Abstract
Anthropogenic nutrient enrichment affects the biogeochemical cycles and nutrient stoichiometry of coastal ecosystems and is often associated with coral reef decline. However, the mechanisms by which dissolved inorganic nutrients, and especially nitrogen forms (ammonium versus nitrate) can disturb the association between corals and their symbiotic algae are subject to controversial debate. Here, we investigated the coral response to varying N : P ratios, with nitrate or ammonium as a nitrogen source. We showed significant differences in the carbon acquisition by the symbionts and its allocation within the symbiosis according to nutrient abundance, type and stoichiometry. In particular, under low phosphate concentration (0.05 µM), a 3 µM nitrate enrichment induced a significant decrease in carbon fixation rate and low values of carbon translocation, compared with control conditions (N : P = 0.5 : 0.05), while these processes were significantly enhanced when nitrate was replaced by ammonium. A combined enrichment in ammonium and phosphorus (N : P = 3 : 1) induced a shift in nutrient allocation to the symbionts, at the detriment of the host. Altogether, these results shed light into the effect of nutrient enrichment on reef corals. More broadly, they improve our understanding of the consequences of nutrient loading on reef ecosystems, which is urgently required to refine risk management strategies.
Keywords: coral, 13C, autotrophy
1. Introduction
Mutualistic symbioses between animals and algae are common in marine habitats [1]. Prominent examples include the associations of cyanobacteria with sponges and ascidians [2], algae with ciliates [3], dinoflagellates with sea anemones, sponges, clams, hydra and reef corals [4,5]. Those associations allow the animal host to exploit otherwise unsuitable food sources by acquiring or synthesizing nutrients lacking in its diet. In particular, algal symbionts fix inorganic carbon and transfer their photosynthates to the host for its own nutritional needs [6]. They can acquire other inorganic nutrients from the environment and recycle the host nitrogenous waste products as a substrate for the synthesis of high-value compounds, which are transferred back to the animal [7,8]. These symbiotic relations, however, rely on a fragile equilibrium, which can be disrupted by many stressors, such as ocean warming or acidification [9], and pollution and eutrophication (i.e. increased nutrient and sedimentation levels) [10].
The most well-known host–algal association in tropical ecosystems is formed by corals and dinoflagellates of the genus Symbiodinium. The ecological relevance of this mutualistic interaction is brought to light by two considerations: it structures reef food webs [11] and the benefits exchanged convey ecological advantages to the partners [1,12]. Coral reefs indeed rank among the most productive ecosystems, although they usually thrive in oligotrophic waters with low nutrient concentrations. Their success is due to the ‘tight recycling of nutrients' both within the ecosystem and the coral–dinoflagellate association. Yet, the nutritional ecology of such symbiosis and in particular the role of inorganic nutrient availability on the coral performances is still poorly understood [13,14]. Investigating those aspects will allow for informed conservation actions to efficiently protect coral reefs which face severe threats through climate change and human activities, including profound modifications in the seawater chemistry and nutrient concentrations [10]. On one hand, global climate change will probably result in higher water temperatures, stronger stratification and severe nutrient shortage in surface waters, limiting growth of all primary producers [15]. On the other hand, many inshore fringing reefs experience increased sedimentation and nutrient levels in response to fertilizer use and land clearing [10]. Consequently, at certain periods of the year, inorganic nitrogen and phosphorus concentrations can be much higher [16–19] than the usual levels measured on reefs (less than 0.5 µM for nitrogen [20–22], less than 0.1 µM for phosphate [23–25]). Furthermore, the geomorphology and the different sources and types of pollution, will also change the abundance of nitrogen sources (nitrate versus ammonium) and the N : P ratios of coastal ecosystems [26,27].
Although it is known that eutrophication can lead to coral overgrowth by macroalgae through the algae's fast nutrient uptake and use [28,29], the impact of nutrient availability on the functioning of the coral–dinoflagellate symbiosis is still poorly understood. To our knowledge, only one study has shown a loss of symbionts under nutrient shortage [13], so that further work is needed to fully understand the minimum nutrient requirements of corals and other comparable symbiotic associations. Conversely, the physiological effects of nutrient enrichment have been more intensively investigated. It is clear now that the supply of phosphorus induces a decrease in calcification rates or skeletal density [23,30,31], while results are more controversial for nitrogen addition. Nitrate reduced calcification and photosynthesis in some coral species [30,32], but had no effect or a positive one on other species [32–34]. The same was observed with ammonium enrichment [30,35]. Furthermore, it has been suggested that ammonium may have a different effect than nitrate, because nitrate use requires an energetically costly reduction [36,37], potentially lowering the amount of photosynthates transferred by symbionts to the host [38]. Altogether, these results suggest that there is a need to better understand the physiological processes and nutrient fluxes within the coral–dinoflagellate association, which will shed light on the functioning of other nutrient-sharing symbioses also affected by nutrient enrichments, such as the plant–mychorrizae, or chlorella–mycobacterium associations [39,40]. Therefore, the first aim of this study was to test the effect of nitrogen enrichment, either supplied in the form of ammonium or nitrate, on the acquisition of carbon and allocation of photosynthates in the tropical coral Stylophora pistillata in symbiosis with Symbiodinium clade A1. In addition, independently of the nitrogen source considered, a recent study has demonstrated that the response of corals to nutrient enrichment also depends on the balance between N and P availability, as a lack of phosphorus, coupled to nitrate enrichment, increased bleaching susceptibility [13]. The second aim of this study was to test the effect of different ammonium–phosphorus ratios on S. pistillata metabolism and photosynthates translocation, to investigate the potential role of nutrient stoichiometry on coral physiology.
2. Material and methods
(a). Experimental set-up
Six colonies of the scleractinian coral S. pistillata (Esper 1797) from the Red Sea (Gulf of Aqaba, Jordan; CITES DCI/89/32) were used to generate 240 nubbins (40 colony−1). Nubbins were suspended on a nylon thread, equally distributed into eight 20 l aquaria (5 nubbins colony−1 aquaria−1) and maintained for six weeks under the following controlled conditions: aquaria were continuously supplied with oligotrophic seawater at a flow rate of 20 l h−1, and metal halide lamps (Philips, HPIT 400 W, Distrilamp, France) provided a constant irradiance of 150 µmol photons m−2 s−1. Seawater temperature was maintained constant at 25 ± 1°C using temperature controllers (Toshniwal N6100, West Instruments, Brighton, UK) and submersible resistance heaters (Aquarium Systems, France). Nubbins were kept unfed to avoid any interaction with the nutrient enrichments.
After this first period, four inorganic nutrient conditions were generated in duplicated tanks: (i) a control condition (called ‘C’) with 0.5 µM nitrogen (N- mainly nitrate) and 0.05 µM phosphorus (P); (ii) a 0.05 µM P and 3 µM N, with addition of 2.5 µM ammonium (called ‘NH4’ condition); (iii) a 0.05 µM P and 3 µM N, with addition of 2.5 µM nitrate (called ‘NO3’); and (iv) a 1 µM P and 3 µM N (with addition of 2.5 µM NH4) enriched condition, called ‘NH4-PO4’. For this purpose, the control tanks received only natural seawater (N : P = 0.5 : 0.05 µM). For the three enriched conditions, tanks were continuously supplied with ammonium chloride (NH4Cl) or sodium nitrate (NaNO3) pumped from a stock solution via a peristaltic pump at a flow rate of 0.3 l h−1. The NH4-PO4 tank was also continuously supplied with sodium dihydrogen phosphate (NaH2PO4), delivered at the same flow rate as for nitrogen. Nutrient concentrations were monitored twice to three times a week using an autoanalyzer (Alliance Instrument, AMS, France). The total nitrogen concentration varied between 0.4 and 0.55 in the control tank, and between 3.1 and 3.5 in the other enriched conditions. Phosphorus levels varied from 0.03 to 0.05 in the control and nitrogen-enriched tanks, and from 0.8 to 1.2 in the NH4-PO4 tanks. Nubbins were incubated for three weeks under these conditions before analyses.
(b). Measurements
To assess the acquisition and exchange of photosynthates under the different conditions, we used the model fully described in Tremblay et al. [41], which follows the acquisition of external 13C-labelled bicarbonate and its allocation into the different compartments of the symbiotic association as well as the carbon lost as mucus (particulate organic carbon (POC) and dissolved organic carbon (DOC)). Carbon fluxes were calculated using the rates of photosynthesis, of host and symbiont respiration and of calcification. Symbionts density and animal tissue growth were also evaluated to complement the model.
(i). Physiological measurements
All measurements were performed on six nubbins per treatment (one per colony). Net photosynthesis (Pn) and respiration (R) rates were assessed at 0 and 150 µmoles photons m−2 s−1 on nubbins incubated in glass chambers filled with filtered seawater (FSW) containing the respective amount of inorganic nutrients. The water was homogenized using a stirring bar and each chamber was equipped with a Unisense optode connected to a computer with Oxy-4 software (Chanel fiber-optic oxygen meter, PreSens, Regensburg, Germany). Optodes were calibrated against nitrogen-saturated and air-saturated seawater for the 0% and 100% oxygen, respectively. Pn and R rates were estimated by regressing oxygen data against time. Gross photosynthetic (Pg) rates were calculated by adding R to Pn. After incubation, nubbins were frozen for the determination of symbiont density, total chlorophyll and protein concentrations according to Hoogenboom et al. [42]. Data were normalized to the skeletal surface area (cm2) of each nubbin, using the wax-dipping technique [43]. Oxygen fluxes were converted to carbon equivalent based on molar weights according to Anthony & Fabricius [44].
Respiration rates of freshly isolated symbionts, as well as their concentration, were also measured according to Tremblay et al. [41]. Rates of calcification were determined using the buoyant weight technique [45], and normalized to the nubbin surface area and the incubation time. Carbon allocation to calcification (Cc) was calculated with the following formula: Cc = Msk × 12/100, where Msk represents the amount (µg) of CaCO3 produced and 12/100 is the ratio of molecular masses of C (12 g mol−1) and CaCO3 (100 g mol−1).
(ii). H13CO3 labelling
Incubations were performed according to Tremblay et al. [41]. For each condition, 18 nubbins (three nubbins per colony) were placed in individual beakers containing 200 ml FSW with the right amount of nutrients, and enriched with 0.6 mM NaH13CO3 (98 atom %13C, no. 372382, Sigma-Aldrich, St Louis, MO, USA). They were incubated for 5 h and then transferred into non-enriched seawater for two chase periods (0 and 24 h). One nubbin per colony was removed after each period and directly frozen at −20°C for 13C measurements in the animal tissue and symbionts. Eighteen control nubbins (three nubbins per colony) were incubated in parallel in non 13C-enriched seawater. The %13C enrichment was measured using a Delta Plus Mass spectrometer coupled to a C/N analyser (Thermofisher Scientific, Bremen, Germany). The equations are described in Tremblay et al. [41]. As the results from the two chase periods (0 and 24 h) were very similar, we only report the carbon budget obtained after 24 h in the following results, for clarity.
(iii). Statistics
Statistical analyses were conducted with R statistical software v. 3.2.0 [46] by the use of the ‘stats' package. Normality and homoscedasticity of the data residuals were tested using Kolmogorov–Smirnov (using Lilliefors corrections) and Levene tests, and data were log-transformed when required. One-way ANOVAs were performed on all response variables with ‘nutrient treatment’ as factor except for the carbon incorporation rates (ρ) and percentage of fixed carbon remaining (Cr), which were tested using a two-way ANOVA, with ‘nutrient treatment’ and ‘coral compartment’ as factors (compartments are symbionts and host tissue; see the electronic supplementary material, S6). When there were significant differences between treatments, an a posteriori test was performed (Tukey's test). The Bonferroni correction was used to account for multiple testing in the 13C labelling part of the experiment. p-values were considered significant for p < 0.05.
3. Results
(a). Physiological measurements
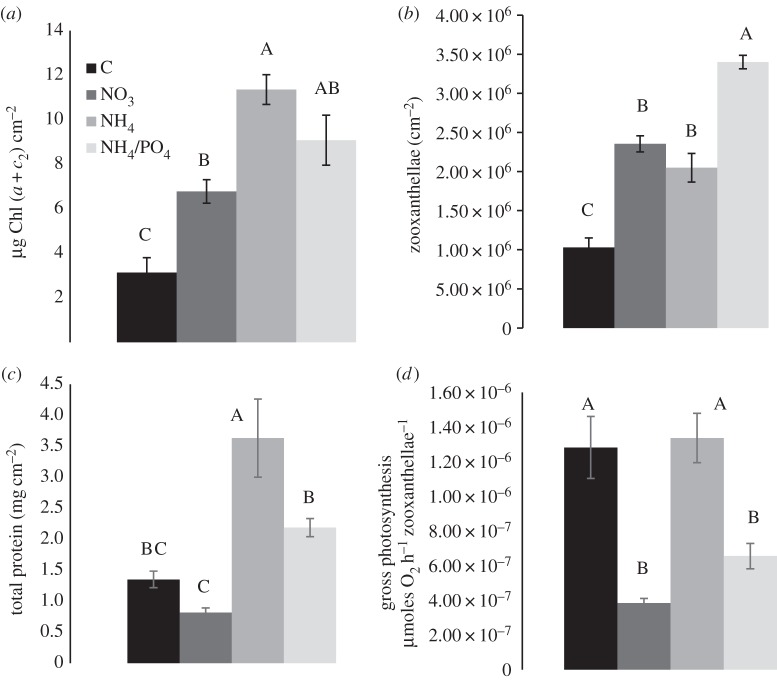
Control corals contained lower concentrations of chlorophyll (Chl) and symbionts than the nutrient-enriched nubbins (figure 1 and the electronic supplementary material, S5, Tukey HSD, p < 0.0001). Symbiont density was not different between NO3 and NH4 conditions (Tukey HSD, p = 0.39), but higher in the NH4-PO4 one (figure 3b and the electronic supplementary material, S5, Tukey HSD, p < 0.001). The amount of total chlorophyll content per symbiont remained equivalent in all conditions except for NO3 nubbins, which showed a lower amount (Tukey HSD, p < 0.001). NH4 corals synthesized the highest amount of total protein compared with the other conditions (figure 3a and the electronic supplementary material, S5, Tukey HSD, p < 0.02). For the other physiological parameters, control and NO3-conditions were different from NH4 and NH4-PO4 conditions. Indeed, control and NO3 groups presented lower calcification rates (electronic supplementary material, S5, Tukey HSD, p < 0.003), as well as lower net (Pn) and gross (Pg) photosynthesis per skeletal surface area (electronic supplementary material, S5, Tukey HSD, p < 0.01 and p < 0.001) than the NH4 groups. Moreover, NO3 corals showed significantly lower respiration rates (R) than the NH4 nubbins (Tukey HSD, p < 0.0001). Mean Pn values normalized to skeletal surface area were equal to 0.35–0.41 µmol O2 cm−2 h−1 in control and NO3 corals versus two to three times more (0.94–1.25 µmol O2 cm−2 h−1) for NH4 and NH4-PO4 nubbins. There was no significant difference in the above parameters between the NH4 and NH4-PO4 treatments (electronic supplementary material, S5, ANOVA, p > 0.05). Pg per symbiont cell was above 1 × 10−6 µmol O2 h−1 symbiont−1 in control and NH4-enriched corals, whereas it was three times lower in NO3 and NH4-PO4 corals (electronic supplementary material, S5, Tukey HSD, p < 0.001).
Figure 1.
(a) Total chlorophyll (µg Chl (a + c2) cm−2), (b) symbiont (no. zoox. cm−2), (c) protein (mg cm−2) content, and (d) gross photosynthesis rate (µmoles O2 h−1 zoox.−1) for the different treatments. Data are expressed as mean ± s.e.
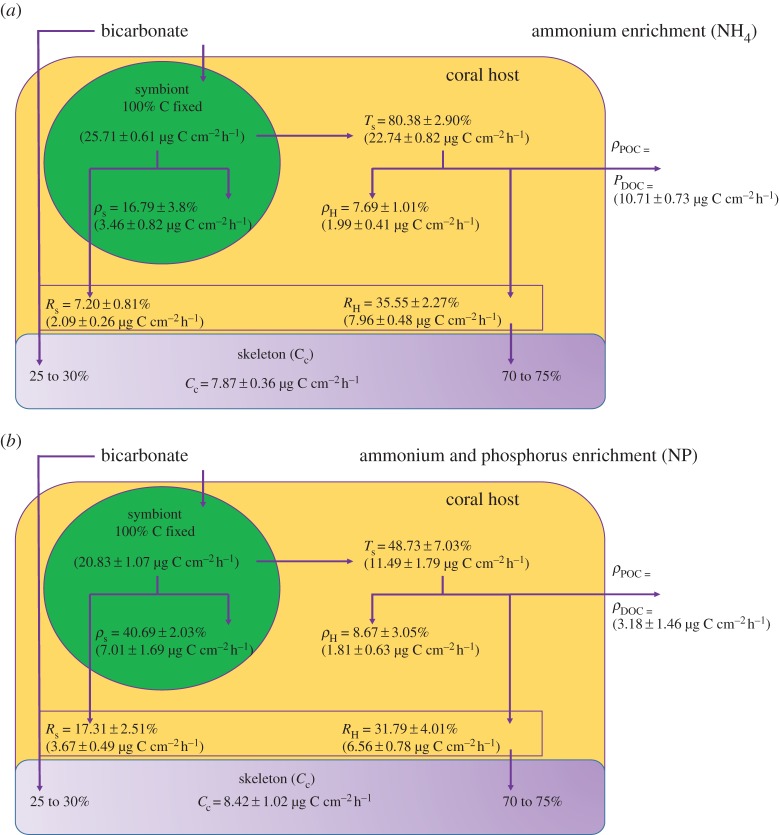
Figure 3.
Carbon budgets for corals maintained under (a) N-NH4 : P = 3 : 0.05 supply and (b) N-NH4 : P = 3 : 1 addition. Data are expressed as mean ± s.e.
(b). Carbon budget
The natural atom %13C measured in non-enriched corals ranged between 1.128 and 1.133% in symbionts, and between 1.125 and 1.130% in coral tissue. After 5 h incubation in 13C-bicarbonate, all nubbins were significantly enriched in 13C compared with control corals (atom% 13C between 1.157 and 1.180% in symbionts, and between 1.136 and 1.152% in host tissues). Only the final carbon budgets under the different conditions are reported in the results, intermediate calculations are shown in the electronic supplementary material.
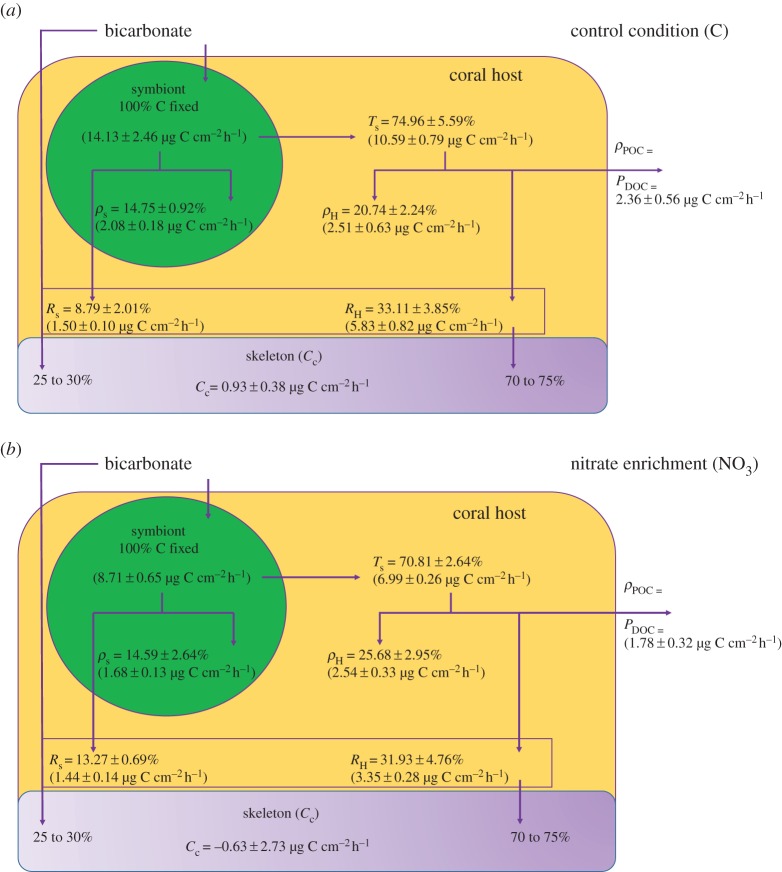
For control corals, the initial carbon fixed by symbionts (gross photosynthesis, Pg) was equal to 14.13 ± 2.46 µg C cm−2 h−1. More than two-thirds (74.96 ± 5.59% or 10.59 ± 0.79 µg C cm−2 h−1) of this carbon was translocated to the host after 24 h (figure 2a) and half was lost as respiration (ca 55%) and mucus (dissolved and particulate organic carbon, ca 23%; figure 2a). Only 20 to 25% remained both in the host and symbionts after 24 h. The final incorporation rates were thus equivalent in symbionts and host tissue (ANOVA, p > 0.05), 2.08 ± 0.18 and 2.51 ± 0.63 µg C cm−2 h−1, respectively.
Figure 2.
Carbon budgets for (a) control corals, N : P = 0.5 : 0.05 and (b) corals maintained under N-NO3 : P = 3 : 0.05 enrichment. Data are expressed as mean ± s.e.
Nutrient enrichment had significant effects on the fate of the autotrophically acquired carbon, with significant differences between NH4 or NO3 enrichments, or when ammonium was combined with PO4. NH4 alone significantly increased the amount of carbon initially fixed by the symbionts (Pg = 25.71 ± 0.61 µg C cm−2 h−1) compared with control nubbins (Tukey HSD, p = 0.001; figure 3a). It also enhanced the amount of carbon translocated after 24 h (Tukey HSD, p < 0.0001; figure 3a) in terms of total quantity (double amount, or 22.74 ± 0.82 µg C cm−2 h−1) and percentage (80.38 ± 2.90%). Eighty per cent of the translocated carbon was lost after 24 h through respiration and mucus release (ca 10.71 ± 0.73 µg C cm−2 h−1 significantly higher than in control corals, Tukey HSD, p < 0.001; figure 3a). However, the amount of carbon remaining in the host and symbionts (5.5 µg C cm−2 h−1) was still significantly higher than in the control condition.
The NH4-PO4 enrichment again changed the carbon budget compared with control corals. The initial carbon fixation rate in symbionts was intermediate between control and NH4 corals (Pg = 20.83 ± 1.07 µg C cm−2 h−1 Tukey HSD, p1 = 0.5 and p2 = 0.2). Carbon translocation rate was significantly lower than in control corals, as only 48.73 ± 7.03% were translocated after 24 h (Tukey HSD, p < 0.05), but the amount of translocated carbon stayed similar (Tukey HSD, p > 0.05). Half of the photosynthesized carbon was lost as respiration and ‘mucus' (figure 3b). The carbon incorporation rate in symbionts (40.69 ± 2.03% of the total photosynthesized carbon or 7.01 ± 1.69 µg C cm−2 h−1) was significantly higher compared with control and nitrogen-enriched corals (Tukey HSD, p < 0.002).
NO3 nubbins presented a significant lower initial carbon fixation rate compared with control corals (Tukey HSD, p < 0.03; figure 2b), with only 8.71 ± 0.65 µg C fixed cm−2 h−1. Carbon translocation rates also tended to be lower, although not significantly. More than half of the photosynthesized carbon (52%) was lost as respiration and mucus (figure 2b). Symbiont respiration and carbon incorporation rates were similar to control conditions (Tukey HSD, p > 0.05). Compared with NH4-enriched corals, initial carbon fixation rates, as well as the percentage and amount of carbon translocated were significantly lower (Tukey HSD, p < 0.002).
4. Discussion
Global climate change and other anthropogenic perturbations affect the biogeochemical cycles and the nutrient stoichiometry of marine ecosystems [47]. As organisms need stable nutrient cycles to flourish in a particular environment [48], any alteration leading to an imbalanced N : P stoichiometry will impact the organisms' health. Our study shows that the coral–dinoflagellate association is very responsive to changes in the nitrogen and phosphorus availability. We found evidence for significant differences in the acquisition of autotrophic carbon and its allocation within the symbiosis depending on the inorganic N : P ratio in seawater as well as the type of nitrogen source available. In addition, our results support the general view that the coral–algal symbiosis is nutrient limited when grown under the low dissolved N and P concentrations observed in oligotrophic environments.
After three weeks of culture at low N-P concentrations (control condition; N : P = 0.5 : 0.05), colonies showed a reduced metabolism and primary signs of nutrient limitation compared with NH4-enriched corals: i.e. lower biomass (protein concentration) and calcification rates, and a reduced symbiont growth, which is comparable to what is observed in other nutrient-limited algae [49,50]. A pronounced decrease in the symbiont density of several coral species was also previously observed under similarly low nutrient levels [13]. Conversely, in the Gulf of Eilat (Israel), colonies of S. pistillata present 30% more symbionts and from identical up to three times more areal chlorophyll content [51]. Although in situ inorganic nutrient concentrations are equivalent to those supplied in this experiment, corals benefit from heterotrophic feeding, which can constitute a significant nutrient source [52]. Interestingly, in this control poor-nutrient condition, a large amount of autotrophic carbon was still transferred to sustain the host respiratory needs. However, corals implemented a nutrient conservation strategy as little of this newly produced carbon was lost as dissolved and particulate material. These observations suggest that recovery from thermal stress-induced bleaching may be more difficult in very oligotrophic environments. Indeed, the lack of nutrient availability may limit symbiont growth and photosynthesis, and in turn the resilience of coral colonies.
The carbon budget comparison under nitrate and ammonium enrichment sheds new light into how both forms of nitrogen affect the symbiosis and helps explaining their different effects on coral metabolism. The general belief is that nitrogen enrichment alleviates the symbiont dependency on the host for nitrogen supply, resulting in higher symbiont density, higher retention of photosynthates in symbionts for their own development, at the expense of host metabolism [53,54] and in a competition between symbiont photosynthesis and host calcification for inorganic carbon use [55,56]. That theory was however challenged by studies showing a positive effect of nutrient enrichment on coral growth [35,57,58] and by the fact that some corals can thrive in high-nutrient waters [58,59]. Our results highlight major differences in the carbon budget of the symbiosis between the two forms of nitrogen enrichment. Although symbiont density under both nitrogen forms doubled compared with control corals, total carbon acquisition and translocation were significantly higher in NH4-enriched corals, inducing an enhancement in host calcification. These results are in agreement with previous studies showing that short-term ammonium enrichment in low concentration tends to enhance coral growth [35,57,58,60] and suggest that inorganic carbon supply was sufficient to cover the needs for symbiont photosynthesis and host calcification. Conversely, nitrate supplementation induced a significant decrease in the amount of carbon acquired compared with control corals, owing to lower rates of photosynthesis per symbiont cell. Compared with ammonium, both carbon acquisition and translocation were significantly and largely decreased. This is explained by the fact that nitrate reduction into ammonium is an energy and electron-consuming process [37]. Indeed, nitrite reductase, which reduces nitrite into ammonium in the chloroplasts of photosynthetic cells, uses the reduced ferredoxin from the photosynthetic chain as an electron donor and consumes six electrons to catalyse the reduction. Thus, in this reaction, electrons are lost for the photosynthetic process. On the long term, or under stress, the reduced carbon fixation under nitrate enrichment may induce a nutrient shortage and may weaken the symbiosis, as observed in corals but also in other dinoflagellate–host associations [61]. Although the detrimental effect of nitrate on photosynthesis has to be confirmed with different coral species and nitrate concentrations, excess nitrate in reef waters, which is mainly brought by industry and agriculture runoff, will probably severely affect the entire coral primary productivity. On the contrary, ammonium is the principal form of recycled nitrogen from fish excretion [60,62], although rain can also be a significant source in some reef environments [27]. Enhancing fish populations above reefs will probably be beneficial for corals, as already observed with resident fish schools [63].
The last treatment (NH4-PO4) reveals how a change in nutrient ratio in seawater can affect the animal–algal symbiosis by shifting nutrient limitation from one partner to the other. Compared to the NH4-treatment, in which P level was kept low, the NH4-PO4 enrichment induced a significant increase in symbiont density (above 3 × 106 cells cm−2). The allocation of autotrophic nutrient thus shifted from the host to the symbionts, which decreased their photosynthate translocation down to 48% and kept a large amount of photosynthetic products for their own use. In addition, owing to their high density in the host tissue, each symbiont cell was less efficient in acquiring carbon owing to a self-shading and light limitation [64,65], and/or owing to a CO2 limitation [53]. Those results suggest that differences in symbiont genotype and/or density within the host tissue may shape the response of corals to nutrient enrichment. Coral species, which host low symbiont densities and/or slow-growing symbiont genotypes are likely to be more resilient than others to eutrophication stress and also to bleaching [66]. Soft corals such as Heteroxenia fuscescens, which harbour rhythmic pulsating tentacles, can represent another potential resilient group. Pulsations indeed maintain an active water movement, which enhances photosynthesis via the fast removal of excess oxygen and the fast supply of inorganic carbon [67]. We therefore suggest that eutrophication may cause a shift in the coral species composition of the reefs, by favouring species, which can avoid carbon limitation of their symbionts.
Taken all together, our results suggest that both the nitrogen source and the N : P ratio in seawater modify the symbiotic relationship between host and algae, by affecting the autotrophic carbon acquisition and allocation. The relevance of seawater N : P ratio stoichiometry for coral health was already pointed out by Wiedenmann et al. [13]. They observed, during thermal stress, a higher decrease in photosynthetic efficiency of the symbionts under imbalanced N(nitrate ) : P ratio (3 : 0.07) than when phosphorus was available. Our data bring another dimension to the conclusions of these authors, as in both N : P imbalanced ratios presented in this study (NH4 : PO4 or NO3 : PO4 ratios: 3 : 0.05), only the nitrate-supplied condition weakened the symbiosis after three weeks. Additionally, we also showed that balanced N(ammonium ) : P ratio significantly decreased the percentage of carbon translocation in S. pistillata at the detriment of the coral host, compared with an imbalanced N(ammonium ) : P ratio, suggesting that the effect of nutrient enrichment on the coral symbiosis is more complex than previously thought. Finally, the carbon budget analysis suggests that not only the N : P but also the C : N : P ratio and the symbiont density have to be taken into account to understand the nutritional relationship between the symbionts and their host.
5. Conclusion
Nutrient-induced changes in host–symbiont interactions have been well studied in terrestrial plant–fungal associations [68], but much less is known about it in aquatic systems. There are still large gaps in our understanding on how nutrients affect marine symbioses. This study showed that nutrient concentrations and ratios differently impact the carbon acquisition and allocation within the coral–dinoflagellate symbiotic association. Future studies should further investigate nutrient combinations and ratios, as well as their synergistic effects with other stressors, such as thermal, light and acidification stresses, which all affect the acquisition and allocation of carbon between the two partners [13]. More, these experiments were run with the coral holobiont S. pistillata, in symbiosis with clade A. As symbionts from different clades present various thermal sensitivity in nitrogen metabolism [69], further research is required to assess the relevance and the effect of variation in N : P stoichiometry on coral health. Finally, our study sheds new light into how marine symbioses face eutrophication compared with other terrestrial and marine species, and is thus essential to predict the responses of communities to anthropogenic disturbances.
Supplementary Material
Acknowledgements
We thank S. Sikorski, C. Rottier and A. Labbe for laboratory assistance, D. Desgré for his great support in the experimental implementation and Prof. D. Allemand, Director of the Centre Scientifique de Monaco, for his scientific support. We are grateful to Prof. J. Wiedenmann from the University of Southampton for his valuable comments on the manuscript as well as to Dr Jean-Olivier Irisson from the Observatoire Océanologique de Villfranche-sur-mer for statistical advices and to H. K. for her proof-reading. We thank two anonymous reviewers and the associate editor for their time and effort in providing helpful suggestions and comments on the manuscript.
Ethics
The care of animals used in experimentation was in accordance with institutional guidelines under the following CITES number: DCI/89/32.
Data accessibility
Additional data supporting this article have been uploaded as part of the electronic supplementary material. All data are accessible via the following Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.361gs [70].
Authors' contributions
Conceived and designed the experiments: C.F.P., L.E. and R.G. Performed the experiments: L.E. and J.F.M. Analysed the data: L.E. Contributed reagents/materials/analysis tools: C.F.P., R.G. and J.F.M. Wrote the paper: L.E., C.F.P. and R.G.
Competing interests
We declare we have no competing interests
Funding
L.E., R.G. and C.F.P. were funded by The Centre Scientifique of Monaco. J.F.M. was funded by Institut Universitaire Européen de la Mer.
References
- 1.Venn A, Loram J, Douglas A. 2008. Photosynthetic symbioses in animals. J. Exp. Bot. 59, 1069–1080. ( 10.1093/jxb/erm328) [DOI] [PubMed] [Google Scholar]
- 2.Simister RL, Deines P, Botté ES, Webster NS, Taylor MW. 2012. Sponge-specific clusters revisited: a comprehensive phylogeny of sponge-associated microorganisms. Environ. Microbiol. 14, 517–524. ( 10.1111/j.1462-2920.2011.02664.x) [DOI] [PubMed] [Google Scholar]
- 3.Johnson MD. 2011. Acquired phototrophy in ciliates: a review of cellular interactions and structural adaptations1. J. Eukaryot. Microbiol. 58, 185–195. ( 10.1111/j.1550-7408.2011.00545.x) [DOI] [PubMed] [Google Scholar]
- 4.Muscatine L, Porter JW. 1977. Reef corals: mutualistic symbioses adapted to nutrient-poor environments. Bioscience 27, 454–460. ( 10.2307/1297526) [DOI] [Google Scholar]
- 5.Norton JH, Shepherd MA, Long HM, Fitt WK. 1992. The zooxanthellal tubular system in the giant clam. Biol. Bull. 183, 503–506. ( 10.2307/1542028) [DOI] [PubMed] [Google Scholar]
- 6.Wang J-T, Douglas AE. 1997. Nutrients, signals, and photosynthate release by symbiotic algae (the impact of taurine on the dinoflagellate alga Symbiodinium from the sea anemone Aiptasia pulchella). Plant Physiol. 114, 631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J, Douglas A. 1999. Essential amino acid synthesis and nitrogen recycling in an alga–invertebrate symbiosis. Mar. Biol. 135, 219–222. ( 10.1007/s002270050619) [DOI] [Google Scholar]
- 8.Rahav O, Dubinsky Z, Achituv Y, Falkowski P. 1989. Ammonium metabolism in the zooxanthellate coral, Stylophora pistillata. Proc. R. Soc. Lond. B 236, 325–337. ( 10.1098/rspb.1989.0026) [DOI] [Google Scholar]
- 9.Doney SC, et al. 2012. Climate change impacts on marine ecosystems. Annu. Rev. Mar. Sci. 4, 11–37. ( 10.1146/annurev-marine-041911-111611) [DOI] [PubMed] [Google Scholar]
- 10.Fabricius KE. 2011. Factors determining the resilience of coral reefs to eutrophication: a review and conceptual model. In Coral reefs: an ecosystem in transition (eds Z Dubinsky, N Stambler), pp. 493–505. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 11.Moberg F, Folke C. 1999. Ecological goods and services of coral reef ecosystems. Ecol. Econ. 29, 215–233. ( 10.1016/S0921-8009(99)00009-9) [DOI] [Google Scholar]
- 12.Yellowlees D, Rees TAV, Leggat W. 2008. Metabolic interactions between algal symbionts and invertebrate hosts. Plant Cell Environ. 31, 679–694. ( 10.1111/j.1365-3040.2008.01802.x) [DOI] [PubMed] [Google Scholar]
- 13.Wiedenmann J, D'Angelo C, Smith EG, Hunt AN, Legiret F-E, Postle AD, Achterberg EP. 2013. Nutrient enrichment can increase the susceptibility of reef corals to bleaching. Nat. Clim. Change 3, 160–164. ( 10.1038/nclimate1661) [DOI] [Google Scholar]
- 14.D'Angelo C, Wiedenmann J. 2014. Impacts of nutrient enrichment on coral reefs: new perspectives and implications for coastal management and reef survival. Curr. Opin. Environ. Sustain. 7, 82–93. ( 10.1016/j.cosust.2013.11.029) [DOI] [Google Scholar]
- 15.Elser JJ, et al. 2007. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 10, 1135–1142. ( 10.1111/j.1461-0248.2007.01113.x) [DOI] [PubMed] [Google Scholar]
- 16.Rouzé H, Lecellier G, Langlade M, Planes S, Berteaux-Lecellier V. 2015. Fringing reefs exposed to different levels of eutrophication and sedimentation can support similar benthic communities. Mar. Pollut. Bull. 92, 212–221. ( 10.1016/j.marpolbul.2014.12.016) [DOI] [PubMed] [Google Scholar]
- 17.Naumann MS, Bednarz VN, Ferse SC, Niggl W, Wild C. 2015. Monitoring of coastal coral reefs near Dahab (Gulf of Aqaba, Red Sea) indicates local eutrophication as potential cause for change in benthic communities. Environ. Monit. Assess. 187, 1–14. ( 10.1007/s10661-014-4257-9) [DOI] [PubMed] [Google Scholar]
- 18.Govers LL, Lamers LP, Bouma TJ, de Brouwer JH, van Katwijk MM. 2014. Eutrophication threatens Caribbean seagrasses: an example from Curaçao and Bonaire. Mar. Pollut. Bull. 89, 481–486. ( 10.1016/j.marpolbul.2014.09.003) [DOI] [PubMed] [Google Scholar]
- 19.Brodie J, Devlin M, Haynes D, Waterhouse J. 2011. Assessment of the eutrophication status of the Great Barrier Reef lagoon (Australia). Biogeochemistry 106, 281–302. ( 10.1007/s10533-010-9542-2) [DOI] [Google Scholar]
- 20.Furnas M, Mitchell AW, Wellington J, Brady B. 1988. Dissolved and particulate nutrients in waters of the Whitsunday Island Group. Report prepared for the Great Barrier Reef Marine Park Authority Townsville, Australia. [Google Scholar]
- 21.Lomas MW, Lipschultz F. 2006. Forming the primary nitrite maximum: nitrifiers or phytoplankton? Limnol. Oceanogr. 51, 2453–2467. ( 10.4319/lo.2006.51.5.2453) [DOI] [Google Scholar]
- 22.Meeder E, Mackey KR, Paytan A, Shaked Y, Iluz D, Stambler N, Rivlin T, Post AF, Lazar B. 2012. Nitrite dynamics in the open ocean-clues from seasonal and diurnal variations. Mar. Ecol. Prog. Ser. 453, 11–26. ( 10.3354/meps09525) [DOI] [Google Scholar]
- 23.Kinsey DW, Davies PJ. 1979. Effects of elevated nitrogen and phosphorus on coral reef growth. Limnol. Oceanogr. 24, 935–940. ( 10.4319/lo.1979.24.5.0935) [DOI] [Google Scholar]
- 24.Atkinson M. 1987. Low phosphorus sediments in a hypersaline marine bay. Estuar. Coast. Shelf Sci. 24, 335–347. ( 10.1016/0272-7714(87)90054-0) [DOI] [Google Scholar]
- 25.Charpy L. 2001. Phosphorus supply for atoll biological productivity. Coral Reefs 20, 357–360. ( 10.1007/s00338-001-0182-9) [DOI] [Google Scholar]
- 26.Conley DJ, Paerl HW, Howarth RW, Boesch DF, Seitzinger SP, Havens KE, Lancelot C, Likens GE. 2009. Controlling eutrophication: nitrogen and phosphorus. Science 323, 1014–1015. ( 10.1126/science.1167755) [DOI] [PubMed] [Google Scholar]
- 27.Altieri K, Hastings M, Peters A, Oleynik S, Sigman D. 2014. Isotopic evidence for a marine ammonium source in rainwater at Bermuda. Glob. Biogeochem. Cycles 28, 1066–1080. ( 10.1002/2014GB004809) [DOI] [Google Scholar]
- 28.Fabricius KE. 2005. Effects of terrestrial runoff on the ecology of corals and coral reefs: review and synthesis. Mar. Pollut. Bull. 50, 125–146. ( 10.1016/j.marpolbul.2004.11.028) [DOI] [PubMed] [Google Scholar]
- 29.Burkepile DE, Allgeier JE, Shantz AA, Pritchard CE, Lemoine NP, Bhatti LH, Layman CA. 2013. Nutrient supply from fishes facilitates macroalgae and suppresses corals in a Caribbean coral reef ecosystem. Sci. Rep. 3, 1493 ( 10.1038/srep01493) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrier-Pages C, Gattuso J-P, Dallot S, Jaubert J. 2000. Effect of nutrient enrichment on growth and photosynthesis of the zooxanthellate coral Stylophora pistillata. Coral Reefs 19, 103–113. ( 10.1007/s003380000078) [DOI] [Google Scholar]
- 31.Dunn JG, Sammarco PW, LaFleur G. 2012. Effects of phosphate on growth and skeletal density in the scleractinian coral Acropora muricata: a controlled experimental approach. J. Exp. Mar. Biol. Ecol. 411, 34–44. ( 10.1016/j.jembe.2011.10.013) [DOI] [Google Scholar]
- 32.Schlöder C, D'Croz L. 2004. Responses of massive and branching coral species to the combined effects of water temperature and nitrate enrichment. J. Exp. Mar. Biol. Ecol. 313, 255–268. ( 10.1016/j.jembe.2004.08.012) [DOI] [Google Scholar]
- 33.Chauvin A, Denis V, Cuet P. 2011. Is the response of coral calcification to seawater acidification related to nutrient loading? Coral Reefs 30, 911–923. ( 10.1007/s00338-011-0786-7) [DOI] [Google Scholar]
- 34.Tanaka Y, Miyajima T, Koike I, Hayashibara T, Ogawa H. 2007. Imbalanced coral growth between organic tissue and carbonate skeleton caused by nutrient enrichment. Limnol. Oceanogr. 52, 1139–1146. ( 10.4319/lo.2007.52.3.1139) [DOI] [Google Scholar]
- 35.Béraud E, Gevaert F, Rottier C, Ferrier-Pagès C. 2013. The response of the scleractinian coral Turbinaria reniformis to thermal stress depends on the nitrogen status of the coral holobiont. J. Exp. Biol. 216, 2665–2674. ( 10.1242/jeb.085183) [DOI] [PubMed] [Google Scholar]
- 36.Patterson K, Cakmak T, Cooper A, Lager I, Rasmusson AG, Escobar MA. 2010. Distinct signalling pathways and transcriptome response signatures differentiate ammonium-and nitrate-supplied plants. Plant Cell Environ. 33, 1486–1501. ( 10.1111/j.1365-3040.2010.02158.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dagenais-Bellefeuille S, Morse D. 2013. Putting the N in dinoflagellates. Front. Microbiol. 4, 369 ( 10.3389/fmicb.2013.00369) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shantz AA, Burkepile DE. 2014. Context-dependent effects of nutrient loading on the coral-algal mutualism. Ecology 95, 1995–2005. ( 10.1890/13-1407.1) [DOI] [PubMed] [Google Scholar]
- 39.Johnson NC, Rowland DL, Corkidi L, Egerton-Warburton LM, Allen EB. 2003. Nitrogen enrichment alters mycorrhizal allocation at five mesic to semiarid grasslands. Ecology 84, 1895–1908. ( 10.1890/0012-9658(2003)084%5B1895%3ANEAMAA%5D2.0.CO%3B2) [DOI] [Google Scholar]
- 40.Kim H-J, et al. 2015. Growth promotion of Chlorella vulgaris by modification of nitrogen source composition with symbiotic bacteria, Microbacterium sp. HJ1. Biomass Bioenergy 74, 213–219. ( 10.1016/j.biombioe.2015.01.012) [DOI] [Google Scholar]
- 41.Tremblay P, Grover R, Maguer JF, Legendre L, Ferrier-Pagès C. 2012. Autotrophic carbon budget in coral tissue: a new 13C-based model of photosynthate translocation. J. Exp. Biol. 215, 1384–1393. ( 10.1242/jeb.065201) [DOI] [PubMed] [Google Scholar]
- 42.Hoogenboom M, Beraud E, Ferrier-Pagès C. 2010. Relationship between symbiont density and photosynthetic carbon acquisition in the temperate coral Cladocora caespitosa. Coral Reefs 29, 21–29. ( 10.1007/s00338-009-0558-9) [DOI] [Google Scholar]
- 43.Veal C, Carmi M, Fine M, Hoegh-Guldberg O. 2010. Increasing the accuracy of surface area estimation using single wax dipping of coral fragments. Coral Reefs 29, 893–897. ( 10.1007/s00338-010-0647-9) [DOI] [Google Scholar]
- 44.Anthony K, Fabricius KE. 2000. Shifting roles of heterotrophy and autotrophy in coral energetics under varying turbidity. J. Exp. Mar. Biol. Ecol. 252, 221–253. ( 10.1016/S0022-0981(00)00237-9) [DOI] [PubMed] [Google Scholar]
- 45.Davies PS. 1989. Short-term growth measurements of corals using an accurate buoyant weighing technique. Mar. Biol. 101, 389–395. ( 10.1007/BF00428135) [DOI] [Google Scholar]
- 46.R Development Core Team. 2015. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 47.Sterner RW, Elser JJ. 2002. Ecological stoichiometry: the biology of elements from molecules to the biosphere. Princeton, NJ: Princeton University Press. [Google Scholar]
- 48.Arrigo KR. 2005. Marine microorganisms and global nutrient cycles. Nature 437, 349–355. ( 10.1038/nature04159) [DOI] [PubMed] [Google Scholar]
- 49.Vanucci S, et al. 2012. Nitrogen and phosphorus limitation effects on cell growth, biovolume, and toxin production in Ostreopsis cf.ovata. Harmful Algae 15, 78–90. ( 10.1016/j.hal.2011.12.003) [DOI] [Google Scholar]
- 50.Moore C, et al. 2013. Processes and patterns of oceanic nutrient limitation. Nat. Geosci. 6, 701–710. ( 10.1038/ngeo1765) [DOI] [Google Scholar]
- 51.Mass T, Kline D, Roopin M, Veal C, Cohen S, Iluz D, Levy O. 2010. The spectral quality of light is a key driver of photosynthesis and photoadaptation in Stylophora pistillata colonies from different depths in the Red Sea. J. Exp. Biol. 213, 4084–4091. ( 10.1242/jeb.039891) [DOI] [PubMed] [Google Scholar]
- 52.Houlbreque F, Tambutte E, Richard C, Ferrier-Pages C. 2004. Importance of a micro-diet for scleractinian corals. Mar. Ecol. Prog. Ser. 282, 151–160. ( 10.3354/meps282151) [DOI] [Google Scholar]
- 53.Dubinsky Z, Stambler N, Ben-Zion M, McCloskey L, Muscatine L, Falkowski P. 1990. The effect of external nutrient resources on the optical properties and photosynthetic efficiency of Stylophora pistillata. Proc. R. Soc. Lond. B 239, 231–246. ( 10.1098/rspb.1990.0015) [DOI] [Google Scholar]
- 54.Wooldridge SA, Done TJ. 2009. Improved water quality can ameliorate effects of climate change on corals. Ecol. Appl. 19, 1492–1499. ( 10.1890/08-0963.1) [DOI] [PubMed] [Google Scholar]
- 55.Stambler N, Popper N, Dubinsky Z, Stimson J. 1991. Effects of nutrient enrichment and water motion on the coral Pocillopora damicornis. Pac. Sci. 45, 299–307. [Google Scholar]
- 56.Marubini F, Davies P. 1996. Nitrate increases zooxanthellae population density and reduces skeletogenesis in corals. Mar. Biol. 127, 319–328. ( 10.1007/BF00942117) [DOI] [Google Scholar]
- 57.Langdon C, Atkinson M. 2005. Effect of elevated pCO2 on photosynthesis and calcification of corals and interactions with seasonal change in temperature/irradiance and nutrient enrichment. J. Geophys. Res. Oceans (1978–2012) 110, C09507 ( 10.1029/2004JC002576) [DOI] [Google Scholar]
- 58.Bongiorni L, Shafir S, Angel D, Rinkevich B. 2003. Survival, growth and gonad development of two hermatypic corals subjected to in situ fish-farm nutrient enrichment. Mar. Ecol. Prog. Ser. 253, 137–144. ( 10.3354/meps253137) [DOI] [Google Scholar]
- 59.Szmant AM. 2002. Nutrient enrichment on coral reefs: is it a major cause of coral reef decline? Estuaries 25, 743–766. ( 10.1007/BF02804903) [DOI] [Google Scholar]
- 60.Meyer JL, Schultz ET. 1985. Migrating haemulid fishes as a source of nutrients and organic matter on coral reefs. Limnol. Oceanogr. 30, 146–156. ( 10.4319/lo.1985.30.1.0146) [DOI] [Google Scholar]
- 61.Uthicke S, Vogel N, Doyle J, Schmidt C, Humphrey C. 2012. Interactive effects of climate change and eutrophication on the dinoflagellate-bearing benthic foraminifer Marginopora vertebralis. Coral Reefs 31, 401–414. ( 10.1007/s00338-011-0851-2) [DOI] [Google Scholar]
- 62.Holbrook SJ, Brooks AJ, Schmitt RJ, Stewart HL. 2008. Effects of sheltering fish on growth of their host corals. Mar. Biol. 155, 521–530. ( 10.1007/s00227-008-1051-7) [DOI] [Google Scholar]
- 63.Meyer JL, Schultz ET, Helfman GS. 1983. Fish schools: an asset to corals. Science 220, 1047–1049. ( 10.1126/science.220.4601.1047) [DOI] [PubMed] [Google Scholar]
- 64.Enríquez S, Méndez ER, Prieto RI. 2005. Multiple scattering on coral skeletons enhances light absorption by symbiotic algae. Limnol. Oceanogr. 50, 1025–1032. ( 10.4319/lo.2005.50.4.1025) [DOI] [Google Scholar]
- 65.Lesser MPL, Mazel C, Phinney D, Yentsch CS. 2000. Light absorption and utilization by colonies of the congeneric hermatypic corals Montastraea faveolata and Montastraea cavernosa. Limnol. Oceanogr. 45, 76–86. ( 10.4319/lo.2000.45.1.0076) [DOI] [Google Scholar]
- 66.Cunning R, Baker AC. 2013. Excess algal symbionts increase the susceptibility of reef corals to bleaching. Nat. Clim. Change 3, 259–262. ( 10.1038/nclimate1711) [DOI] [Google Scholar]
- 67.Kremien M, Shavit U, Mass T, Genin A. 2013. Benefit of pulsation in soft corals. Proc. Natl Acad. Sci. USA 110, 8978–8983. ( 10.1073/pnas.1301826110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ryan GD, Rasmussen S, Xue H, Parsons AJ, Newman JA. 2014. Metabolite analysis of the effects of elevated CO2 and nitrogen fertilization on the association between tall fescue (Schedonorus arundinaceus) and its fungal symbiont Neotyphodium coenophialum. Plant Cell Environ. 37, 204–212. ( 10.1111/pce.12146) [DOI] [PubMed] [Google Scholar]
- 69.Baker DM, Andras JP, Jordán-Garza AG, Fogel ML. 2013. Nitrate competition in a coral symbiosis varies with temperature among Symbiodinium clades. ISME J. 7, 1248–1251. ( 10.1038/ismej.2013.12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ezzat L, Maguer JF, Grover R, Ferrier-Pagès C. Data from: new insights into carbon acquisition and exchanges within the coral-dinoflagellate symbiosis under NH 4 + and NO 3 − supply. Dryad Data Repository. See 10.5061/dryad.361gs. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Additional data supporting this article have been uploaded as part of the electronic supplementary material. All data are accessible via the following Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.361gs [70].