Abstract
Anthropogenic vectors have moved marine species around the world leading to increased invasions and expanded species' ranges. The biotic resistance hypothesis of Elton (in The ecology of invasions by animals and plants, 1958) predicts that more diverse communities should have greater resistance to invasions, but experiments have been equivocal. We hypothesized that species richness interacts with other factors to determine experimental outcomes. We manipulated species richness, species composition (native and introduced) and availability of bare space in invertebrate assemblages in a marina in Monterey, CA. Increased species richness significantly interacted with both initial cover of native species and of all organisms to collectively decrease recruitment. Although native species decreased recruitment, introduced species had a similar effect, and we concluded that biotic resistance is conferred by total species richness. We suggest that contradictory conclusions in previous studies about the role of diversity in regulating invasions reflect uncontrolled variables in those experiments that modified the effect of species richness. Our results suggest that patches of low diversity and abundance may facilitate invasions, and that such patches, once colonized by non-indigenous species, can resist both native and non-indigenous species recruitment.
Keywords: biotic resistance, invasions, marine fouling community, species richness
1. Introduction
Human activities over the last century have led to major changes in the worldwide distribution of marine species. Shipping, transport within the aquarium trade and deliberate introduction for mariculture have changed or expanded species ranges and transported non-indigenous species (NIS) (e.g. [1–4]). NIS can negatively impact mariculture, recreational and commercial fishing, restoration efforts, native habitats and the populations of local species (e.g. [5,6]). Given these impacts, there is increased interest in understanding the processes that allow successful introductions of NIS to occur.
Researchers have long noted that certain regions and habitats appear to be particularly susceptible to invasions [7,8]. This variability was originally explained by Elton as the ability of species-rich systems to safeguard against invasions [1] (see also [9–12]). Several experimental studies have investigated the role of native species diversity in resistance to invasions, and indicated reduced success of NIS with increased native species diversity [7,13–15], while observational studies have shown the opposite [9,16].
Despite these equivocal results, biotic resistance has become one of the guiding paradigms of invasion biology (e.g. [17]). The ‘insurance hypothesis’, a variant of biotic resistance, holds that species diversity provides assurance that some native species will use the majority of resources even if others fail to thrive, which leads to decreased opportunities for NIS [18,19]. For example, unoccupied space within a community could be a crucial limiting resource for NIS in some sessile invertebrate assemblages [20,21]. In previous studies, cover and survival of sessile invertebrate NIS decreased with increasing native species richness because at least one native species was always occupying space in the high-diversity communities [7,8,19]. However, if diversity and cover are correlated, it is not clear whether diversity or limited resources each contribute to invasion outcomes.
The large number of factors possibly influencing the invasion process has motivated a search for which are most important (e.g. native diversity, free space, prior presence of NIS) [22]. Field experiments often investigate NIS success by manipulating one or two treatment variables and often yield conflicting results (e.g. [7,8,19,23]). These contradictory conclusions highlight the need for more complex experiments.
The objective of this study was to explore the relationship between NIS success and (i) species richness, (ii) available space and (iii) the percentage of native species within the community. For the purpose of this study, NIS success was defined as recruitment and growth of an NIS individual to a size large enough to be seen in photographs (greater than 1 mm). Monterey Harbor in Monterey Bay, California (36.602278° N, 121.891250° W) was chosen for ease of access and documented presence of NIS. We predicted that (i) recruitment of all species (both native and NIS) will be greater on monoculture assemblages than assemblages with greater species richness; (ii) recruitment of all species will be greater on assemblages with more available bare space than on assemblages with less available bare space; and (iii) recruitment of all species will be greater to assemblages containing a lower percentage of native species than to those assemblages with a higher percentage of native species.
2. Material and methods
Adult individuals of 16 species (nine native and seven NIS) that inhabit Monterey Harbor were collected in June 2012 (table 1; electronic supplementary material, figure S1; California Department of Fish and Wildlife Permit no. SC-11868) and attached to PVC tiles (20 × 20 cm) with different levels of richness, free space and percentage of native species. Adults from each species varied in size because of differences in growth form, but were randomly distributed among treatments to avoid bias because of this variation (electronic supplementary material, table S1). Hard-bodied organisms were attached to PVC tiles with marine epoxy (Water Weld JB weld and 3M marine sealant) and soft-bodied organisms were tied to tiles with dental floss (electronic supplementary material, figure S1). No losses were evident after the first sampling period.
Table 1.
Species biogeographic status (based on [24]) and combinations used in experimental treatments. Native status is designated as N for native or I for introduced.
| monoculture | medium diversity | high diversity |
|---|---|---|
| Botrylloides violaceus (I) | Botryllus ‘schlosseri’ (I) W. subtorquata (I) M. senile (N) Barentsia ramosa (N) | Botryllus ‘schlosseri’ (I) Watersipora subtorquata (I) Bugula neritina (I) D. listerianum (I) Mytilus californianus (N) M. senile (N) C. californica (N) B. crenatus (N) |
| Watersipora subtorquata (I) | W. subtorquata (I) B. violaceus (I) Eudistylia polymorpha (N) B. crenatus (N) | W. subtorquata (I) B. violaceus (I) Bugula neritina (I) Ciona savignyi (I) Eudistylia polymorpha (N) B. crenatus (N) A. ceratodes (N) M. senile (N) |
| Mytilus galloprovincialis (I) | M. galloprovincialis (I) B. violaceus (I) A. ceratodes (N) C. californica (N) | M. galloprovincialis (I) B. violaceus (I) Bugula neritina (I) Botryllus ‘schlosseri’ (I) A. ceratodes (N) C. californica (N) Barentsia ramosa (N) Distaplia occidentalis (N) |
| Diplosoma listerianum (I) | Ciona savignyi (I) D. listerianum (I) Aplidium californicum (N) Barentsia ramosa (N) | Ciona savignyi (I) D. listerianum (I) W. subtorquata (I) B. violaceus (I) Aplidium californium (N) Barentsia ramosa (N) Distaplia occidentalis (N) Mytilus californianus (N) |
| Ascidia ceratodes (N) | C. californica (N) Balanus glandula (N) Aplidium californicum (N) Mytilus californianus (N) | A. ceratodes (N) B. crenatus (N) Barentsia ramosa (N) Eudistylia polymorpha (N) C. californica (N) Distaplia occidentalis (N) Mytilus californianus (N) M. senile (N) |
| Balanus crenatus (N) | M. senile (N) Eudistylia polymorpha (N) Barentsia ramosa (N) B. crenatus (N) | M. senile (N) Eudistylia polymorpha (N) Mytilus californianus (N) Distaplia occidentalis (N) B. crenatus (N) A. ceratodes (N) Aplidium californium (N) C. californica (N) |
| Corynactis californica (N) | A. ceratodes (N) Distaplia occidentalis (N) Corynactis californica (N) Barentsia ramosa (N) | |
| Metridium senile (N) | Distaplia occidentalis (N) M. senile (N) Mytilus californianus (N) A. ceratodes (N) |
The experiment manipulated three factors: diversity (one, four and eight species), amount of free space (low, 20–49%; high, 50–90%), and percentage of native species (0% in monoculture treatments only, 50% or 100%) (table 2). Percentage of native species was based on richness in each treatment (i.e. when eight species were used and four were native, percentage of native species was 50%).
Table 2.
Categorical experimental treatments. Treatment levels of diversity, free space and percentage of native species yielded 12 treatment levels, each executed with different species combinations. Final analysis treated each variable as continuous because of losses of organisms prior to the first date of sampling.
| diversity level | free space | percentage of native species |
|---|---|---|
| monoculture | low (20–50%) | low (0%) |
| high (100%) | ||
| high (50–90%) | low (0%) | |
| high (100%) | ||
| medium (four species) | low (20–50%) | low (50%) |
| high (100%) | ||
| high (50–90%) | low (50%) | |
| high (100%) | ||
| high (eight species) | low (20–50%) | low (50%) |
| high (100%) | ||
| high (50–90%) | low (50%) | |
| high (100%) |
All of the 12 treatments were assembled with random selections of native and/or NIS. Table 1 shows the 22 species combinations; each of these was executed at low and high cover (44 treatments). For example, the monoculture treatments included a tile of Ascidea ceratodes at low cover and high cover, a tile of Watersipora subtorquata at low cover and high cover, etc. Random species combinations spread species-specific effects among treatments, thereby reducing the likelihood of bias because of key inhibitors or facilitators [19,25]. Blank tiles (hung horizontally and vertically) were deployed as recruitment controls, and tiles with glue as controls for potential effects of the glue (three tiles). Tiles were placed in six blocks; each contained one of all treatments and controls in a randomized arrangement (47 tiles × 6 blocks). All treatment tiles were hung horizontally 0.2–0.4 m below five separate floating docks (the largest dock contained two blocks separated by greater than 5 m); tiles were placed facing down to encourage invertebrate recruitment, as many larvae are photonegative [26–28]. To account for potential variability of currents, light and sediment load, blocks were spaced across multiple docks. The distances between the closest and most separated blocks were 10 and 50 m, respectively.
The experiment was started in late June 2012 and photographed every two weeks for four months. Recruitment was measured by noting new individuals (not detected on that tile previously) appearing under randomly assigned points on tiles (see below) whether that point fell on bare space or other organisms. The size of the individual or colony was used to identify a new recruit from a previously settled individual, as two weeks of growth was sufficient to differ from new settlers (electronic supplementary material, table S2). An exception was the tiny worm Spirorbis, which was considered a new recruit solely upon first appearance of any individual, as their growth was not measurable in photos.
Photographs were taken with a Panasonic Lumix TS20 waterproof camera mounted on a 40 cm rod, to capture the entire tile in a single frame. These photos were edited using Adobe Photoshop CS3 to remove background and adjust exposure as needed. Percentage cover of the community was estimated using Photogrid v. 1.0 beta, where each photo received 50 points generated in a fully random fashion [29,30]. Organisms under each point were identified to the lowest taxonomic level through morphological identification, and confirmed using genetic methods (when morphological identifications were not feasible) prior to statistical analysis (see the electronic supplementary material).
Owing to low light caused by turbidity from an approaching storm, one block was not photographed on 24 September. This block was removed from all analyses, and there was no loss of power with the decreased sample size [31]. Owing to loss of organisms in the first 3 days after deployment, categorical treatment levels were set aside. Instead, species richness (one to eight species), free space (0–78%) and percentage native species (0–100%) were treated as continuous variables based on photographs taken three days after initial deployment; after this time there was no visible mortality or loss of the original organisms. Failure of attachment seemed to affect all taxa and blocks equally with the exception of monocultures of the sea anemone Metridium senile, in which most individuals migrated off the tiles. Tiles that lost all organisms were excluded from analyses, leaving 227 tiles.
Recruitment (counts of recruits, with Poisson distribution) was analysed with a generalized linear mixed effects (GLMM) model (log-link) [32–34] fitted by Laplace approximation with treatments as fixed factors, and time and blocks as random factors [33]. The exact combination of factors in the best-fit model varied based on time point and/or the response variable being tested. Predictor variables were centred (the mean was subtracted from each value) and the best-fit models determined based on the Akaike information criterion (AIC) [35]. R2 values reported were based on formulae for conditional and marginal R2 measurements [36].
3. Results
During the 100-day deployment (30 June–7 October 2012), 10 species recruited to tiles, five of which were not used in assembling any original treatment (electronic supplementary material, figure S2; figure 1d). All these species were previously observed in Monterey Harbor and represented four phyla. The majority of all recruits were ascidians, which are known to reproduce year-round in Monterey Harbor (e.g. Botryllus 'schlosseri' [35]). The ascidians Diplosoma listerianum and Botrylloides violaceus recruited to experimental tiles multiple times during the experiment. Blank and glue controls did not significantly differ in recruitment (F1,18 = 0.982, p = 0.335) and were excluded from the analyses below.
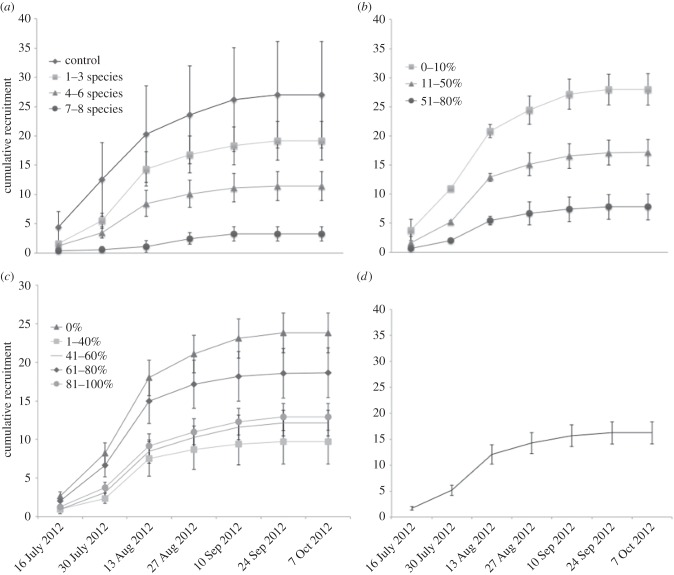
Figure 1.
Cumulative recruitment over time grouped by community attributes. Cumulative recruitment is the total count of individual recruits that occur up to that date of both native and NIS. Each main factor was binned based on initial photographs and pooled across other factors. Cumulative recruitment versus (a) species richness, (b) initial percentage cover, (c) initial percentage of native species and (d) cumulative recruitment over the course of the experiment. Error bars represent ±2 s.e. except in (c) (±1 s.e.).
The GLMM revealed that all factors tested (richness, percentage cover and percentage of native species) and one interaction had significant effects on recruitment (regardless of native status) during this experiment (table 3 and figure 2). This best-fit model (lowest AIC value) incorporated block as the random factor, allowing for assessment of block specific responses. This full GLMM had a conditional R2 (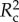 ) value of 0.845, while the fixed effects alone on the latent scale had a marginal R2 (
) value of 0.845, while the fixed effects alone on the latent scale had a marginal R2 (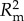 ) of 0.465 (electronic supplementary material, table S3). This difference shows that block and time have large effects in the model and explain an additional 38% of the variability.
) of 0.465 (electronic supplementary material, table S3). This difference shows that block and time have large effects in the model and explain an additional 38% of the variability.
Table 3.
Results of GLMM on total cumulative recruitment. Only significant factors remained in the final model.
| parameter | estimate | s.e. | p-value | variance | |
|---|---|---|---|---|---|
| fixed | intercept | 1.4716 | 0.2853 | 5.33 × 10−6 | — |
| richness (R) | −0.0349 | 0.0047 | 7.71 × 10−12 | — | |
| percentage cover (PC) | −0.0202 | 0.0004 | <2 × 10−16 | ||
| percentage native species (PNS) | −0.0034 | 0.0003 | <2 × 10−16 | — | |
| R : PC | −0.0058 | 0.0001 | <2 × 10−16 | — | |
| time | 0.0169 | 0.0009 | <2 × 10−16 | ||
| random | block | — | — | — | 3.547 × 10−6 |
| time | — | — | — | 5.208 × 10−01 |
Figure 2.
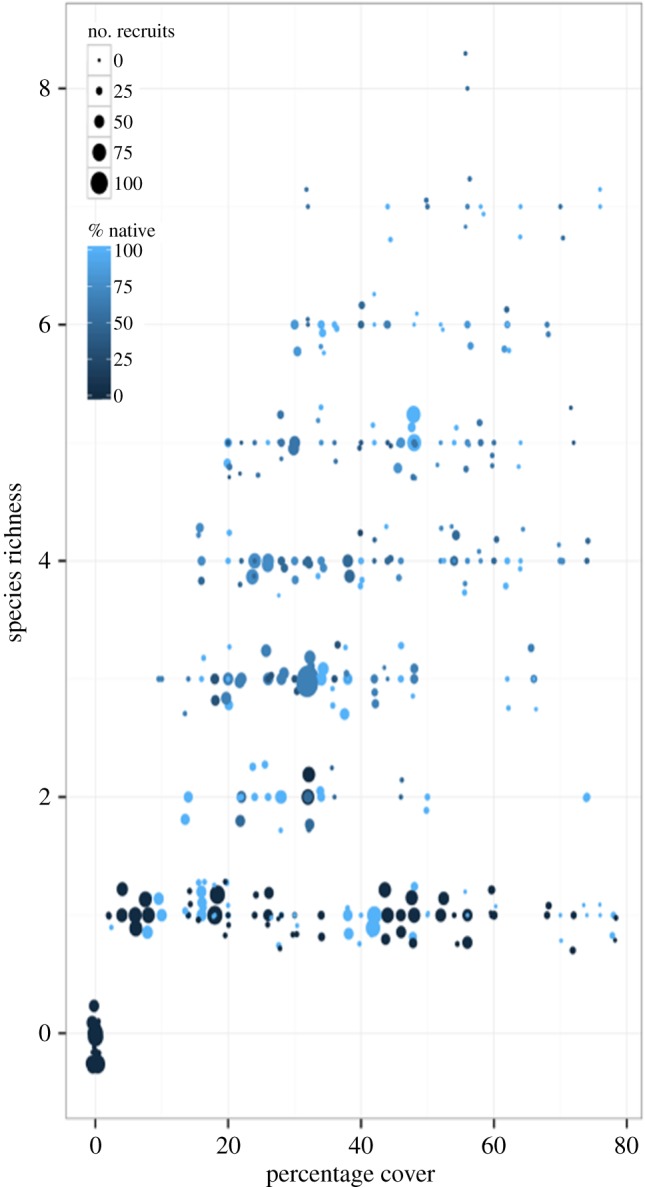
The effects of community attributes on total recruitment after 100 days. Cumulative recruitment as a response of experimental treatments, highlighting the interaction of the species richness and cover. Communities with high cover and high richness had the least recruitment, while native status of the community had a variable effect.
Species-rich communities experienced less recruitment than species-poor communities, supporting the hypothesis, but there was a significant interaction with the initial amount of cover (table 3 and figure 1a). Recruitment to tiles with low levels of richness decreased with increasing cover, while tiles with high richness received little recruitment, regardless of level of cover, resulting in the significant interaction between these factors (figure 2).
Over the course of the experiment, percentage cover on tiles did not reach 100%; the highest recorded was 80%, suggesting that this is the maximum ‘winter’ cover for tiles of the size used. Overall, treatments with less free space received less recruitment over time, supporting the hypothesis that free space facilitates recruitment. However, the interaction of species richness and cover, discussed above, indicates that the effect of space can be modified by other factors (figures 1b, 2; table 3).
Assemblages with initially more native species had significantly less successful recruitment than assemblages with more NIS adults already present (table 3). This result supports the hypothesis that increased native richness decreased successful recruitment of all species studied (figure 1c; electronic supplementary material, figure S2c). However, the coefficient for this factor was an order of magnitude smaller than that of the others, suggesting that is a weak predictor of recruitment success (table 3).
Because the majority of recruitment occurred in the first two months of the experiment, a GLMM was applied separately to the first 57 days of data. This revealed a positive relationship between total recruitment and both higher species richness and higher percentage cover, with a significant interaction between these factors. This result is opposite of the relationships seen after 100 days (electronic supplementary material, table S4). However, the GLMM also showed that communities with initially higher percentage of native species received less recruitment than communities with initially more adult NIS, as shown when all time points were included (above).
During the first 71 days of this experiment (the same time frame as in the study by Stachowicz et al. [7], the GLMM revealed that predictor variables had a significant negative relationship with cumulative recruitment, and a significant interaction of richness and initial percentage cover (electronic supplementary material, table S5). At this time, speciose communities had less recruitment than their species-poor counterparts, but this only held true at some levels of cover, as again there was a significant interaction of manipulated factors.
The majority of recruitment by NIS occurred during the first month of the experiment (electronic supplementary material, figure S2). A GLMM revealed that high prior cover and high percentage of native species were significant predictors of low NIS recruitment during this time period (electronic supplementary material, figure S1) [7]. Dense communities dominated by native species were more resistant to successful recruitment by NIS. Species richness again significantly interacted with percentage cover to affect NIS recruitment. Recruits of an unidentified colonial ascidian were excluded from all these analyses, as their native status could not be resolved.
4. Discussion
Our results indicate that interactions among all manipulated factors determine the risk of invasion at the time and spatial scales investigated. In particular, we found that species richness (including both NIS and native species) consistently interacts with available space, though the relationship varied over time. Assemblages with high richness received less recruitment than monocultures regardless of their initial cover, but communities with high richness and high prior cover received the least amount of recruitment (figure 1a). These results support the premise of Elton's biotic resistance hypothesis, but also indicate that factors other than species richness contribute to invasion patterns. At most time points, communities with higher total resident species richness (native and NIS) received less recruitment, suggesting that both native and NIS richness contribute to a community's stability.
The probability of a successful invasion depends on resource availability and species interactions, as well as the timing of the recruitment by NIS [36]. Previous experiments suggested that complementary resource utilization allowed more diverse communities to resist invasions, as species-rich communities were better able to pre-empt space, leaving little resources for invaders [7,8,15]. Prior results, however, may confound available space and diversity. In our study, diverse communities received little recruitment at all levels of cover and communities contained some level of available space throughout the experiment. In our study, species-rich communities resisted new recruitment by mechanisms other than space exploitation, and space was not the only limiting factor in these communities.
Factors not tested here (e.g. neighbour growth, productivity, or trophic group) may explain the significant interactions between species richness and initial cover found in this study (e.g. [37–39]). While available primary space was negatively related to recruitment in this experiment, adult organisms (both native and NIS) can provide settlement cues or substrates [40]. For example, D. listerianum and B. violaceus, both NIS, were observed settling on adult native species such as Mytilus californianus and A. ceratodes. Thus, high prior cover did not act as absolute barrier to new recruitment.
Propagule pressure, here the availability of larvae, may also be extremely important in determining the likelihood of successful invasions [41,42]. In this study, adult NIS may have provided a source of larvae settling on the tiles. For example, the ascidian D. listerianum releases short-lived larvae that are immediately competent to settle [40]. Limited dispersal would lead to increased settlement near the parent and onto neighbouring treatment tiles. Differences in fecundity of adults in each treatment and on nearby structures may explain the differences between treatment blocks (table 3). The timing and size of these pulses in propagules could explain differences in treatment effects at different time points in this experiment. Specifically, we suggest that the switch in effect of predictor variables between 57 and 71 days was because of a large pulse of recruitment seen at 45 days. A strong pulse of larvae could have overcome the inhibitory effects of species richness. Yet this effect was not long lasting; at the 71-day time point the negative influence of species richness returned. To account for the influence of episodically important factors on invasion success, we suggest that communities be monitored throughout an experiment.
During a 70-day fouling community study on the east coast of the United States, Stachowicz et al. [7] argued that native species richness protected against invasions. It is now known that many of the putatively native species used in their experiment were misclassified, and actually represent a mixture of cryptogenic and NIS [43,44]. Given this, their results show that as total species richness increased, invasion success decreased. However, too few combinations of strictly native species were tested to determine if native species were better able to resist new settlement than mixed or NIS communities. In our study, native species suppressed recruitment, but the effect was small when compared with other factors. Total species richness is more important than species origins for resistance to invasions.
The interaction of resident diversity and prior cover in resisting recruitment demonstrates one example of the complex mechanisms that determine the success of invasions. Artificial structures in coastal waters, like those studied here, provide a point of entry for newly arrived NIS and an advantageous place to study marine invasions [24,44,45]. While this study specifically addressed marine invasions, we suggest that interactions among contributing processes are important for controlling invasions in other ecosystems. This new understanding may explain contradictory conclusions seen in previous literature [9,17]. Finally, this experiment investigated invasion processes at a patch scale in an already invaded landscape; how these communities would resist entirely new NIS remains undetermined, but we predict that patches of communities with low cover and low diversity provide opportunities for new NIS to become established.
Supplementary Material
Acknowledgements
We would like to thank the dive programmes at Moss Landing Marine Laboratories, Marilyn Cruickshank and volunteers who helped collect this data. We would also like to acknowledge Hannelore Rose and Max Butensky for their help with photo analysis, and Deasy Lontoh, Tomo Eguchi and Scott Hamilton for their assistance with the analysis. We thank Greg Ruiz, Scott Hamilton, Andy Chang and anonymous reviewers for their comments and insight on this manuscript.
Ethics
Collection of organisms was approved by the California Department of Fish and Wildlife, permit no. SC-11868.
Data accessibility
Raw data are available on Dryad (http://dx.doi.org/10.5061/dryad.f9801).
Authors' contributions
M.L.M. conceived and designed the study, carried out field and molecular laboratory work, collected and analysed data, and drafted the manuscript; J.B.G. participated in the design of this study and helped draft the manuscript. Both the authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
Funding sources of Harvey Fellowship, MLML Wave Award, International Women's Fishing Hall of Fame Scholarship and Meyers’ Oceanographic Trust Scholarship generously gave funds associated with the collection of this data.
References
- 1.Elton CS. 1958. The ecology of invasions by animals and plants. London, UK: Methuen. [Google Scholar]
- 2.Carlton J, Geller J. 1993. Ecological roulette: the global transport of nonindigenous marine organisms. Biol. Conserv. 67, 280. [DOI] [PubMed] [Google Scholar]
- 3.Carlton JT, Ruiz GM. 2005. The magnitude and consequences of bioinvasions in marine ecosystems: implications for conservation biology. In Marine conservation biology: the science of maintaining the sea's biodiversity (eds Norse EA, Crowder LB), pp. 123–148. Washington, DC: Island Press. [Google Scholar]
- 4.Bax N, Williamson A, Aguero M, Gonzalez E, Geeves W. 2003. Marine invasive alien species: a threat to global biodiversity. Mar. Policy 27, 313–323. ( 10.1016/S0308-597X(03)00041-1) [DOI] [Google Scholar]
- 5.California Department of Fish and Game. 2008. California aquatic invasive species management plan. Aquatic Sciences. [Google Scholar]
- 6.Pimentel D, Zuniga R, Morrison D. 2004. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol. Econ. 52, 273–288. ( 10.1016/j.ecolecon.2004.10.002) [DOI] [Google Scholar]
- 7.Stachowicz JJ, Whitlatch RB, Osman RW. 1999. Species diversity and invasion resistance in a marine ecosystem. Science 286, 1577–1579. ( 10.1126/science.286.5444.1577) [DOI] [PubMed] [Google Scholar]
- 8.Stachowicz JJ, Fried H, Osman RW, Whitlatch RB. 2002. Biodiversity, invasion resistance, and marine ecosystem function: reconciling pattern and process. Ecology 83, 2575–2590. ( 10.1890/0012-9658(2002)083%5B2575%3ABIRAME%5D2.0.CO%3B2) [DOI] [Google Scholar]
- 9.Marchetti MP, Light T, Moyle PB, Viers JH. 2004. Fish invasions in California watersheds: testing hypotheses using landscape patterns. Ecol. App. 14, 1507–1525. ( 10.1890/03-5173) [DOI] [Google Scholar]
- 10.Fox MD, Fox BJ. 1986. The susceptibility of natural communities to invasion. In Ecology of biological invasions: an Australian perspective (eds Groves RH, Burdon JJ), pp. 57–66. Canberra, Austrailia: Austrailian Academy of Science. [Google Scholar]
- 11.Brown JH. 1989. Patterns, modes, and extents of invasions by vertebrates. In Biological invasions: a global perspective (eds Drake JA, Mooney HA, F di Castri, Groves RH, Kruger FJ, Rejmanek M, Williamson M), pp. 85–109. Chichester, UK: John Wiley. [Google Scholar]
- 12.Case TJ. 1990. Invasion resistance arises in strongly interacting spices-rich model, competition communities. Proc. Natl Acad. Sci. USA 87, 9610–9614. ( 10.1073/pnas.87.24.9610) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giller P, et al. 2004. Biodiversity effects on ecosystem functioning: emerging issues and their experimental test in aquatic environments. Oikos 104, 423–436. ( 10.1111/j.0030-1299.2004.13253.x) [DOI] [Google Scholar]
- 14.Hooper D, et al. 2005. Effects of biodiversity on ecosystem functioning: a consesus of current knowledge. Ecol. Monogr. 75, 3–35. ( 10.1890/04-0922) [DOI] [Google Scholar]
- 15.Tilman D. 1997. Community invasibility, recruitment limitation, and grassland biodiversity. Ecology 78, 81–92. ( 10.1890/0012-9658(1997)078%5B0081%3ACIRLAG%5D2.0.CO%3B2) [DOI] [Google Scholar]
- 16.Fridley JD, Brown RL, Bruno JF. 2004. Null models of exotic invasion and scale-dependent patterns of native and exotic species richness. Ecology 85, 3215–3222. ( 10.1890/03-0676) [DOI] [Google Scholar]
- 17.Sax DF, Brown JH. 2000. The paradox of invasion. Glob. Ecol. Biogeogr. 9, 363–371. ( 10.1046/j.1365-2699.2000.00217.x) [DOI] [Google Scholar]
- 18.Yachi S, Loreau M. 1999. Biodiversity and ecosystem productivity in a fluctuating environment: the insurance hypothesis. Proc. Natl Acad. Sci. USA 96, 1463–1468. ( 10.1073/pnas.96.4.1463) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stachowicz JJ, Bruno JF, Duffy JE. 2007. Understanding the effects of marine biodiversity on communities and ecosystems. Annu. Rev. Ecol. Evol. Syst. 38, 739–766. ( 10.1146/annurev.ecolsys.38.091206.095659) [DOI] [Google Scholar]
- 20.Dayton P. 1971. Comepetition, disturbance and community organization: the provision and subsequent utilization of space in a rocky intertidal community. Ecol. Monogr. 41, 351–389. ( 10.2307/1948498) [DOI] [Google Scholar]
- 21.Sutherland JP, Karlson RH. 1977. Development and stability of the fouling community at Beaufort NC. Ecol. Monogr. 47, 425–446. ( 10.2307/1942176) [DOI] [Google Scholar]
- 22.Fridley JD, Stachowicz JJ, Naeem S, Sax DF, Seabloom EW, Smith MD, Stohlogren TJ, Tilman D, Von Holle B. 2007. The invasion paradox: reconciling pattern and process in species invasions. Ecology 88, 3–17. ( 10.1890/0012-9658(2007)88%5B3%3ATIPRPA%5D2.0.CO%3B2) [DOI] [PubMed] [Google Scholar]
- 23.Shea K, Chesson P. 2002. Community ecology theory as a framework for biological invasions. Trends Ecol. Evol. 17, 170–176. ( 10.1016/S0169-5347(02)02495-3) [DOI] [Google Scholar]
- 24.Lockwood J, Hoopes M, Marchetti M. 2007. Invasion ecology. Oxford, UK: Blackwell Publishing. [Google Scholar]
- 25.Huston M. 1997. Hidden treatments in ecological experiments: re-evaluating the ecosystem function of biodiversity. Oecologia 110, 449–460. ( 10.1007/s004420050180) [DOI] [PubMed] [Google Scholar]
- 26.Glasby T. 1999. Interactive effects of shading and proximity to the seafloor on the development of subtidal epibiotic assemblages. Mar. Ecol. Prog. Ser. 190, 113–124. ( 10.3354/meps190113) [DOI] [Google Scholar]
- 27.Glasby T. 2001. Development of sessile marine assemblages on fixed versus moving substrata. Mar. Ecol. Prog. Ser. 215, 37–47. ( 10.3354/meps215037) [DOI] [Google Scholar]
- 28.Glasby T, Connell S. 2001. Orientation and position of substrata have large effects on epibiotic assemblages. Mar. Ecol. Prog. Ser. 214, 127–135. ( 10.3354/meps214127) [DOI] [Google Scholar]
- 29.Bird C. 2002. PhotoGrid ecological analysis of digital photos. See http//www.photogrid.netfirm.com/. [Google Scholar]
- 30.Foster MS, Harrold C, Hardin DD. 1991. Point vs. photo quadrat estimates of the cover of sessile marine organisms. J. Exp. Mar. Biol. Ecol. 146, 193–203. ( 10.1016/0022-0981(91)90025-R) [DOI] [Google Scholar]
- 31.Faul F, Erdfelder E, Buchner A, Lang AG. 2009. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav. Res. Methods 41, 1149–1160. ( 10.3758/BRM.41.4.1149) [DOI] [PubMed] [Google Scholar]
- 32.Bates D, Maechler M, Bolker B, Walker S, Christensen RHB, Singmann H, Dai B. 2014. Package ‘lme4′ version 1.1-7. See https://github.com/lme4/lme4. [Google Scholar]
- 33.Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R, pp. 101–502. New York, NY: Springer. [Google Scholar]
- 34.Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MH, White JS. 2008. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol. Evol. 24, 127–135. ( 10.1016/j.tree.2008.10.008) [DOI] [PubMed] [Google Scholar]
- 35.Akaike H. 1973. Information theory and an extension of the maximum likelihood principle. In 2nd Int. Symp. on Information Theory (eds Petrov BN, Csaki F), pp. 267–281. Budapest, Hungary: Akademiai Kiado. [Google Scholar]
- 36.Nakagawa S, Schielzeth H. 2013. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 4, 133–142. ( 10.1111/j.2041-210x.2012.00261.x) [DOI] [Google Scholar]
- 37.Chadwick-Furman N, Weissman I. 1995. Life history plasticity in chimaeras of the colonial ascidian Botryllus schlosseri. Proc. R. Soc. Lond. B 262, 157–162. ( 10.1098/rspb.1995.0190) [DOI] [PubMed] [Google Scholar]
- 38.Stohlgren T, et al. 1999. Exotic plant species invade hot spots of native plant diversity. Ecol. Monogr. 69, 25–46. ( 10.1890/0012-9615(1999)069%5B0025%3AEPSIHS%5D2.0.CO%3B2) [DOI] [Google Scholar]
- 39.Kennedy TA, Naeem S, Howe KM, Knops JM, Tilman D, Reich P. 2002. Biodiversity as a barrier to ecological invasion. Nature 417, 636–638. ( 10.1038/nature00776) [DOI] [PubMed] [Google Scholar]
- 40.Marshall D, Pechenik J, Keough M. 2003. Larval activity levels and delayed metamorphosis affect post-larval performance in the colonial ascidian Diplosoma listerianum. Mar. Ecol. Prog. Ser. 246, 153–162. ( 10.3354/meps246153) [DOI] [Google Scholar]
- 41.Ruiz GM, Fofonoff PW, Carlton JT, Wonham MJ, Hines AH. 2000. Invasion of coastal marine communities in North America: apparent patterns, processes, and biases. Annu. Rev. Ecol. Evol. Syst. 31, 481–531. ( 10.1146/annurev.ecolsys.31.1.481) [DOI] [Google Scholar]
- 42.Hurlbut C. 1991. Larval substratum selection and postsettlement mortality as determinants of the distribution of two bryozoans. J. Exp. Mar. Biol. Ecol. 147, 103–119. ( 10.1016/0022-0981(91)90040-4) [DOI] [Google Scholar]
- 43.Stachowicz JJ, Byrnes J. 2006. Species diversity, invasion success, and ecosystem functioning: disentangling the influence of resource competition, facilitation, and extrinsic factors. Mar. Ecol. Prog. Ser. 311, 251–262. ( 10.3354/meps311251) [DOI] [Google Scholar]
- 44.Carlton JT. 1996. Biological invasions and cryptogenic species. Ecology 77, 1653–1655. ( 10.2307/2265767) [DOI] [Google Scholar]
- 45.Occhipinti-Ambrogi A. 2007. Global change and marine communities: alien species and climate change. Mar. Pollut. Bull. 55, 342–352. ( 10.1016/j.marpolbul.2006.11.014) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data are available on Dryad (http://dx.doi.org/10.5061/dryad.f9801).