Abstract
During the twentieth century, Amazonia was widely regarded as relatively pristine nature, little impacted by human history. This view remains popular despite mounting evidence of substantial human influence over millennial scales across the region. Here, we review the evidence of an anthropogenic Amazonia in response to claims of sparse populations across broad portions of the region. Amazonia was a major centre of crop domestication, with at least 83 native species containing populations domesticated to some degree. Plant domestication occurs in domesticated landscapes, including highly modified Amazonian dark earths (ADEs) associated with large settled populations and that may cover greater than 0.1% of the region. Populations and food production expanded rapidly within land management systems in the mid-Holocene, and complex societies expanded in resource-rich areas creating domesticated landscapes with profound impacts on local and regional ecology. ADE food production projections support estimates of at least eight million people in 1492. By this time, highly diverse regional systems had developed across Amazonia where subsistence resources were created with plant and landscape domestication, including earthworks. This review argues that the Amazonian anthrome was no less socio-culturally diverse or populous than other tropical forested areas of the world prior to European conquest.
Keywords: plant domestication, landscape domestication, Amazonian dark earths, population estimates, complex societies
1. Introduction
The word ‘Amazonia’ conjures images of dense rainforests, painted and feathered natives, exotic fauna and flora, as well as rampant deforestation, biodiversity extinction, and climate change. These fragmentary images seldom coalesce into robust understanding of this vast area, which is partially a legacy of eighteenth to nineteenth century descriptions with already decimated human populations [1]. Over the past few decades, archaeology has revealed numerous fairly large-scale complex societies across all major regions, which contrast with the small-scale twentieth century societies described by anthropologists, but agree well with initial European eye witness accounts from the sixteenth to seventeenth century [2–6]. The current consensus among historical ecologists suggests that Amazonia is a complex mosaic of coupled human-natural systems, typical of anthropogenic biomes or anthromes globally [7], refuting earlier claims of uniform environmental limitations [8,9]. Here, we summarize the nature and extent of these transformations during the Holocene to show that Amazonia was no more limiting than tropical forested regions elsewhere across the globe. Amazonia was domesticated before European conquest.
As elsewhere, human societies dramatically modified species composition in many ecosystems, beginning in some areas by the early Holocene and giving rise to complex and sophisticated systems of land management associated with large, settled populations by European conquest. Specifically, large pre-Columbian societies domesticated large portions of their landscape to make them more productive and congenial [10], as expected in cultural niche construction theory [11]. The modifications of species and ecosystems are due to domestication, both of plant and animal populations and of landscapes [10,12–14]. Growing populations caused long-term modifications in soils, creating Amazonian dark earths (ADEs), and transformed naturally biodiverse forests into anthropogenic forest landscapes [3,12,15,16].
This revisionist view of a domesticated Amazonia is contested by some natural and social scientists [17–20]. These critiques are based on small samples that are used to extrapolate across the region, often without engagement with the full breadth of scholarship on pre-Columbian Amazonia. Most commentators agree that Amazonia was occupied by societies with different levels of complexity [2] and each had different impacts on their landscapes. There were dense populations along some resource-rich sections of major rivers, less dense populations along minor rivers and sparse populations between rivers [15]. Given the antiquity and intensity of these impacts, few—if any—pristine landscapes remained in 1492. There were anthropogenic forests throughout the basin, and an overall population and landscape footprint far greater than argued recently. Resolving these views has obvious implications for indigenous cultural heritage.
2. Plant management and domestication
Amazonia is a major world centre of plant domestication, where selection began in the Late Pleistocene to Early Holocene in peripheral parts of the basin [10,21–24]. By European contact, at least 83 native species were domesticated to some degree, including manioc, sweet potato, cacao, tobacco, pineapple and hot peppers, as well as numerous fruit trees and palms, and at least another 55 imported neotropical species were cultivated [10]. Plant domestication is a long-term process in which natural selection interacts with human selection driving changes that improve usefulness to humans and adaptations to domesticated landscapes [10,25,26]. Hence, there is a continuum from incipient change to fully domesticated status, where the plants depend upon humans for their survival [25]. In Amazonia, plant management was a particularly important part of subsistence strategies [27], including 3000–5000 exploited non-domesticated species [28], following the expectations of cultural niche construction theory [29,30].
Small-scale societies practiced foraging and casual horticulture across Amazonia throughout the Early and Mid-Holocene, and substantially altered forest composition through diverse activities around villages and camp sites, along trails and in fallow fields, and via the unintentional interactions and changes in local ecology precipitated by these activities [12,31–38]. Foragers acted throughout Amazonia [17], and their promotion and management of forest resources—although not intensive locally—is more spatially extensive than that of farmers [38,39]. These changes favour useful plants and animals and, although subtle, this minimal level of landscape domestication results in enduring and dramatic anthropogenic footprints in a variety of settings, particularly when considered at centennial and millennial scales [10,16,38]. While plant domestication is driven by selection and propagation, landscape domestication concerns the demography of a variety of useful and domesticated plants, and their interactions with settlement features, soils, earthworks and fluvial works [10,14,15,40].
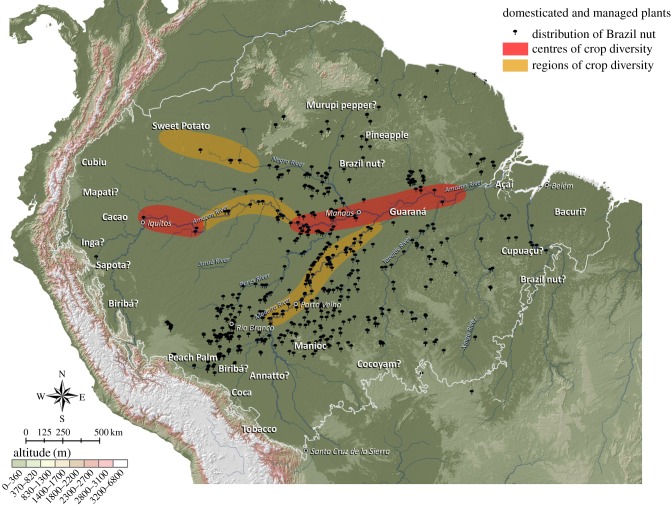
In Amazonia, the transition from primarily foraging to developed farming systems occurred by ca 4000 BP, as formerly casual cultivation in home gardens and managed forests was transformed by larger and more settled populations [23,41], although the timing and intensity of these changes varied significantly across the basin [27]. As populations expanded, they accumulated crop genetic resources, creating centres of crop genetic diversity (figure 1). These centres provide strong evidence that pre-conquest human populations had intensively transformed and diversified their plant resources [10,21]. Large-scale human population expansions during the Holocene generally depended upon farming technologies, which often provided an adaptive advantage over small foraging groups [42,43]. In Amazonia, this included fairly intensive arboriculture, as well as staple root and seed crops [10]. The first Amazon River chroniclers reported an abundance of well-fed populations along the bluffs, surrounded by orchards on the uplands and seasonal fields in the floodplains [5,6,44].
Figure 1.
Plant management and domestication in Amazonia. The names of species identify known or suspected (with ‘?’) origins of domestication of 20 native Amazonian crop species [24]. The centres and regions of crop genetic diversity contain significant or moderate concentrations of accumulated crop genetic resources [21]. See the electronic supplementary material for Brazil nut.
Fully domesticated species comprised part of emerging farming systems, including arboriculture, but incipient or semi-domesticated species were often managed in forests [10,16,38]. Some forests were highly modified, such as the widely dispersed Brazil nut stands [37], whereas others became high-diversity anthropogenic forests [12,16,38]. Other forests are oligarchic—dominated by a single species—and occupy extensive areas across Amazonia; some of these were managed to enhance yields [45]. For instance, Açaí-do-pará (Euterpe oleracea), which dominates thousands of square kilometres in the Amazon River estuary [45], was a major subsistence resource for the mound-building Marajoara society [3,13]. Many present Amazonian forests, while seemingly natural, are domesticated to varying degrees in terms of altered plant distributions and densities [16,37,38], because trees are long lived [10,16].
The degree of vegetation modification around villages varied significantly, with cultivated fields and orchards close by, surrounded by managed forests with decreasing evidence of management as distance from the village increased [16]. This is also supported by palaeo-ecological and archeobotanical evidence [18–20,46]. The extent of these ‘low-intensity’ anthropogenic forests is considerable: recent data from the Purus–Madeira interfluve suggest that the concentration of useful species is detectable as much as 40 km from major and even minor rivers [16]. Barlow et al. [17] suggest that these interfluvial forests, which comprise the vast majority of the region, were used for foraging but not actively managed, and are therefore viewed as essentially natural. However, they ignore the fact that foragers modify forests along trails and at campsites (see above), and that we are discussing thousands of years of activities. Considering the dense river and stream network that covers most of Amazonia [47], that tributaries often have as many archaeological sites as the main rivers [48,49], that tributaries often have as many Brazil nut stands as main rivers (figure 1) and that these stands are often associated with ADE sites [50], it is likely that a significant portion of Amazonian forests was modified to some degree and remains so today.
These conclusions are critiqued in two recent studies [19,20], based on sampling in three and four locations in western Amazonia, respectively, where phytolith and charcoal analysis did not identify extensive land-use change. Curiously, these studies ignore the expectation that land-use change is more pronounced near settlements than further away [16,18]. Although the authors affirm that phytoliths are diagnostic in a small number of families, they conclude that interfluvial forests were little modified during the last millennia and that therefore the entire western Amazon was sparsely populated. They fail to engage with the evidence presented here, which seldom is visible with phytoliths and seldom requires extensive use of fire, but is visible with other botanical and ecological techniques.
3. Anthropogenic soils and earthworks
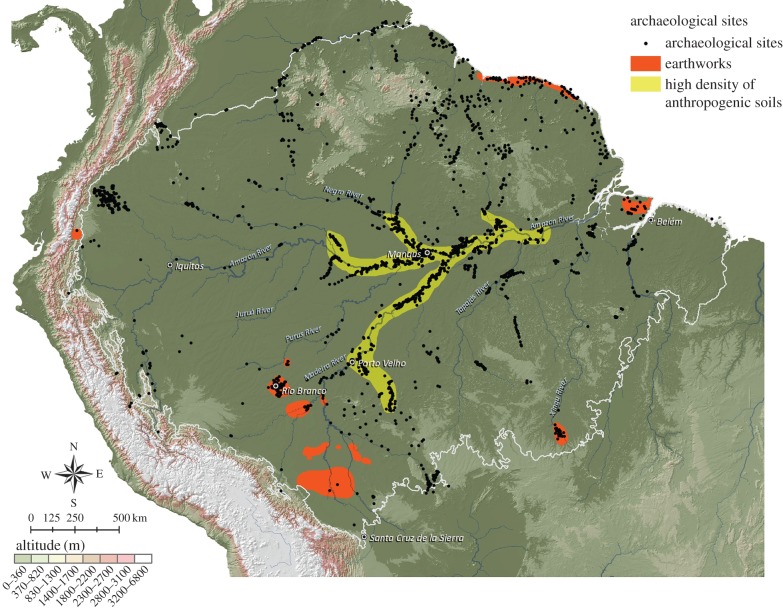
Amazonia is dominated by nutrient-poor soils in uplands, including dystrophic Ferralsols and Acrisols in central and northwestern Amazonia and moderately fertile Cambisols in southwestern Amazonia [51]. However, greater than 10% of Amazonian soils are naturally nutrient-sufficient or even nutrient-rich, such as Gleysols and Fluvisols in floodplains and palaeo-floodplains that total greater than 40 000 km2 in Brazil alone. Recent studies have documented the frequent presence of anthrosols [52] associated with fairly settled societies, with significantly enhanced nutrients and carbon, as is true across the globe during the Late Holocene [53]. These ADEs are concentrated along bluffs overlooking major and minor rivers [44,49], but are also found in higher floodplain levels [54] and interfluves. ADE sites are widely dispersed across a mosaic of landscapes (figure 2) and have the potential to feed millions of inhabitants.
Figure 2.
Selected archaeological sites in lowland South America, including concentrations of known earthworks and anthropogenic soils (based on WinklerPrins & Aldrich [52] and two decades of survey work by E.G. Neves and colleagues from the University of São Paulo).
ADEs are the result of human waste management in and around settlements, and intentional burning, mulching and composting in agricultural areas [53,55,56]. ADE sites appear in parts of the Amazon in the sixth millennia BP [57], but increase rapidly in number and size after ca 2500 BP, associated with the expansion of sedentary societies [58]. Native Amazonians used an array of technologies and plant species in a multitude of food production systems, and some of these included intentional and non-intentional improvement of soil quality [15,41,55,59]. It is now well accepted that dump heaps around human settlements gave rise to the extremely dark ADE, called terra preta [58]. Surrounding the ‘core’ areas of ADE sites are often found extensive anthropogenic soils with lighter colour and lower concentrations of nutrients and ceramic fragments, which reflect the residues of farming systems around settlements [41,60].
The extent of soil modification in situ, while extensive, is only a fraction of pre-Columbian domesticated landscapes, which often involved regional planning and sophisticated local engineering. Diverse earthworks (ceremonial, habitation, monumental, burial, agricultural), all highly visibly features of these landscapes, have been identified in dozens of areas, although most were only discovered in recent decades with the development of in-depth archaeology, remote sensing and deforestation. Identified are tens of thousands of raised fields in the Llanos de Mojos of Bolivia, the Guiana coasts, Amapá in northeast Brazil and the Orinoco Llanos; many hundreds of kilometres of causeways and roads in the Xingu, Mojos, Orinoco, Guianas and Central Amazonia regions; canals, artificial cuts between river meander bends, artificial ponds and fish weirs in the Mojos, Xingu, middle Amazon, Belterra Plateau and Marajó Island regions and integrated networks of settlement features, including mounds, plazas, ditches, walls and roads, in numerous areas [3,13,15,40,61–65].
4. Population
The scale of pre-European human impacts on Amazonian landscapes of Amazonia, in terms of intensity, form and distribution, are related to native population totals and densities. Estimates of 1492 human population vary widely, given the minimal documentary evidence prior to recent times. Conservative estimates of one to two million people are based on current or recent (past 200 years) information (tribal counts or estimates, and densities), which do not account for demonstrable catastrophic depopulation from epidemics, starvation, slavery and brutality soon after 1492. Most pre-1996 estimates for Greater Amazonia suggest up to six million people [66].
Soil creation and landscape engineering for settlements and production of domesticated and managed crops fuelled population expansion. Recent estimates of the extent of ADE suggest even larger population totals. Sombroek et al. [67] estimated that 0.1–0.3% of forested Amazonia contains ADE, although this estimate may be too conservative considering that some tributaries have high densities of ADE [48,49]. Using 0.2% (12 600 km2 out of 6.3 million km2), 10% in cultivation in any 1 year, and three methods based on a maize staple, a manioc staple and rates of phosphorus deposition, produces estimated ADE populations of 3.1, 3.8 and 3.3 million, respectively [68]. With a tentative five to six million for the remainder of Greater Amazonia, the estimated minimum population would be in the range of 8–10 million, with an average density of 0.66–0.81 per km2 [68].
A recent model suggests that ‘terra pretas are likely to be found throughout ca 154 063 square kilometers or 3.2% of the [Amazonian] forest’ [49, p. 1]. Although the model has limitations, including the presence of ADE in a wide range of settings that do not conform to model expectations, notably interfluvial areas of both western and eastern Amazonia, it strongly supports Levis et al.'s [48] observation that ADE sites are very abundant on tributaries. It also supports the evidence that occupation sites were concentrated on river bluffs [44], also supported by early eyewitness accounts of linear bluff villages extending for several leagues and numbering in the thousands of people along the major rivers in the 1500–1600s [4–6]. Another problem with McMichael et al.'s [49] conclusions is that using the Woods et al. [68] methods just outlined, 3.2% ADE would mean an unlikely 50 million people, hence large areas where ADE is likely to occur in fact do not contain these anthropogenic soils.
Interfluve settlements are generally believed to have consisted of small, constantly shifting villages and nomadic or semi-nomadic hunter–gatherers. While such were undoubtedly common, this is an excessive generalization. There are numerous reports from the sixteenth century to the early-twentieth century of interfluve villages numbering 1000–1500 people, with some having as many as 5000–10 000 [66]. In the upper Xingu region, numerous late pre-Columbian villages as large as 50 ha with populations of 800–1000 or more, organized in densely spaced clusters allow a conservative estimate of a regional population of 50 000 [69]. In seasonally flooded savannahs (Marajó Island, Llanos de Mojos, Orinoco Llanos), village sites associated with earthworks have been suggested to have had 1000 or more people [66]. Tributaries of the Madeira and upper Amazon contain ADE sites up to 40 ha in area, similar to the lower range of larger sites along the main rivers [48]. Given that 30% of Amazonia is occupied by wetlands [47], often associated with bluffs suitable for large villages [49], large unstudied areas throughout Amazonia could have supported complex societies.
5. Late Holocene domesticated landscapes
The transition from subsistence based principally on foraging and small-scale food production to farming started by approximately 4000 BP [3,23], with regional variation [27]. During the Late Holocene, regional population and socio-political complexity within integrated polities increased in numerous areas and networks of interaction were intensified and formalized, linking societies in broad regional political economies [2,3,70]. The net effects, the result of millennia of occupation in many cases, were highly domesticated subregions across Amazonia within networks of greater and lesser anthropogenic impacts criss-crossing the tropical forests. The current consensus is that numerous large pre-Columbian societies existed by the Late Holocene, with regional socio-political integration and broad interaction networks typical of socio-cultural and geo-political variation observed in other world areas [71].
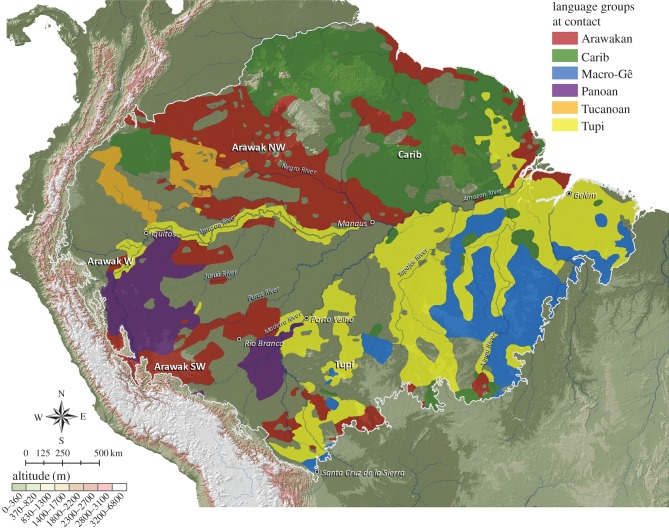
The initial impetus for these changes, alongside development in regional systems, was the influence and actual movements of early farmers associated with ancestors of major linguistic families, especially Arawak, Tupi–Guarani and Carib, and several smaller groups, e.g. Pano, Tukano [72–78]. The Arawak family originated in broadly defined western Amazonia and expanded across much of riverine Amazonia, which was associated with early development of farming villages, an Amazonian case of farmer language expansion [2,42,72,79]. They dominated significant areas along major rivers and their headwaters, and were recognized for diverse semi-intensive and intensive landscape management strategies and broad interaction networks they maintained across vast areas [80] (figure 3; see also map in Eriksen [78, p. 222]).
Figure 3.
Distribution of major and some minor Amazonian linguistic groups at contact, following Eriksen ([78]; used with permission). Language names in the map represent suspected origins [78]. Apparently empty areas were inhabited by other minor language groups and linguistic isolates.
Expansions of speakers of the Macro-Tupi and, particularly, Tupi–Guarani family languages (originating in southwestern Amazonia) and the Carib family (in northern Amazonia) were primarily in upland areas [76,77,81]. Movements along small rivers and across interfluvial areas expanded through significant parts of Amazonia, somewhat after the Arawak expansion started [78]. Although they were already horticultural, they may not represent farmer-language expansions similar to Arawak [42]. In many regions, there was substantial presence of diverse groups, including interaction networks and multi-ethnic societies. In central Amazonia, Tupi–Guarani speakers occupied Arawak villages and subsequent Tupi villages were smaller [70,75,82].
By Late Holocene times, enclaves of socio-politically linked peer-polities existed across Greater Amazonia, particularly in three broad macroregions: the Amazon River floodplains and adjacent areas, including the estuary, and the broad northern and southern borderland areas [2]. Earlier mound-building complexes were occupied from 3000 BP, including Sangay in Ecuador, Guyana, the upper Madeira and Purus, and the lower Amazon [3,13,63,65,82]. Substantial domestic and ceremonial earthworks dating to the past two millennia have been identified along the Amazon River floodplain and in northern and southern Amazonian borderlands, particularly in seasonally inundated areas [3,13,65,78,80].
Early descriptions mentioned numerous villages along the Ucayali [4] and Amazon Rivers [5,6]. Each occupied 10–50 ha and numbered several thousand people; some were linked by roads to inland areas [6]. There were larger centres, such as Santarém, at the mouth of the Tapajós River, which comprised a network of occupation areas (up to 50 ha) that together occupied 400 ha [3,13,83]. In Central Amazonia, an eight millennia history of occupations culminated on the eve of conquest in a multi-ethnic regional polity similar in settlement patterns to those documented in the sixteenth century [70,84]. These large centres were among the first native societies to succumb to European conquest [1].
In the northern borderlands, including Marajó Island, Amapá, coastal Guianas and middle-lower Orinoco, there are well-documented cases of settled regional polities by 2000 BP with agricultural and wetland earthworks and monumental architecture [63,80]. By 1500–500 BP, these typically Arawak-speaking societies included large, powerful regional peer-polities that extended into upland areas of the Guiana plateau and pre-Andean areas, notably the western Orinoco [85] and the coastal Guianas [63]. Core settlements were structurally elaborated in production, communication and ritual landscapes. Complex heterarchical polities and regional confederations were typical of areas away from major rivers, and were linked in sacred geographies that connected regions across many parts of Amazonia [13,80].
In the southern borderlands, complex settled regions are focused on major headwater basins of the Xingú, Tapajós, Madeira and Purus, as well as densely settled areas along the eastern margins, including the Tocantins. In these areas, there is substantial landscape modification related to large, permanent settlements, intensive agriculture, well-established communication networks, including monumental sites with regional interaction. The best known cases are the Llanos de Mojos in Bolivia [40,61,86], Acre in southwestern Brazilian Amazonia [13] and the upper Xingú in central Brazil [69,87] (figures 2 and 3), but ethno-history and preliminary archaeological surveys suggest wider distributions across southern Amazonia, including the upper Tapajós and Paraguay watersheds [13,40,61,69,86–88]. Like the floodplain and northern borderlands, these settled peer polities created pockets of intensive anthropogenic influence consisting of diffuse but highly planned and integrated regional populations.
6. Conclusion
The emerging multidisciplinary picture of Amazonia is one of great diversity through time and across space. Throughout the Holocene, significant anthropogenic influences occurred in portions of all major subregions. The process and geographical extent of landscape domestication accelerated dramatically with transitions to food production in village gardens, cultivated fields, orchards, domesticated forests, associated anthropogenic soils and earthworks. After 3000 BP, several major Amazonian language families expanded widely across the humid tropical forest and adjacent areas with increasingly diversified inventories of domesticated and managed plants. These societies developed complex systems of regional interaction as they adapted to and modified regional social and biophysical landscapes. Over the past two millennia, these diverse regional trajectories, including substantial internal variation in all areas from large, settled populations to sparsely populated areas within discrete regions, became increasingly articulated within and between regions, and promoted distinctive patterns of land use with related ecological knowledge, but also widespread interaction and connectivity in broad regional political economies.
At the time of European conquest, this variation included a patchy distribution of socio-politically complex systems, semi-intensive techno-economic infrastructure and domesticated landscapes set within a mosaic that also included cultural systems with ‘minimalist’ socio-political organization, simple techno-economies and with less domesticated landscapes. The scales of plant and landscape domestication across Amazonia are comparable to those in other tropical and subtropical regions, and they also fuelled population expansion and social complexity. Larger regional populations clearly fall into the range of medium-sized pre-Columbian polities elsewhere, with population densities well within the range of medium pre-modern urbanized forested landscapes during the Late Holocene in most world areas.
Archaeologists, ecologists and crop geneticists have studied only a small fraction of Amazonia, so the apparently empty areas in our maps represent opportunities for research rather than assumed lack of domestication by pre-conquest peoples, as suggested recently based on a small number of phytolith and charcoal cores in western Amazonia. Engagement with the full range of scholarship on the pre-history of Amazonia reviewed here suggests that western Amazonia is no different than any other major part of Amazonia, although it is different in the lack of an intensive research effort. This is especially true when considering the origins of the Arawak language family and ethnohistorical reports from the region, as well as new archaeology on western Amazonian earthworks. Interdisciplinary studies of coupled natural-human systems reveal that some areas were sparsely occupied but not far away other areas were densely occupied.
The idea of a domesticated Amazonia, i.e. the immense diversity of social, cultural and historical processes that shaped Amazonia during the Holocene, situates this vast area in the company of other world anthromes. It contrasts strongly with reports of empty forests, which continue to captivate scientific and popular media. This view thus problematizes rather than dismisses the human factor in any and all parts of the region, with the corollary that the potential of human influence requires recognition of cultural and historical continuity with many indigenous peoples today. Descendant populations have intrinsic rights to this history and the places it occurred, not simply as disenfranchised groups, but as active partners [89]. They provide a longitudinal view of how human populations actually adapted to changes in the past and how this effected forest composition and distributions. Past systems provide clues to how people responded to opportunities and challenges created by climate change, and offer ideas for present efforts to ameliorate global warming [90]. Indigenous technologies were not only adaptations to changing forest conditions, but also intentional actions to manage those changes. Further resolution of differing views through integrated fieldwork has great global significance given the importance of Amazonia and its sensitivity to climate and human interventions.
Acknowledgements
We are also grateful to numerous colleagues (listed in the electronic supplementary material) who kindly contributed unpublished data on the distribution of Brazil nut and of archaeological sites. We thank Dr Love Eriksen for sharing the shape files for the language map.
Data accessibility
The electronic supplementary material file contains information about the sources of data used to create figures 1–3.
Authors' contributions
C.R.C. coordinated the review and co-wrote the plant section; W.M.D. wrote the population section; M.J.H. co-wrote the Late Holocene section; A.B.J. co-wrote the plant section, compiled and designed the maps; E.G.N. co-wrote the Late Holocene section; W.G.T. and W.I.W. co-wrote the soils section. All authors gave final approval for publication.
Competing interests
The authors declare no competing interests.
Funding
We have been supported by numerous funding agencies, among which the Brazilian Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq, the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and the Archaeology Programme at the National Science Foundation are particularly acknowledged for support of research summarized here.
References
- 1.Hemming J. 1995. Red gold: the conquest of the Brazilian Indians, 2nd edn London, UK: Papermac. [Google Scholar]
- 2.Heckenberger MJ, Neves EG. 2009. Amazonian archaeology. Annu. Rev. Anthropol. 38, 251–266. ( 10.1146/annurev-anthro-091908-164310) [DOI] [Google Scholar]
- 3.Roosevelt AC. 2014. The Amazon and the Anthropocene: 13,000 years of human influence in a tropical rainforest. Anthropocene 4, 69–87. ( 10.1016/j.ancene.2014.05.001) [DOI] [Google Scholar]
- 4.Myers TP. 1974. Spanish contacts and social change on the Ucayali River, Peru. Ethnohistory 21, 135–157. ( 10.2307/480948) [DOI] [Google Scholar]
- 5.Porro A. 1996. Os povos das águas: ensaios de etno-história amazônica. Rio de Janeiro, RJ: Editora Vozes. [Google Scholar]
- 6.Medina JT. 1934. The discovery of the Amazon according to the account of Friar Gaspar de Carvajal and other documents. New York, NY: The American Geographical Society. [Google Scholar]
- 7.Ellis EC. 2011. Anthropogenic transformation of the terrestrial biosphere. Phil. Trans. R. Soc. A 369, 1010–1035. ( 10.1098/rsta.2010.0331) [DOI] [PubMed] [Google Scholar]
- 8.Meggers BJ. 1996. Amazonia: man and culture in a counterfeit paradise, revised edition, 2nd edn, 214 p. Washington, DC: Smithsonian Institution Press. [Google Scholar]
- 9.Meggers BJ. 1954. Environmental limitation on the development of culture. Am. Anthropol. 56, 801–824. ( 10.1525/aa.1954.56.5.02a00060) [DOI] [Google Scholar]
- 10.Clement CR. 1999. 1492 and the loss of Amazonian crop genetic resources. I. The relation between domestication and human population decline. Econ. Bot. 53, 188–202. ( 10.1007/BF02866498) [DOI] [Google Scholar]
- 11.Smith BD. 2011. General patterns of niche construction and the management of ‘wild’ plant and animal resources by small-scale pre-industrial societies. Phil. Trans. R. Soc. B 366, 836–848. ( 10.1098/rstb.2010.0253) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balée W. 2013. Cultural forests of the Amazon: a historical ecology of people and their landscapes, 268 p. Tuscaloosa, AL: The University of Alabama Press. [Google Scholar]
- 13.Schaan DP. 2011. Sacred geographies of ancient Amazonia: historical ecology of social complexity. Walnut Creek, CA: Left Coast Press. [Google Scholar]
- 14.Erickson CL. 2008. Amazonia: the historical ecology of a domesticated landscape. In Handbook of South American archaeology (eds Silverman H, Isbell W), pp. 157–183. New York, NY: Springer. [Google Scholar]
- 15.Denevan WM. 2001. Cultivated landscapes of native Amazonia and the Andes, 396 p. Oxford, UK: Oxford University Press. [Google Scholar]
- 16.Levis C, Souza PF, Schietti J, Emilio T, Pinto JLPdV, Clement CR, Costa FRC. 2012. Historical human footprint on modern tree species composition in the Purus–Madeira interfluve, central Amazonia. PLoS ONE 7, e48559. ( 10.1371/journal.pone.0048559) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barlow J, Gardner TA, Lees AC, Parry L, Peres CA. 2012. How pristine are tropical forests? An ecological perspective on the pre-Columbian human footprint in Amazonia and implications for contemporary conservation. Biol. Conserv. 151, 45–49. ( 10.1016/j.biocon.2011.10.013) [DOI] [Google Scholar]
- 18.Bush MB, Silman MR. 2007. Amazonian exploitation revisited: ecological asymmetry and the policy pendulum. Front. Ecol. Environ. 5, 457–465. ( 10.1890/070018) [DOI] [Google Scholar]
- 19.McMichael CH, Piperno DR, Bush MB, Silman MR, Zimmerman AR, Raczka MF, Lobato LC. 2012. Sparse pre-Columbian human habitation in western Amazonia. Science 336, 1429–1431. ( 10.1126/science.1219982) [DOI] [PubMed] [Google Scholar]
- 20.Piperno DR, McMichael CH, Bush MB. In press. Amazonia and the Anthropocene: what was the spatial extent and intensity of human landscape modification in the Amazon Basin at the end of prehistory? The Holocene ( 10.1177/0959683615588374) [DOI] [Google Scholar]
- 21.Clement CR. 1999. 1492 and the loss of Amazonian crop genetic resources. II. Crop biogeography at contact. Econ. Bot. 53, 203–216. ( 10.1007/BF02866499) [DOI] [Google Scholar]
- 22.Piperno DR. 2011. The origins of plant cultivation and domestication in the New World Tropics. Curr. Anthropol. 52, S453–S470. ( 10.1086/659998) [DOI] [Google Scholar]
- 23.Piperno DR, Pearsall DM. 1998. The origins of agriculture in the lowland neotropics, 400 p. San Diego, CA: Academic Press. [Google Scholar]
- 24.Clement CR, de Cristo-Araújo M, d'Eeckenbrugge GC, Alves Pereira A, Picanço-Rodrigues D. 2010. Origin and domestication of native Amazonian crops. Diversity 2, 72–106. ( 10.3390/d2010072) [DOI] [Google Scholar]
- 25.Harlan JR. 1992. Crops & man, 2nd edn Madison, WI: American Society of Agronomy & Crop Science Society of America. [Google Scholar]
- 26.Rindos D. 1984. The origins of agriculture: an evolutionary perspective, 325 p. San Diego, CA: Academic Press. [Google Scholar]
- 27.Neves EG. 2013. Was agriculture a key productive activity in pre-colonial Amazonia? The stable productive basis for social equality in the Central Amazon. In Human–environment interactions: current and future directions (eds Brondízio ES, Moran EF), pp. 371–388. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 28.Lleras Pérez E. 2012. Plant diversity in Amazonia and world genetic heritage. In Domestication and breeding—Amazonian species (eds Borém A, Lopes MTG, Clement CR, Noda H), pp. 39–52. Viçosa, Minas Gerais: Editora da Universidade Federal de Viçosa. [Google Scholar]
- 29.Smith BD. 2012. A cultural niche construction theory of initial domestication. Biol. Theory 6, 260–271. ( 10.1007/s13752-012-0028-4) [DOI] [Google Scholar]
- 30.Smith BD, Zeder MA. 2013. The onset of the Anthropocene. Anthropocene PLoS ONE 7, e48559. ( 10.1016/j.ancene.2013.05.001) [DOI] [Google Scholar]
- 31.Denevan WM. 2007. Pre-European human impacts on tropical lowland environments. In The physical geography of South America (eds Veblen TT, Young KR, Orme AR), pp. 265–278. Oxford, UK: Oxford University Press. [Google Scholar]
- 32.Peters CM. 2000. PreColumbian silviculture and indigenous management of Neotropical forests. In Imperfect balance: landscape transformations in the preColumbian Americas (ed. Lentz DL.), pp. 203–224. New York, NY: Columbia University Press. [Google Scholar]
- 33.Politis GG. 2007. Nukak: ethnoarchaeology of an Amazonian people. Walnut Creek, CA: Left Coast Press. [Google Scholar]
- 34.Posey DA. 1985. Indigenous management of tropical forest ecosystems: the case of the Kayapo Indians of the Brazilian Amazon. Agroforestry Syst. 3, 139–158. ( 10.1007/BF00122640). [DOI] [Google Scholar]
- 35.Rival LM. 2007. Domesticating the landscape, producing crops and reproducing society in Amazonia. In Emergence and convergence: towards a new holistic anthropology? (eds Parkin D, Ulijaszek S), pp. 72–90. Oxford, UK: Berghahm. [Google Scholar]
- 36.Rival LM. 2002. Trekking through history: the Huaorani of Amazonian Ecuador. New York, NY: Columbia University Press. [Google Scholar]
- 37.Shepard GH Jr, Ramirez H. 2011. ‘Made in Brazil’: human dispersal of the Brazil nut (Bertholletia excelsa, Lecythidaceae) in ancient Amazonia. Econ. Bot. 65, 44–65. ( 10.1007/s12231-011-9151-6) [DOI] [Google Scholar]
- 38.Stahl PW. In press. Interpreting interfluvial landscape transformations in the pre-Columbian Amazon. The Holocene. ( 10.1177/0959683615588372) [DOI] [Google Scholar]
- 39.Stahl PW. 2008. The contributions of zooarchaeology to historical ecology in the Neotropics. Quat. Int. 180, 5–16. ( 10.1016/j.quaint.2007.08.028) [DOI] [Google Scholar]
- 40.Erickson CL. 2006. The domesticated landscapes of the Bolivian Amazon. In Time and complexity in historical ecology: studies in the Neotropical lowlands (eds Balée W, CL. Erickson), pp. 235–278. New York, NY: Columbia University Press. [Google Scholar]
- 41.Arroyo-Kalin M. 2012. Slash-burn-and-churn: landscape history and crop cultivation in pre-Columbian Amazonia. Quat. Int. 249, 4–18. ( 10.1016/j.quaint.2011.08.004) [DOI] [Google Scholar]
- 42.Blench R. 2012. The role of agriculture in explaining the diversity of Amerindian languages. In The past ahead. Language, culture and identity in the Neotropics (ed. Isendahl C.), pp. 13–38. Uppsala, Sweden: Acta Universitatis Upsaliensis. [Google Scholar]
- 43.Renfrew C, Bellwood P. 2002. Examining the farming/language dispersal hypothesis, 436 p. Cambridge, UK: McDonald Institute for Archaeological Research. [Google Scholar]
- 44.Denevan WM. 1996. A bluff model of riverine settlement in prehistoric Amazonia. Ann. Assoc. Am. Geograph. 86, 654–681. ( 10.1111/j.1467-8306.1996.tb01771.x) [DOI] [Google Scholar]
- 45.Peters CM, Balick MJ, Kahn F, Anderson AB. 1989. Oligarchic forests of economic plants in Amazonia: utilization and conservation of an important tropical resource. Conserv. Biol. 3, 341–349. ( 10.1111/j.1523-1739.1989.tb00240.x) [DOI] [PubMed] [Google Scholar]
- 46.Mayle FE, Iriarte J. 2014. Integrated palaeoecology and archaeology: a powerful approach for understanding pre-Columbian Amazonia. J. Archaeol. Sci. 51, 54–64. ( 10.1016/j.jas.2012.08.038) [DOI] [Google Scholar]
- 47.Junk WJ, Piedade MTF, Schöngart J, Cohn-Haft M, Adeney JM, Wittmann F. 2011. A classification of major naturally-occurring Amazonian lowland wetlands. Wetlands 31, 623–640. ( 10.1007/s13157-011-0190-7) [DOI] [Google Scholar]
- 48.Levis C, Silva MS, Silva MA, Moraes CP, Neves EG, Tamanaha EK, Flores BM, Clement CR. 2014. What do we know about the distribution of Amazonian dark earth along tributary rivers in Central Amazonia? In III Encuentro Internacional de Arqueología Amazónica (ed. Rostain S.), pp. 305–311, 542. Quito, EC: Instituto Francés de Estudios Andinos. [Google Scholar]
- 49.McMichael CH, Palace MW, Bush MB, Braswell B, Hagen S, Neves EG, Silman MR, Tamanaha EK, Czarnecki C. 2014. Predicting pre-Columbian anthropogenic soils in Amazonia. Proc. R. Soc. B 281, 20132475 ( 10.1098/rspb.2013.2475) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomas E, Alcázar Caicedo C, McMichael CH, Corvera R, Loo J, Linder P. In press. Uncovering spatial patterns in the natural and human history of Brazil nut (Bertholletia excelsa) across the Amazon basin. J. Biogeogr. ( 10.1111/jbi.12540) [DOI] [Google Scholar]
- 51.Santos H, Carvalho Júnior W, Dart R, Áglio M, Sousa J, Pares J, Fontana A, Martins A, Oliveira A. 2011. O novo mapa de solos do Brasil—legenda atualizada. Rio de Janeiro, Brazil: Embrapa Solos. [Google Scholar]
- 52.WinklerPrins AMGA, Aldrich SP. 2010. Locating Amazonian dark earths: creating an interactive GIS of known locations. J. Latin Am. Geogr. 9, 33–50. ( 10.1353/lag.2010.0029) [DOI] [Google Scholar]
- 53.Glaser B, Birk JJ. 2012. State of the scientific knowledge on properties and genesis of anthropogenic dark earths in Central Amazonia (terra preta de Índio). Geochim. Cosmochim. Acta 82, 39–51. ( 10.1016/j.gca.2010.11.029) [DOI] [Google Scholar]
- 54.Teixeira WG, Martins G, Lima HN. 2006. An Amazonian dark earth profile description from a site located in the floodplain (Várzea) in the Brazilian Amazon. In Pueblos y paisajes antiguos de la selva Amazónica (eds Morcote-Rios G, Mora Camargo S, Franky Calvo C), pp. 293–300. Bogotá, Colombia: Universidad Nacional de Colombia/Taracaxum. [Google Scholar]
- 55.Arroyo-Kalin M. 2010. The Amazonian formative: crop domestication and anthropogenic soils. Diversity 2, 473–504. ( 10.3390/d2040473) [DOI] [Google Scholar]
- 56.Schmidt MJ, et al. 2014. Dark earths and the human built landscape in Amazonia: a widespread pattern of anthrosol formation. J. Archaeol. Sci. 42, 152–165. ( 10.1016/j.jas.2013.11.002) [DOI] [Google Scholar]
- 57.Miller ET. 1992. Arqueologia nos empreendimentos hidrelétricos da Eletronorte: resultados preliminares, 93 p. Brasília, DF: Eletronorte. [Google Scholar]
- 58.Neves EG, Petersen JB, Bartone RN, Silva CA. 2003. Historical and socio-cultural origins of Amazonian dark earth. In Amazonian dark earths: origin, properties, management (eds Lehmann J, Kern DC, Glaser B, Woods WI), pp. 29–50. Dordrecht, The Netherlands: Kluwer. [Google Scholar]
- 59.Denevan WM. 2004. Semi-intensive pre-European cultivation and the origins of anthropogenic dark earths in Amazonia. In Amazonian dark earths: explorations in space and time (eds Glaser B, Woods WI), pp. 135–143. New York, NY: Springer. [Google Scholar]
- 60.Fraser JA, Teixeira WG, Falcão NPS, Woods WI, Lehmann J, Junqueira AB. 2011. Anthropogenic soils in the Central Amazon: from categories to a continuum. Area 43, 264–273. ( 10.1111/j.1475-4762.2011.00999.x) [DOI] [Google Scholar]
- 61.Denevan WM. 1966. The aboriginal cultural geography of the Llanos de Mojos of Bolivia, vol. 48 Berkeley, Ibero-Americana: University of California Press. [Google Scholar]
- 62.Rostain S. 2012. Between Sierra and Selva: landscape transformations in upper Ecuadorian Amazonia. Quat. Int. 249, 31–42. ( 10.1016/j.quaint.2011.08.031) [DOI] [Google Scholar]
- 63.Rostain S. 2012. Islands in the rainforest: landscape management in pre-Columbian Amazonia. Walnut Creek, CA: Left Coast Press. [Google Scholar]
- 64.Lombardo U, Canal-Beeby E, Veit H. 2011. Eco-archaeological regions in the Bolivian Amazon. Geogr. Helv. 66, 173–182. ( 10.5194/gh-66-173-2011) [DOI] [Google Scholar]
- 65.Roosevelt AC. 1991. Moundbuilders of the Amazon: geophysical archaeology on Marajo Island, Brazil. San Diego, CA: Academic Press. [Google Scholar]
- 66.Denevan WM. 2014. Estimating Amazonian Indian numbers in 1492. J. Latin Am. Geogr. 13, 203–217. ( 10.1353/lag.2014.0036) [DOI] [Google Scholar]
- 67.Sombroek W, Ruivo ML, Fearnside PM, Glaser B, Lehmann J. 2003. Amazonian dark earths as carbon stores and sinks. In Amazonian dark earths: origin, properties, management (eds J Lehmann, DC Kern, B Glaser, WI Woods), pp. 125–139. Dordrecht, The Netherlands: Kluwer.
- 68.Woods WI, Denevan WM, Rebellato L. 2013. Population estimates for anthropogenically enriched soils (Amazonian dark earths). In Soils, climate and society: archaeological investigations in ancient America (eds Wingard JD, Hayes SE), pp. 1–20. Boulder, CO: University Press of Colorado. [Google Scholar]
- 69.Heckenberger MJ, Russell JC, Fausto C, Toney JR, Schmidt MJ, Pereira E, Franchetto B, Kuikuro A. 2008. Pre-Columbian urbanism, anthropogenic landscapes, and the future of the Amazon. Science 321, 1214–1217. ( 10.1126/science.1159769) [DOI] [PubMed] [Google Scholar]
- 70.Neves EG. 2012. Sob os tempos do equinócio: oito mil anos de história na Amazônia Central (6.500 AC—1.500 DC). São Paulo, Brazil: Universidade de São Paulo. [Google Scholar]
- 71.Rostain S. 2014. Antes de Orellana—Actas del 3er Encuentro Internacional de Arqueología Amazónica. Quito, EC: Instituto Francés de Estudios Andinos. [Google Scholar]
- 72.Epps P, Salanova AP. 2013. The languages of Amazonia. Tipití: J. Soc. Anthropol. Lowland South Am. 11, 1–28. [Google Scholar]
- 73.Heckenberger MJ. 2002. Rethinking the Arawakan diaspora: hierarchy, regionality, and the Amazonian formative. In Comparative Arawakan histories: rethinking language family and culture area in Amazonia (eds Hill JD, Santos-Granero F), pp. 99–122. St. Louis, MO: University of Illinois Press. [Google Scholar]
- 74.Heckenberger MJ, Russell JC, Toney JR, Schmidt MJ. 2007. The legacy of cultural landscapes in the Brazilian Amazon: implications for biodiversity. Phil. Trans. R. Soc. B 362, 197–208. ( 10.1098/rstb.2006.1979) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Neves EG. 2008. Prehispanic chiefdoms of the Amazonian floodplain: long-term history and political changes. In Handbook of South American archaeology (eds Silverman H, Isbell W), pp. 359–379. New York, NY: Springer. [Google Scholar]
- 76.Noelli FS. 2008. The Tupi expansion. In Handbook of South American archaeology (eds Silverman H, Isbell W), pp. 659–670. New York, NY: Springer. [Google Scholar]
- 77.Urban G. 2002. A história da cultura brasileira segundo as línguas nativas. In História dos Índios no Brasil, (ed. Carneiro da Cunha M.), 2nd edn, pp. 87–102. São Paulo, Brazil: Companhia das Letras. [Google Scholar]
- 78.Eriksen L. 2011. Nature and culture in prehistoric Amazonia: using GIS to reconstruct ancient ethnogenetic processes from archaeology, linguistics, geography, and ethnohistory Lund, Sweden: Lund University. [Google Scholar]
- 79.Aikhenvald AY. 1999. The Arawak language family. In The Amazonian languages (eds Dixon RMW, Aikhenvald AY), pp. 65–106. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 80.Eriksen L, Danielsen S. 2014. The Arawakan matrix. In The native languages of South America: origins, development, typology (eds O'Connor L, Muysken P), pp. 152–176. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 81.Walker RS, Wichmann S, Mailund T, Atkisson CJ. 2012. Cultural phylogenetics of the Tupi language family in lowland South America. PLoS ONE 7, e35025. ( 10.1371/journal.pone.0035025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moraes CdP, Neves EG. 2012. O ano 1000: adensamento populacional, interação e conflito na Amazônia Central. Amazônica—Revista de Antropologia 4, 122–148. [Google Scholar]
- 83.Gomes DMC. 2008. Cotidiano e poder na Amazônia pré-colonial. São Paulo, Brazil: Editora da Universidade de São Paulo. [Google Scholar]
- 84.Neves EG, Petersen JB. 2006. The political economy of pre-Columbian Amerindians: landscape transformations in Central Amazonia. In Time and complexity in historical ecology: studies in the Neotropical lowlands (eds Balée WL, Erickson CL), pp. 279–310. New York, NY: Columbia University Press. [Google Scholar]
- 85.Redmond EM, Spencer CS. 2007. Archaeological survey in the high llanos and Andean piedmont of Barinas, Venezuela. New York, NY: American Museum of Natural History. [Google Scholar]
- 86.Walker JH. 2004. Agricultural change in the Bolivian Amazon. Pittsburgh, PA: Center for Comparative Archaeology, University of Pittsburgh. [Google Scholar]
- 87.Heckenberger MJ. 2005. The ecology of power: culture, place, and personhood in the southern Amazon, AD 1000–2000. New York, NY: Routledge. [Google Scholar]
- 88.Walker JH. 2008. The llanos de Mojos. In The handbook of South American archaeology (eds Silverman H, Isbell W), pp. 927–939. New York, NY: Springer. [Google Scholar]
- 89.Heckenberger MJ. 2013. Who is Amazonia? The ‘salt of the matter’ for indigenous sustainability. Environ. Res. Lett. 8, 041007 ( 10.1088/1748-9326/8/4/041007) [DOI] [Google Scholar]
- 90.Carson JF, Whitney BS, Mayle FE, Iriarte J, Prumers H, Soto JD, Watling J. 2014. Environmental impact of geometric earthwork construction in pre-Columbian Amazonia. Proc. Natl Acad. Sci. USA 111, 10 497–10 502. ( 10.1073/pnas.1321770111) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The electronic supplementary material file contains information about the sources of data used to create figures 1–3.