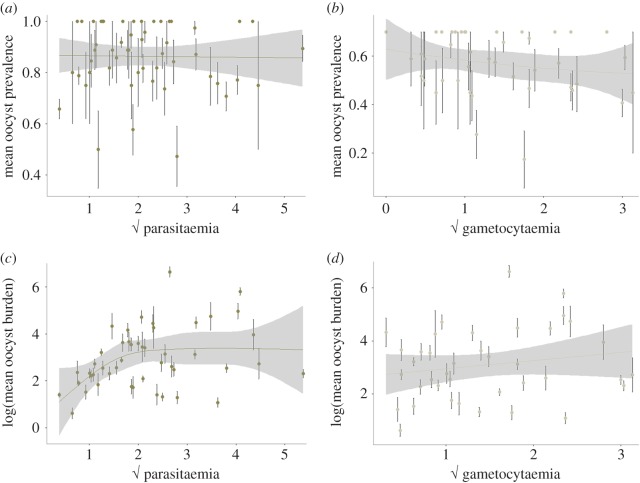
Bird parasitaemia was not correlated with oocyst prevalence (model 17: 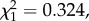 p = 0.3240; figure 4a) but was a good predictor of oocyst burden (model 21:
p = 0.3240; figure 4a) but was a good predictor of oocyst burden (model 21: 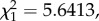 p = 0.0175; figure 4c). Fitting the quadratic term (parasitaemia2) significantly improved the model fit (model 21:
p = 0.0175; figure 4c). Fitting the quadratic term (parasitaemia2) significantly improved the model fit (model 21: 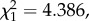 p = 0.0362), suggesting that oocyst burden is a decelerating polynomial function of bird parasitaemia (figure 4b). The relationship between higher bird parasitaemia and higher oocyst burden in mosquitoes may be mediated by blood intake. Interestingly, we found a positive association between the density of parasites in the blood and the volume of blood ingested by the mosquitoes (model 25:
p = 0.0362), suggesting that oocyst burden is a decelerating polynomial function of bird parasitaemia (figure 4b). The relationship between higher bird parasitaemia and higher oocyst burden in mosquitoes may be mediated by blood intake. Interestingly, we found a positive association between the density of parasites in the blood and the volume of blood ingested by the mosquitoes (model 25: 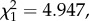 p = 0.019; figure 5).
p = 0.019; figure 5).
Figure 4.
Mean infection prevalence and oocyst burden in mosquitoes as a function of (square root transformed) parasitaemia (a,c) and gametocytaemia (b,d) in the bird. Bars represent standard errors around the mean. Shaded areas on either side of the regression line represent the 95% CI. (Online version in colour.)
Figure 5.
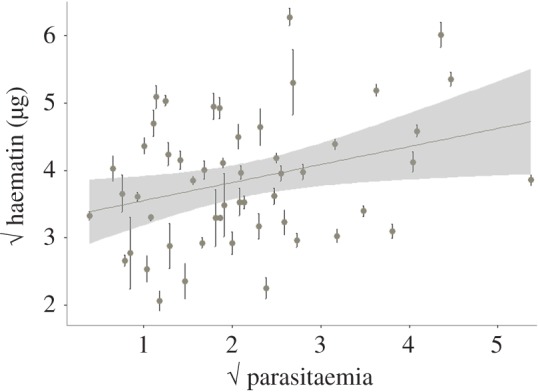
Mean haematin excreted by mosquitoes as a function of bird parasitaemia (total % of red blood cells infected). Bars represent standard errors around the mean. Both variables have been square root transformed to improve the fit of the model. (Online version in colour.)
The patterns for gametocytaemia were, however, different. Although there was a trend for higher oocyst burdens as gametocytaemia increased (figure 4d), it was not statistically significant (model 22: 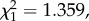 p = 0.2436). Also, unexpectedly, we found a weak but statistically significant negative relationship between bird gametocytaemia and the probability of mosquito infection (model 18:
p = 0.2436). Also, unexpectedly, we found a weak but statistically significant negative relationship between bird gametocytaemia and the probability of mosquito infection (model 18: 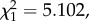 p = 0.0239; figure 4b). Anaemia, on the other hand, did not explain either parasite prevalence (model 19:
p = 0.0239; figure 4b). Anaemia, on the other hand, did not explain either parasite prevalence (model 19: 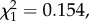 p = 0.695) or burden (model 24:
p = 0.695) or burden (model 24: 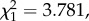 p = 0.052) in mosquitoes.
p = 0.052) in mosquitoes.
(ii). Mosquito-related factors
We then analysed the mosquito-related factors that may determine transmission. Previous work has shown that one of the main predictors of oocyst prevalence and burden is the amount of blood meal ingested by the mosquito [10]. Our results confirmed the existence of very strong relationships between haematin (a standard proxy for blood meal quantification) and oocyst prevalence (model 26: 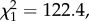 p < 0.0001; figure 6a) and between haematin and oocyst burden (model 27:
p < 0.0001; figure 6a) and between haematin and oocyst burden (model 27: 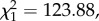 p < 0.0001; figure 6b).
p < 0.0001; figure 6b).
Figure 6.
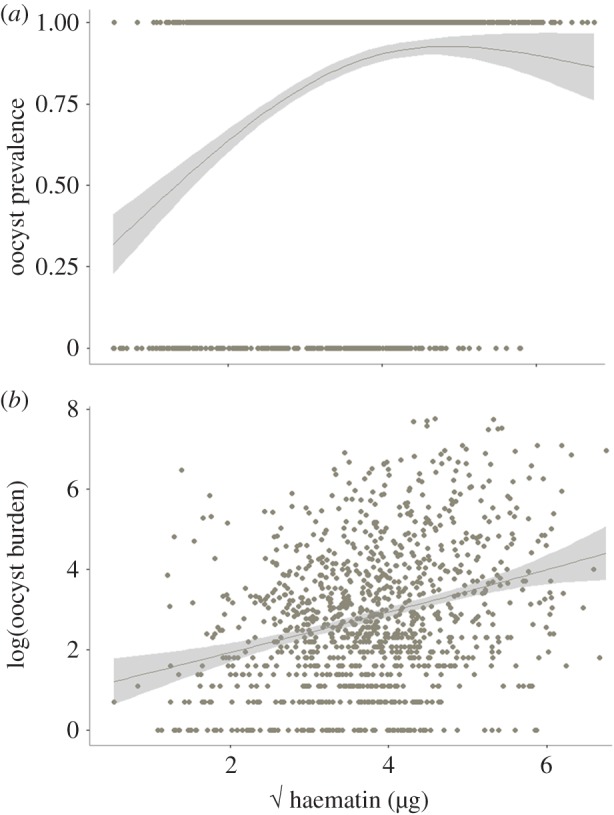
Oocyst prevalence (a) and burden (b) as a function of haematin. Haematin has been square root transformed to improve the fit of the model. (Online version in colour.)
Haematin itself was dependent on several factors, foremost of which were the anaemic status of the bird and the size of the mosquito. Unsurprisingly, larger mosquitoes took larger blood meals (electronic supplementary material, figure S6). The increase in haematin production with mosquito size was slightly greater for mosquitoes feeding on uninfected than on infected birds, as evidenced by a significant interaction between size and infection status (model 29: 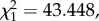 p < 0.0001). Bird anaemia was also important: there was a highly significant relationship between the packed cell volume in the bird and the amount of haematin excreted by mosquitoes feeding on that bird (model 28:
p < 0.0001). Bird anaemia was also important: there was a highly significant relationship between the packed cell volume in the bird and the amount of haematin excreted by mosquitoes feeding on that bird (model 28:  p < 0.0001; electronic supplementary material, figure S7). It follows that mosquitoes feeding on infected birds which were highly anaemic produced less haematin than mosquitoes feeding on uninfected birds (on average 34% less).
p < 0.0001; electronic supplementary material, figure S7). It follows that mosquitoes feeding on infected birds which were highly anaemic produced less haematin than mosquitoes feeding on uninfected birds (on average 34% less).
(d). Predictors of mosquito fitness
We determined which bird or mosquito-related factors predicted the fitness (fecundity, longevity) of mosquitoes. One of the key predictors of mosquito fecundity was the infection status of the bird on which they fed: females feeding on an infected bird laid significantly fewer eggs (11% fewer, on average) than those feeding on uninfected ones (model 30: 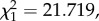 p < 0.0001; figure 7a). This could be due to either a direct effect of the parasite on fecundity (on average 80–90% of mosquitoes that fed on an infected bird became infected) or the indirect result of feeding on blood with a lower protein content (anaemic blood). To test for this, we added haematin, a proxy for the amount of haemoglobin ingested, into the model. As expected, both haematin and its quadratic term (haematin2) were key predictors of mosquito fecundity, indicating that egg production was a saturating function of the amount of blood ingested. However, the presence of haematin in the model did not reduce the significance of the infection effect (model 31:
p < 0.0001; figure 7a). This could be due to either a direct effect of the parasite on fecundity (on average 80–90% of mosquitoes that fed on an infected bird became infected) or the indirect result of feeding on blood with a lower protein content (anaemic blood). To test for this, we added haematin, a proxy for the amount of haemoglobin ingested, into the model. As expected, both haematin and its quadratic term (haematin2) were key predictors of mosquito fecundity, indicating that egg production was a saturating function of the amount of blood ingested. However, the presence of haematin in the model did not reduce the significance of the infection effect (model 31: 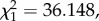 p < 0.0001; figure 7b) suggesting that the reduction in egg production was not entirely explained by the decrease in blood quality.
p < 0.0001; figure 7b) suggesting that the reduction in egg production was not entirely explained by the decrease in blood quality.
Figure 7.
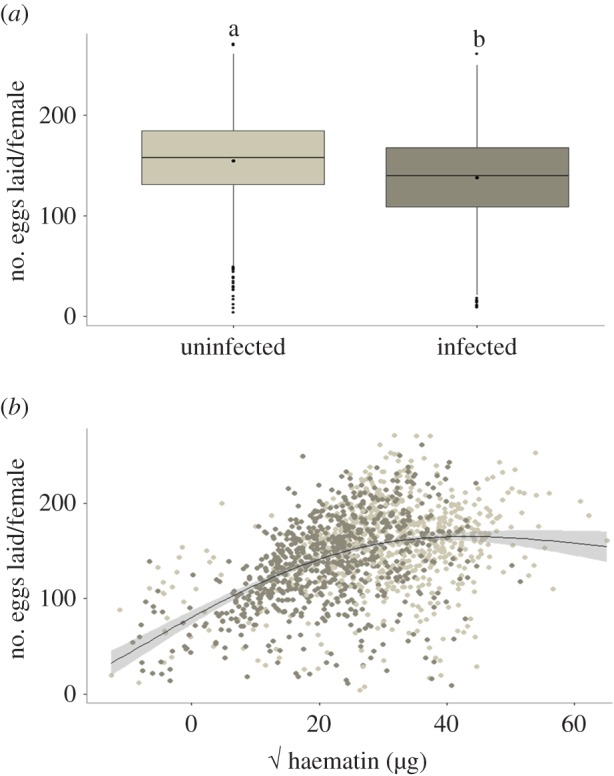
Number of eggs laid by females as a function of their infection status (a) and the amount of blood meal ingested (b). Light grey box plot and dots represent uninfected females, dark grey box plot and dots represent infected females. In (b), haematin has been square root transformed to improve the fit of the model. (Online version in colour.)
In contrast to fecundity, variations in mosquito lifespan were not explained either by the infection status of the bird (model 32: 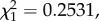 p = 0.6309), or by the size of the blood meal ingested (model 32:
p = 0.6309), or by the size of the blood meal ingested (model 32: 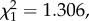 p = 0.2531). Interestingly, lifespan was positively correlated with bird parasitaemia: mosquitoes fed on birds with higher parasitaemias were longer lived (model 33:
p = 0.2531). Interestingly, lifespan was positively correlated with bird parasitaemia: mosquitoes fed on birds with higher parasitaemias were longer lived (model 33: 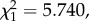 p = 0.0143; figure 8).
p = 0.0143; figure 8).
Figure 8.
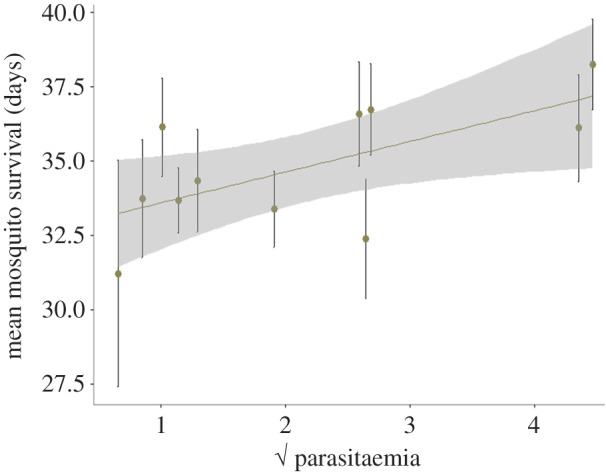
Mean mosquito survival as a function of bird parasitaemia (total % of red blood cells infected). Bars represent standard errors around the mean. (Online version in colour.)
4. Discussion
(a). Serial passages
Previous work has shown that when parasites of various Plasmodium species are serially passaged, they often generate higher parasite densities [20]. These results fit in with the general prediction that, in the absence of mosquitoes, serial passages should select for higher rates of asexual reproduction and thus for higher parasitaemia [20,27]. In some cases, this increase in parasitaemia has been accompanied by an increase in transmission potential, measured as gametocyte production [19], whereas in others gametocyte production has been reduced [28].
Our results show that, despite regular passages through the mosquitoes, parasitaemia increased slightly but significantly over the course of the passages, yet no significant change in gametocytaemia with time was detected. A more detailed analysis of our serial passage time series indicated, however, that passage through a mosquito did indeed result in a significant, but short lived, decrease in parasitaemia. Transmission through mosquitoes is very different from intraperitoneal injections in many ways, the most obvious of which is inoculum size. This alone could explain the differences in parasitaemia observed [29]. Another, and perhaps more important difference, is that while in intraperitoneal injections the blood-stage parasites are injected more or less directly into the bloodstream, mosquitoes inject sporozoites which need to go through the whole exoerythrocytic merogony in the reticuloendothelial cells of several organs (liver, spleen) before reaching the blood [21]. The decrease in parasitaemia we observed after a mosquito passage may simply be due to a delay in the arrival of parasites in the bloodstream. However, we also detected a negative carry-over effect of the mosquito passage in the subsequent standard (intraperitoneal) passage immediately following it. This suggests that the mosquito effect goes beyond a mere dose or delay issue, and that passage through mosquitoes could durably alter the outcome of evolution through passage experiments. Similar results have recently been obtained in the rodent malaria model, where passage through mosquitoes reduced parasitaemia for five passages before increasing again [30]. In addition, lower parasitaemia was associated with a reduced virulence and a modification of the expression of genes involved in antigenic variation [30]. Clearly, more detailed experiments could be carried out with our system to decipher the impact of mosquito passages on the experimental evolution of P. relictum.
Further experiments will also be needed to address two important issues that hinder the interpretation of our results and make it difficult to compare them with those obtained in rodent malaria [20]. First, rodent malaria experiments have been carried out using single genotype isolates [31], while our strain is a field isolate containing a single P. relictum lineage (as defined by its cyt-b sequence [5]) but with an unknown amount of genetic variability. It is therefore not impossible that the genetic diversity of our strain may have changed through the serial passages, an interesting possibility that will be worth exploring when the molecular tools become available with the upcoming the full genome sequence of the SGS1 strain. Second, for bird welfare reasons (wing vein punctures result in scabbing that takes a few days to heal), parasitaemia was measured only once during the acute phase of the infection. Based on preliminary (unpublished) results showing that in our system peak parasitaemia is attained on average 10–12 days post infection, all our acute parasitaemia measurements were therefore taken within this time window. This has three obvious shortcomings. First, any between-bird variability in peak parasitaemia cannot be accounted for. Second, as mentioned above, the timing of peak parasitaemia may be different in mosquito-triggered infections (our time estimates were based on intraperitoneal infections). Finally, the peak parasitaemia may have shifted with time as a by-product of the serial passages. All of these issues are worthy of study and we are currently developing new blood sampling methods that will allow us to monitor parasitaemia daily to explore them further.
(b). Bird infection traits
One of the most troubling facts when working with avian malaria is the large variance in bird parasitaemias we obtain, much of which can be explained by unavoidable variations in conditions between experiments. However, even within a single experiment, birds infected concomitantly from the same pool of blood show a large variance in both their parasitaemia and gametocytaemia (electronic supplementary material, figure S4). The reasons for this variation are not known but may be numerous. Birds vary widely in their susceptibility to infection according to, among other things, their age [7,32,33], body condition [34,35] and any previous or concurrent infections [36,37]. Canaries are not a standard experimental animal; hence we lack information about many of these key parameters, and their potential role in the observed variance in parasitaemias and gametocytaemias. Further work is required to explore the influence of these factors on susceptibility to malaria infections.
In our experiments, gametocyte conversion ratios also showed a great degree of variation between birds. At the acute stage of the infection, gametocytes constituted, on average, a third of the total P. relictum parasite population, which is much higher than the values obtained in other Plasmodium species. The difference is particularly striking with the rodent malaria species P. chabaudi, where gametocytes represent at most 3% of the parasites present in an infection [38,39]. Conversion ratios in human malaria, on the other hand, vary between 0.3 and 21% depending on host age [40] and genotype [41]. The largest conversion ratios recorded are observed in wild lizards, where gametocytes represent between 50 and 85% of the total parasite population, depending on the season [42]. Whether the high conversion ratios we observed are a distinctive feature of our experimental system or whether they are representative of other avian malaria parasites is not known. We know of only one other study that has quantified conversion ratios in an avian malaria species [43]. In P. juxtanucleare, gametocytes were found to represent around 3% of the total parasite population, but the particular experimental protocol employed (old chronic infections and several doses of corticosteroids to impair the birds' immunity) make this result difficult to generalize [43]. We clearly need more studies that quantify the plasticity in gametocyte conversion ratios in avian malaria in response to different environmental conditions. The possibility of doing this both in the wild and in controlled experimental infections in the laboratory is unparalleled among currently available malaria systems.
(c). Virulence in birds and mosquito transmission
It is widely accepted that avian malaria parasites impose fitness costs to their hosts, although the nature of these costs vary depending on the bird and Plasmodium species. A well-known example of the potentially devastating effects of avian malaria on host fitness is the decline and extinction of naive populations of honeycreepers after the accidental introduction of the parasite to the Hawaiian Islands in the early twentieth century [44]. Detecting fitness costs on endemic infections in the wild has, however, proved more challenging. Long-term monitoring of a wild population of blue tits has revealed strong associations between malaria infection on bird survival and recapture rates [45]. More recently, malaria infection has been found to accelerate the senescence of great reed warblers through telomere degradation [46]. Experimental infections, on the other hand, have consistently shown anaemia to be one of the primary virulence determinants in avian malaria infections [23,36,42]. Likewise, in this analysis, P. relictum infections resulted, on average, in a 30% reduction in the red blood cell count during the acute phase of the infection (10–12 days after inoculation). However, in contrast with a report on rodent malaria [19], we found no phenotypic association between parasitaemia and virulence. Unlike this earlier study, however, we did not monitor the temporal dynamics of the infection: our estimates of parasitaemia are based on single measurements 10–12 days after the infection. The relationship between parasitaemia and virulence deserves further studies that should also consider other measures of virulence (e.g. weight loss [35]).
Parasitaemia was found to be a good predictor of transmission to mosquitoes. This is most apparent through the striking differences in oocyst prevalence and burden in mosquito infections issued from acutely and chronically infected birds (figure 3). This raises the important question of the relative contribution of acute versus chronic infections to transmission in the field, given that acute Plasmodium infections are very short lived, and the vast majority of wild-caught infected birds harbour chronic infections [33]. Additionally, there is a marked seasonal variation in avian malaria prevalence in birds [47,48] and the proportion of birds with an acute infection in a population may fluctuate with the number of infected vectors throughout the year.
Within the range of parasitaemias observed in acute infections, we also found a clear association between parasite burden in the host and in the mosquito, even though the number of oocysts saturates beyond 4% of red blood cells infected (figure 4b). We failed, however, to detect a clear relationship between parasitaemia and the probability of infection of mosquitoes (figure 4c). As has been found in other systems, and most notably in rodent and human malaria [20,39], parasitaemia and gametocytaemia during the acute stage of the infection were strongly correlated (figure 2). It is therefore surprising that we failed to detect a positive association between gametocytaemia and the prevalence or intensity of Plasmodium infection in mosquitoes (figure 4b,d). This result echoes those from other malaria studies, which also reported a lack of a clear relationship between gametocytes and mosquito infection in Plasmodium [49,50] (but see [51,52] for two studies where such a relationship was found). This problem may be due in large part to the estimation of gametocyte densities by microscopy, which misses a significant proportion of the gametocytes present [49,50]. Molecular tools based on the amplification of gametocyte-specific RNA have been developed for rodent and human malaria and allow the quantification (and sexing) of gametocytes with greater accuracy (reviewed in [53]). Similar tools in avian malaria are urgently needed, as they will offer new opportunities to study phenotypic plasticity in gametocyte density and sex ratio in response to different ecological and evolutionary scenarios, both in the field and in the laboratory.
The question remains as to why parasitaemia is a better predictor of mosquito infection rates than gametocytaemia (see also [17]). It is unlikely that parasitaemia bears directly on mosquito infectivity. Parasitaemia, however, is correlated with several in-host factors, including host immunity and metabolic profiles which may affect the likelihood of mosquito infection [54]. Interestingly, we found a significant positive relationship between blood parasitaemia and the volume of blood ingested by mosquitoes (figure 5). The mechanisms underlying this positive relationship are not known. One possibility is that parasitaemia may correlate negatively with blood quality, and that mosquitoes compensate for poorly nutritious blood by feeding more, which would result in higher infection levels. In human malaria, it is well established that peak parasitaemia is associated with decreased concentrations of several blood metabolites, including lipid and sugar levels [55]. In our experiments, the only measure of blood quality we recorded was the number of red blood cells, which are known to be an important source of protein for mosquitoes. This variable did not, however, explain the observed variation in parasitaemia. An alternative explanation for the observed increase in blood meal size with parasitaemia could be a dose-dependent manipulation of mosquito behaviour by the parasite. Plasmodium parasites have been shown to manipulate their hosts at different stages of the parasite's life cycle ([16,56], but see [57]). In particular, we previously reported that infected birds attract significantly more mosquitoes than uninfected ones, possibly through the emission of distinct olfactory signals [16]. None of these reported cases of parasite manipulation has, however, been shown to be dependent on parasite numbers.
(d). Mosquito fitness
Malaria transmission depends critically on the fitness of its infected vectors. For this reason, the effects of Plasmodium infection on mosquito life-history traits such as fecundity and lifespan have received a lot of attention [58,59]. The largest effect of P. relictum parasites on Culex mosquitoes is a drastic reduction in their fecundity, which can result in a loss of up to 40% of eggs laid [12]. Our findings are consistent with these previous results qualitatively, if not quantitatively: infected females laid on average 11.5% fewer eggs than their uninfected counterparts. This reduction in egg production could be due to two non-mutually exclusive factors. First, haemoglobin is the main source of protein for egg production in mosquitoes [60], and infected birds are anaemic. The reduction in fecundity may therefore simply be an indirect by-product of anaemia. Second, there is evidence from rodent malaria suggesting that Plasmodium induces a direct cost on female fecundity. Plasmodium yoelii induces apoptosis in mosquito ovaries [61] and increases egg resorption [62]. Although no molecule of Plasmodium origin has been identified that would justify talking about parasite manipulation, it has been widely assumed that reproductive curtailment is an adaptive strategy of the parasite to increase mosquito survival through a trade-off in energy allocation between reproduction and survival [63,64].
The effect of Plasmodium on mosquito survival is still highly contentious and seems to be extremely sensitive to the particular mosquito–Plasmodium combination used and to the specific laboratory conditions under which it is measured [12,59]. Despite earlier results showing that, under certain experimental conditions, P. relictum infections are associated with an increase in mosquito longevity [12], here we found no clear association between Plasmodium infection and mosquito longevity, although slight differences in the experimental protocols may have blurred the picture. We did, however, find an intriguing positive relationship between bird parasitaemia and mosquito survival (figure 8). Given that parasitaemia is a good predictor of oocystaemia (figure 4b), one possibility is that this positive relationship is the result of mosquitoes with higher oocyst burdens living longer. It is, unfortunately, impossible to verify this with the current dataset as longevity and oocyst burden cannot be quantified in the same individual (mosquitoes need to be killed to quantify oocysts).
5. Conclusion and perspectives
This study represents a comprehensive review of the knowledge we have accumulated regarding between-host trait variability and trait associations in malaria parameters and how they affect transmission. Some of the statistical relationships obtained, such as the relationship between parasitaemia and gametocyte production, or that between blood meal size and oocyst number, are consistent with findings in other experimental systems [20,65]. Others, however, such as the relationship between gametocytaemia and mosquito infection rates, were expected but not found. We need to take a leaf out of the rodent malaria studies and develop markers that will allow us to quantify gametocyte numbers and sex ratios more accurately. This will open the way for novel studies looking at the genetic and environmental determinants underpinning gametocyte conversion rates and sex ratios in avian malaria both in the laboratory [66] and, crucially, in the field. Finally, we also obtained results that were unexpected and that require further study. This is the case for the positive relationship between parasitaemia in the bird and both the haematin excreted by mosquitoes, an imperfect proxy for blood meal size (figure 5), and their subsequent longevity (figure 8). Further experiments are needed to disentangle the potential mechanisms underlying these relationships before any conclusions can be drawn. Finding a satisfactory method to quantify blood meal size that is independent of the amount of haemoglobin in the blood, the main drawback of haematin, would go a long way towards disentangling the effects of anaemia and clarifying the central role seemingly played by blood meal size in these interactions.
The analysis of our serial passage experiments also revealed very interesting patterns. In particular, as shown in earlier studies [30] we show that the mode of transmission (intraperitoneal or via mosquitoes) affects subsequent within-bird dynamics and deserves further investigation. In addition, the number of serial passages had an effect on parasitaemia but not on gametocytaemia. Could this pattern result from the genetic or epigenetic modifications of the gametocyte conversion ratio? The P. relictum genome will provide the framework for tracking mutations that may be involved in the control of within-host dynamics. Unlike previous studies based on multiple clones of P. chabaudi [19,20], we are currently unable to unravel phenotypic from genetic correlations, and hence we cannot examine the genetic constraints moulding avian malaria evolution. It is time to expand our work to assess new malaria lineages and explore the life-history variation associated with the large genetic diversity of avian malaria in the field [5]. Ultimately, the ability to combine empirical observations in the field and in the laboratory on different avian malaria genotypes in both the bird and the mosquito vector will, we believe, generate major contributions to the study of the evolutionary ecology of Plasmodium.
Supplementary Material
Ethics
Experiments were approved by the Ethical Committee for Animal Experimentation established by the authors' institution (permit no. CEEA-LR-1051).
Data accessibility
Dryad website (http://dx.doi.org/10.5061/dryad.35hs3).
Authors' contributions
R.P., J.V., S.C., F.Z., S.G. and A.R. conceived and designed the experiments cited in this paper. R.P., J.V., S.C., F.Z. and A.N. performed the experiments. R.P. compiled and analysed the data. P.P. was instrumental in developing the experimental canary infections. R.P., S.G. and A.R. wrote the paper. All authors read and commented on earlier drafts of the paper and approved the final version of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
J.V. was funded through an FCT (GABBA) grant; F.Z. through a CNRS-Languedoc Roussillon Region grant; S.G. by ERC Starting Grant EVOLEPID 243054 and A.R. by an ANR-SEST grant.
References
- 1.Ross R. 1911. The prevention of malaria. London, UK: Murray. [Google Scholar]
- 2.Huff CG, Bloom W. 1935. A malaria parasite infecting all blood and blood-forming cells of birds. J. Infect. Dis. 57, 315–336. ( 10.1093/infdis/57.3.315) [DOI] [Google Scholar]
- 3.Raffaela G, Marchiafava E. 1944. Considerations on the relationship between exoerythrocytic forms and relapse in malaria. Ann. Soc. Belg. Med. Trop., 323–330. [Google Scholar]
- 4.Marshall EK. 1942. Chemotherapy of avian malaria. Physiol. Rev. 22, 190–204. [Google Scholar]
- 5.Bensch S, Hellgren O, Pérez-Tris J. 2009. MalAvi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol. Ecol. Resour. 9, 1353–1358. ( 10.1111/j.1755-0998.2009.02692.x) [DOI] [PubMed] [Google Scholar]
- 6.Outlaw DC, Ricklefs RE. 2014. Species limits in avian malaria parasites (Haemosporida): how to move forward in the molecular era. Parasitology 141, 1223–1232. ( 10.1017/S0031182014000560) [DOI] [PubMed] [Google Scholar]
- 7.Bichet C, Sorci G, Robert A, Julliard R, Lendvai ÁZ, Chastel O, Garnier S, Loiseau C. 2014. Epidemiology of Plasmodium relictum infection in the house sparrow. J. Parasitol. 100, 59–65. ( 10.1645/12-24.1) [DOI] [PubMed] [Google Scholar]
- 8.Zélé F, Vézilier J, L'Ambert G, Nicot A, Gandon S, Rivero A, Duron O. 2014. Dynamics of prevalence and diversity of avian malaria infections in wild Culex pipiens mosquitoes: the effects of Wolbachia, filarial nematodes and insecticide resistance. Parasit. Vectors 7, 1–16. ( 10.1186/1756-3305-7-437) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ventim R, Ramos JA, Osório H, Lopes RJ, Pérez-Tris J, Mendes L. 2012. Avian malaria infections in western European mosquitoes. Parasitol. Res. 111, 637–645. ( 10.1007/s00436-012-2880-3) [DOI] [PubMed] [Google Scholar]
- 10.Vézilier J, Nicot A, Gandon S, Rivero A. 2010. Insecticide resistance and malaria transmission: infection rate and oocyst burden in Culex pipiens mosquitoes infected with Plasmodium relictum. Malar. J. 9, 379 ( 10.1186/1475-2875-9-379) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rivero A, Magaud A, Nicot A, Vézilier J. 2011. Energetic cost of insecticide resistance in Culex pipiens mosquitoes. J. Med. Entomol. 48, 694–700. ( 10.1603/ME10121) [DOI] [PubMed] [Google Scholar]
- 12.Vézilier J, Nicot A, Gandon S, Rivero A. 2012. Plasmodium infection decreases fecundity and increases survival of mosquitoes. Proc. R. Soc. B 279, 4033–4041. ( 10.1098/rspb.2012.1394) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vézilier J, Nicot A, Lorgeril J, Gandon S, Rivero A. 2013. The impact of insecticide resistance on Culex pipiens immunity. Evol. Appl. 6, 497–509. ( 10.1111/eva.12037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornet S, Gandon S, Rivero A. 2013. Patterns of phenoloxidase activity in insecticide resistant and susceptible mosquitoes differ between laboratory-selected and wild-caught individuals. Parasit. Vectors 6, 315 ( 10.1186/1756-3305-6-315) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zélé F, Nicot A, Duron O, Rivero A. 2012. Infection with Wolbachia protects mosquitoes against Plasmodium-induced mortality in a natural system. J. Evol. Biol. 25, 1243–1252. ( 10.1111/j.1420-9101.2012.02519.x) [DOI] [PubMed] [Google Scholar]
- 16.Cornet S, Nicot A, Rivero A, Gandon S. 2013. Malaria infection increases bird attractiveness to uninfected mosquitoes. Ecol. Lett. 16, 323–329. ( 10.1111/ele.12041) [DOI] [PubMed] [Google Scholar]
- 17.Cornet S, Nicot A, Rivero A, Gandon S. 2014. Evolution of plastic transmission strategies in avian malaria. PLoS Pathog. 10, e1004308 ( 10.1371/journal.ppat.1004308) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mideo N, Alizon S, Day T. 2008. Linking within- and between-host dynamics in the evolutionary epidemiology of infectious diseases. Trends Ecol. Evol. 23, 511–517. ( 10.1016/j.tree.2008.05.009) [DOI] [PubMed] [Google Scholar]
- 19.Mackinnon MJ, Read AF. 1999. Selection for high and low virulence in the malaria parasite Plasmodium chabaudi. Proc. R. Soc. Lond. B 266, 741–748. ( 10.1098/rspb.1999.0699) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mackinnon MJ, Read AF. 2004. Virulence in malaria: an evolutionary viewpoint. Phil. Trans. R. Soc. Lond. B 359, 965–986. ( 10.1098/rstb.2003.1414) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valkiunas G. 2004. Avian malaria parasites and other haemosporidia. Boca Raton, FL: CRC Press. [Google Scholar]
- 22.Emami SN, Ranford-Cartwright LC, Ferguson HM. 2013. The impact of low erythrocyte density in human blood on the fitness and energetic reserves of the African malaria vector Anopheles gambiae. Malar. J. 12, 45 ( 10.1186/1475-2875-12-45) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palinauskas V, Valkiūnas G, Bolshakov CV, Bensch S. 2008. Plasmodium relictum (lineage P-SGS1): effects on experimentally infected passerine birds. Exp. Parasitol. 120, 372–380. ( 10.1016/j.exppara.2008.09.001) [DOI] [PubMed] [Google Scholar]
- 24.Crawley MJ. 2012. The R book. New York, NY: John Wiley & Sons. [Google Scholar]
- 25.Bolker BM. 2008. Ecological models and data in R. Princeton, NJ: Princeton University Press. [Google Scholar]
- 26.Carter LM, Kafsack BFC, Llinas M, Mideo N, Pollitt LC, Reece SE. 2013. Stress and sex in malaria parasites. Why does commitment vary? Evol. Med. Public Health 2013, 135–147. ( 10.1093/emph/eot011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ebert D. 1998. Experimental evolution of parasites. Science 282, 1432–1436. ( 10.1126/science.282.5393.1432) [DOI] [PubMed] [Google Scholar]
- 28.Dearsly AL, Sinden RE, Self IA. 1990. Sexual development in malarial parasites: gametocyte production, fertility and infectivity to the mosquito vector. Parasitology 100, 359–368. ( 10.1017/S0031182000078628) [DOI] [PubMed] [Google Scholar]
- 29.Timms R, Colegrave N, Chan BH, Read AF. 2001. The effect of parasite dose on disease severity in the rodent malaria Plasmodium chabaudi. Parasitology 123, 1–11. ( 10.1017/S0031182001008083) [DOI] [PubMed] [Google Scholar]
- 30.Spence PJ, Jarra W, Lévy P, Reid AJ, Chappell L, Brugat T, Sanders M, Berriman M, Langhorne J. 2013. Vector transmission regulates immune control of Plasmodium virulence. Nature 498, 228–231. ( 10.1038/nature12231) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walliker D. 1981. Cloning of malaria parasites. WHO/MAL/81.939. Geneva, Switzerland: World Health Organization.
- 32.Hudson PJ, Dobson AP. 1997. Transmission dynamics and host-parasite interactions of Trichostrongylus tenuis in red grouse (Lagopus lagopus scoticus). J. Parasitol. 83, 194–202. ( 10.2307/3284438) [DOI] [PubMed] [Google Scholar]
- 33.Knowles SCL, Palinauskas V, Sheldon BC. 2010. Chronic malaria infections increase family inequalities and reduce parental fitness: experimental evidence from a wild bird population. J. Evol. Biol. 23, 557–569. ( 10.1111/j.1420-9101.2009.01920.x) [DOI] [PubMed] [Google Scholar]
- 34.Krasnov BR, Mouillot D, Khokhlova IS, Shenbrot GI, Poulin R. 2005. Covariance in species diversity and facilitation among non-interactive parasite taxa: all against the host. Parasitology 131, 557–568. ( 10.1017/S0031182005007912) [DOI] [PubMed] [Google Scholar]
- 35.Cornet S, Bichet C, Larcombe S, Faivre B, Sorci G. 2014. Impact of host nutritional status on infection dynamics and parasite virulence in a bird-malaria system. J. Anim. Ecol. 83, 256–265. ( 10.1111/1365-2656.12113) [DOI] [PubMed] [Google Scholar]
- 36.Cellier-Holzem E, Esparza-Salas R, Garnier S, Sorci G. 2010. Effect of repeated exposure to Plasmodium relictum (lineage SGS1) on infection dynamics in domestic canaries. Int. J. Parasitol. 40, 1447–1453. ( 10.1016/j.ijpara.2010.04.014) [DOI] [PubMed] [Google Scholar]
- 37.Palinauskas V, Valkiūnas G, Bolshakov CV, Bensch S. 2011. Plasmodium relictum (lineage SGS1) and Plasmodium ashfordi (lineage GRW2): the effects of the co-infection on experimentally infected passerine birds. Exp. Parasitol. 127, 527–533. ( 10.1016/j.exppara.2010.10.007) [DOI] [PubMed] [Google Scholar]
- 38.Reece SE, Ramiro RS, Nussey DH. 2009. SYNTHESIS: Plastic parasites: sophisticated strategies for survival and reproduction? Evol. Appl. 2, 11–23. ( 10.1111/j.1752-4571.2008.00060.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wargo AR, de Roode JC, Huijben S, Drew DR, Read AF. 2007. Transmission stage investment of malaria parasites in response to in-host competition. Proc. R. Soc. B 274, 2629–2638. ( 10.1098/rspb.2007.0873) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ouédraogo AL, Bousema T, de Vlas SJ, Cuzin-Ouattara N, Verhave J-P, Drakeley C, Luty AJF, Sauerwein R. 2010. The plasticity of Plasmodium falciparum gametocytaemia in relation to age in Burkina Faso. Malar. J. 9, 281 ( 10.1186/1475-2875-9-281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gouagna LC, Bancone G, Yao F, Yameogo B, Dabiré KR, Costantini C, Simporé J, Ouedraogo JB, Modiano D. 2010. Genetic variation in human HBB is associated with Plasmodium falciparum transmission. Nat. Genet. 42, 328–331. ( 10.1038/ng.554) [DOI] [PubMed] [Google Scholar]
- 42.Bromwich CR, Schall JJ. 1986. Infection dynamics of Plasmodium mexicanum, a malaria parasite of lizards. Ecology 67, 1227–1235. ( 10.2307/1938678) [DOI] [Google Scholar]
- 43.Silveira P, Vashist U, Cabral A, Amaral KB, Soares GLG, Dagosto M. 2009. Effect of rutin and chloroquine on white leghorn chickens infected with Plasmodium (Bennettinia) juxtanucleare. Trop. Anim. Health Prod. 41, 1319–1323. ( 10.1007/s11250-009-9317-8) [DOI] [PubMed] [Google Scholar]
- 44.Atkinson CT, Dusek RJ, Woods KL, Iko WM. 2000. Pathogenicity of avian malaria in experimentally-infected Hawaii Amakihi. J. Wildl. Dis. 36, 197–204. ( 10.7589/0090-3558-36.2.197) [DOI] [PubMed] [Google Scholar]
- 45.Lachish S, Knowles SCL, Alves R, Wood MJ, Sheldon BC. 2011. Fitness effects of endemic malaria infections in a wild bird population: the importance of ecological structure. J. Anim. Ecol. 80, 1196–1206. ( 10.1111/j.1365-2656.2011.01836.x) [DOI] [PubMed] [Google Scholar]
- 46.Asghar M, Hasselquist D, Hansson B, Zehtindjiev P, Westerdahl H, Bensch S. 2015. Hidden costs of infection: chronic malaria accelerates telomere degradation and senescence in wild birds. Science 347, 436–438. ( 10.1126/science.1261121) [DOI] [PubMed] [Google Scholar]
- 47.Cosgrove CL, Wood MJ, Day KP, Sheldon BC. 2008. Seasonal variation in Plasmodium prevalence in a population of blue tits Cyanistes caeruleus. J. Anim. Ecol. 77, 540–548. ( 10.1111/j.1365-2656.2008.01370.x) [DOI] [PubMed] [Google Scholar]
- 48.Grillo EL, Fithian RC, Cross H, Wallace C, Viverette C, Reilly R, Mayer DCG. 2012. Presence of Plasmodium and Haemoproteus in breeding prothonotary warblers (Protonotaria citrea: Parulidae): temporal and spatial trends in infection prevalence. J. Parasitol. 98, 93–102. ( 10.1645/GE-2780.1) [DOI] [PubMed] [Google Scholar]
- 49.Churcher TS, Bousema T, Walker M, Drakeley C, Schneider P, Ouédraogo AL, Basáñez M-G. 2013. Predicting mosquito infection from Plasmodium falciparum gametocyte density and estimating the reservoir of infection. eLife 2, e00626 ( 10.7554/eLife.00626) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bousema T, Drakeley C. 2011. Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clin. Microbiol. Rev. 24, 377–410. ( 10.1128/CMR.00051-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buckling AG, Read AF. 1999. The effect of chloroquine treatment on the infectivity of Plasmodium chabaudi gametocytes. Int. J. Parasitol. 29, 619–625. ( 10.1016/S0020-7519(98)00230-6) [DOI] [PubMed] [Google Scholar]
- 52.Tchuinkam T, Mulder B, Dechering K, Stoffels H, Verhave JP, Cot M, Carnevale P, Meuwissen JH, Robert V. 1993. Experimental infections of Anopheles gambiae with Plasmodium falciparum of naturally infected gametocyte carriers in Cameroon: factors influencing the infectivity to mosquitoes. Trop. Med. Parasitol. Off. 44, 271–276. [PubMed] [Google Scholar]
- 53.Babiker HA, Schneider P. 2008. Application of molecular methods for monitoring transmission stages of malaria parasites. Biomed. Mater. 3, 034007 ( 10.1088/1748-6041/3/3/034007) [DOI] [PubMed] [Google Scholar]
- 54.Gouagna LC, Bonnet S, Gounoue R, Verhave JP, Eling W, Sauerwein R, Boudin C. 2004. Stage-specific effects of host plasma factors on the early sporogony of autologous Plasmodium falciparum isolates within Anopheles gambiae. Trop. Med. Int. Health TM IH 9, 937–948. ( 10.1111/j.1365-3156.2004.01300.x) [DOI] [PubMed] [Google Scholar]
- 55.Planche T, Dzeing A, Ngou-Milama E, Kombila M, Stacpoole PW. 2005. Metabolic complications of severe malaria. Curr. Top. Microbiol. Immunol. 295, 105–136. ( 10.1007/3-540-29088-5_5) [DOI] [PubMed] [Google Scholar]
- 56.Koella JC, Sørensen FL, Anderson RA. 1998. The malaria parasite, Plasmodium falciparum, increases the frequency of multiple feeding of its mosquito vector, Anopheles gambiae. Proc. R. Soc. Lond. B 265, 763–768. ( 10.1098/rspb.1998.0358) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cator LJ, George J, Blanford S, Murdock CC, Baker TC, Read AF, Thomas MB. 2013. ‘Manipulation’ without the parasite: altered feeding behaviour of mosquitoes is not dependent on infection with malaria parasites. Proc. R. Soc. B 280, 20130711 ( 10.1098/rspb.2013.0711) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hurd H. 2009. Evolutionary drivers of parasite-induced changes in insect life-history traits: from theory to underlying mechanisms. In Advances in parasitology (eds Webster Joanne, P.), ch. 4, pp. 85–110. New York, NY: Academic Press. [DOI] [PubMed] [Google Scholar]
- 59.Ferguson HM, Mackinnon MJ, Chan BH, Read AF. 2003. Mosquito mortality and the evolution of malaria virulence. Evolution 57, 2792–2804. ( 10.1111/j.0014-3820.2003.tb01521.x) [DOI] [PubMed] [Google Scholar]
- 60.Hurd H, Hogg JC, Renshaw M. 1995. Interactions between blood feeding, fecundity and infection in mosquitoes. Parasitol. Today 11, 411–416. ( 10.1016/0169-4758(95)80021-2) [DOI] [Google Scholar]
- 61.Hopwood JA, Ahmed AM, Polwart A, Williams GT, Hurd H. 2001. Malaria-induced apoptosis in mosquito ovaries a mechanism to control vector egg production. J. Exp. Biol. 204, 2773–2780. [DOI] [PubMed] [Google Scholar]
- 62.Carwardine SL, Hurd H. 1997. Effects of Plasmodium yoelii nigeriensis infection on Anopheles stephensi egg development and resorption. Med. Vet. Entomol. 11, 265–269. ( 10.1111/j.1365-2915.1997.tb00405.x) [DOI] [PubMed] [Google Scholar]
- 63.Hurd H. 2003. Manipulation of medically important insect vectors by their parasites. Annu. Rev. Entomol. 48, 141–161. ( 10.1146/annurev.ento.48.091801.112722) [DOI] [PubMed] [Google Scholar]
- 64.Schwartz A, Koella JC. 2001. Trade-offs, conflicts of interest and manipulation in Plasmodium–mosquito interactions. Trends Parasitol. 17, 189–194. ( 10.1016/S1471-4922(00)01945-0) [DOI] [PubMed] [Google Scholar]
- 65.Lyimo EO, Koella JC. 1992. Relationship between body size of adult Anopheles gambiae s.l. and infection with the malaria parasite Plasmodium falciparum. Parasitology 104, 233–237. ( 10.1017/S0031182000061667) [DOI] [PubMed] [Google Scholar]
- 66.Reece SE, Drew DR, Gardner A. 2008. Sex ratio adjustment and kin discrimination in malaria parasites. Nature 453, 609–614. ( 10.1038/nature06954) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Dryad website (http://dx.doi.org/10.5061/dryad.35hs3).