Abstract
Many prey species rely on conspecifics to gather information about unknown predation threats, but little is known about the role of varying environmental conditions on the efficacy of social learning. We examined predator-naive minnows that had the opportunity to learn about predators from experienced models that were raised in either a low- or high-risk environment. There were striking differences in behaviour among models; high-risk models showed a weaker response to the predator cue and became neophobic in response to the control cue (a novel odour, NO). Observers that were previously paired with low-risk models acquired a strong antipredator response only to the predator cue. However, observers that interacted with high-risk models, displayed a much weaker response to the predator odour and a weak neophobic response to the NO. This is the first study reporting such different outcomes of social learning under different environmental conditions, and suggests high-risk environments promote the cultural transmission of neophobia more so than social learning. If such a transfer can be considered similar to secondary traumatization in humans, culturally transmitted neophobia in minnows may provide a good model system for understanding more about the social ecology of fear disorders.
Keywords: social learning, uncertainty, neophobia, secondary traumatization, vicarious traumatization, Pimephales promelas
1. Introduction
Predation risk can induce many phenotypically plastic responses in prey [1,2]. For instance, some prey modify their morphology (e.g. body size or depth, armour thickness, tail depth) to decrease the likelihood of predator attacks or to increase their chance of survival after an attack (e.g. [3,4]). Some prey alter life-history traits such as age or size of sexual maturation [5] or the timing of life-history switches such as metamorphosis, to decrease their risk of predation at a particular life stage [6,7]. However, behavioural responses to risky cues are generally the first lines of defence, as they are more plastic, not so costly and are expressed on a much shorter timescale than other alternatives [8]. Like other types of defences, displaying efficient antipredator behaviour has costs, as it reduces the time and energy available for other fitness-enhancing activities, such as foraging, mate courting and territory defence. To optimize this cost–benefit trade-off, prey should display antipredator responses that match the level of threat posed by the predator (i.e. threat-sensitive responses; e.g. [9,10]). However, the inherent variability of predation risk, both in time and space, creates uncertainty for prey as to whether the cues used to gauge risk do in fact reliably represent the actual risk present in the environment [11]. This uncertainty is what drives prey to adopt a ‘play it safe’ strategy and usually over-respond to predation threats [12].
For many prey species, predator recognition learning is a plastic mechanism for managing predator uncertainty, giving prey a better chance at survival in future predator encounters. For example, prey can learn the level of threat posed by novel predators [13,14] and the spatial and temporal patterns of risk in their habitats (e.g. [15,16]). They can acquire this information either via a direct encounter with a predator, or indirectly via the use of predation-related cues. These cues can be released directly by the predator (predator kairomones, diet cues or injured conspecific cues) or may indirectly indicate the presence of a predator (specific predator habitat or microhabitat, or time of day when predators are active). Such individual assessment allows prey to learn relevant information, but it can be dangerous, time consuming and quickly outdated [17]. By contrast, social learning (or cultural transmission) allows animals to rapidly adjust to uncertainty while limiting danger [18]. Either via ‘eavesdropping’ on conspecific-mediated information, or through direct teaching by more experienced conspecifics, social learning has been shown to occur in a number of ecological contexts, such as foraging, mate choice and predator avoidance [14,19,20]. While this type of learning was initially thought to be limited to highly social species, successful social learning has been demonstrated in a wide diversity of taxa, both within and across species [14]. Typically, a naive observer will learn from a more experienced individual (the model) by observing the response of the model to a novel situation or stimulus. Many studies have looked at factors affecting the efficiency of social learning, such as the model's age, size or status or the composition of groups (group size, model-to-observer ratio). However, little is known about the effect of extrinsic factors (environmental conditions) on the efficiency of learning.
Some prey living in high-risk environments are known to display avoidance or fear responses towards novel stimuli (i.e. neophobia), while those from low-risk environments do not. The concept of neophobia was initially developed in a foraging context, whereby populations of birds and mammals from different environments have been shown to differ in their willingness to sample novel food (reviewed in [21], e.g. [22,23]). The dangerous niche hypothesis posits that neophobia is adaptive because extra caution protects animals from potential risk [21]. In the context of predation, some fish and larval amphibians living in high-risk environments display fearful responses towards novel cues (whether predatory or not) while those from low-risk environments do not. Interestingly, these neophobic responses were inducible in only a few days in low-risk individuals that experienced high-risk conditions [24]. Neophobia may be beneficial when prey lack information about the identity of novel threats in their environment, as it increases their likelihood of surviving their first encounter with an unknown predator [25]. However, this response could be costly if prey unnecessarily responded to a high number of non-risky stimuli in a relatively low-risk environment. Hence, it is not surprising that neophobia appears plastic in its expression [24,26]. Brown et al. [26] demonstrated that the intensity of fear towards novel cues was modulated by the level of risk perceived in the environment, with higher risk environments leading to individuals displaying stronger and longer avoidance responses towards the novel cues compared with those in lower risk environments. Neophobia can also be viewed as a lack of learning that risk is absent [21]. In an experiment with damselfish (Pomacentrus chrysurus), neophobic individuals failed to learn that a novel odour (NO) was safe after experiencing it repeatedly in non-threatening situations (i.e. failed latent inhibition) [27].
Our objectives in this study were to determine whether social learning in a high-risk environment leads to different outcomes than social learning in low-risk environments. Until now, all studies focusing on social learning have done so in a relatively risk-free environment. However, with the emergence of new behavioural patterns such as neophobia, one can wonder whether social learning is equally beneficial in all types of environments, and whether the efficiency of information transmission can be modulated by certain environmental conditions. Specifically, we ask: (i) whether different background levels of risk affect the propensity of naive observers to learn from conspecifics and (ii) whether naive observers distinguish between responses of models towards known risky cues (a known predator) and those displayed via neophobia (an unknown cue). We hypothesized that models living in high-risk environments display heightened vigilance behaviours, which may dampen the increase in intensity of antipredator response given to known threats, making the response less obvious to a naive observer. Thus, we predicted that observers paired with high-risk models may not learn as well as those paired with low-risk models, or they may learn to recognize predators as a lower threat. We also hypothesized that neophobic responses might convey uncertainty to observers via subtle changes in the behaviour of the model. Consequently, observers might be able to distinguish between neophobic and informed responses, and thus may rely less on information observed from uncertain models. To achieve our goals, we paired low-risk predator-naive fathead minnows (Pimephales promelas) with predator-experienced models that were raised in either a low- or high-risk environment. We exposed the pair to either a predator cue known to the model or to a novel cue, giving the naive observer an opportunity to learn from the model. The observer was later tested alone for its response to the cues experienced with the models or a novel control cue.
2. Methods
(a). Test species, collection and maintenance
Fathead minnows are a group-living species that respond to predation risk with reduced activity and increased shelter use [28]. Like many other aquatic species, they display overt antipredator behaviours in response to injured conspecific cues (i.e. alarm cues, ACs) and can be conditioned to recognize novel threats via a single pairing of ACs with a novel predator cue (sight, smell or sound of a predator) [13]. Minnows can also learn socially to recognize novel predators. When a predator-naive observer is paired with an experienced conspecific, again only a single pairing of the frightened conspecific with a predator cue is enough for the observer to learn to recognize this predator cue as a threat [29,30].
In September 2013, we collected adult minnows from Feedlot Pond on the University of Saskatchewan campus using Gee's inverted minnow traps. Minnows from this site are exposed to a variety of predators, including birds, snakes and beetles, but are naive to fish predators (e.g. [31,32]). Before the experiment began, minnows were housed in 76-l flow-through tanks with gravel substrate, aeration, 15 L : 9 D cycle, and a daily 30% flush with filtered dechlorinated tap water via a flow-through system. All minnows were fed flake food every morning throughout the experiment.
(b). Minnow alarm cues
We used standard procedures for making ACs [30], sacrificing five minnows (51–66 mm total length) with a blow to the head in accordance with the Canadian Council on Animal Care. We then removed a total of 11.9 cm2 of skin from the minnows. The skin was placed into a beaker with 20 ml of system water and homogenized (Polytron PT-2500E). The resulting solution was then diluted according to an established protocol to reach a concentration of approx. 1 cm2 of skin per 40 l [30]. The water containing ACs was then frozen at −20°C in 100 ml aliquots.
(c). Odours
Two northern pike (Esox lucius) and two lake sturgeon (Acipenser fulvescens) were loaned to us from other research laboratories at the University of Saskatchewan. The fishes were starved for 4 days prior to their odour being collected. They were transferred into individual 38-l tanks containing dechlorinated tap water for 24 h to capture their odour. The tank water was not filtered during this phase. Because individuals varied in body size (25–35 cm total length and 150–350 g mass), we standardized the odour concentration among tanks so as to obtain 100 ml of water per gram of fish. The odours were frozen into 600-ml aliquots at −20°C. The fish were returned to their holding tanks and fed immediately. During the experiment, we randomized which odour served as the predator odour (PO) and the NO in order to assess any potential bias of minnows to the pike and sturgeon cues. Therefore, half the trials were conducted with pike odour as PO, and sturgeon odour as NO, while the other half of trials were conducted with the cues having opposite roles.
(d). Experimental overview
The experiment was divided into three phases. The first phase consisted of obtaining predator-experienced models that were maintained under either a low or a high background level of risk. The second phase was the conditioning phase, whereby naive observers were paired with predator-experienced models coming from low- or high-risk backgrounds and then exposed to either the PO or NO. The third phase consisted of testing the observer on their own to determine whether they had acquired predator recognition from the experienced model.
(i). Phase 1: background risk for models
We first used an established protocol to obtain ACs which are, by nature, reliable indicators of risk [24,26]. They provide non-specific information about predation risk, and hence do not bias the responses of prey towards one type of predator. Minnows were exposed in groups of four in 37-l experimental tanks containing a gravel substrate, an airstone and a shelter object (10 × 10 cm ceramic tile with three 2-cm plastic legs). Each minnow was only used once as a model in the subsequent social learning trials. After 1 day of acclimation, 5 ml of ACs (high risk) or blank water (W—low risk) was slowly injected into each tank through an injection hose attached to the air stone. These cues were injected 3× per day for 4 days, once in the morning (08.00–11.00), at midday (11.00–14.00) and in the afternoon (14.00–17.00) with a minimum of 2 h between each exposure. There was one exception to this treatment routine: on the morning of day 4, all fish were exposed to 5 ml of AC paired with an additional 20 ml of PO, to achieve the learning paradigm described above. This allowed us to train the predator-naive models to recognize the PO as a threat, hence making them predator-experienced, regardless of their background level of risk. A 100% water flush was performed 1 h following the last exposure each day. At the end of this phase, we obtained predator-experienced models that were maintained in either a low- or high-risk environment.
(ii). Phase 2: conditioning trials
All observers were maintained in a low-risk environment and were predator-naive (76-l housing tanks, approx. 50 fish per tank). They were moved individually into 37-l tanks containing gravel, shelter and an airstone, and were allowed to acclimate for 24 h. Each minnow was then randomly assigned to a single predator-experienced model from either the low- or high-risk environment. At this point, the shelter object was removed to avoid shelter competition and to facilitate shoaling. The pair was then allowed to acclimate for 24 h. At the start of the conditioning trial, we injected either 20 ml of PO (true conditioning) or 20 ml of NO (pseudo-conditioning). One benefit of this pseudo-conditioning is that it allowed us to assess the baseline responses of predator-naive observers to the pike and sturgeon odours. Behavioural observations occurred during this phase. Data were recorded on both fish for 8 min prior to the injection of the cue, to obtain a baseline activity level for the fish, and again for 8 min following the injection of the cue to measure the change in behaviour due to exposure to the cue. During each observation period, we recorded the number of lines crossed by the model and the observer using a grid (6.3 × 6.3 cm) drawn on the outer wall of the tank. Additionally, shoaling behaviour was quantified every 15 s; a score of 1 (no shoaling) was given when the two fish were more than a body length apart, and a score of 2 (shoaling) when they were within one body length. Decreases in activity and increases in shoaling are well-documented antipredator responses of minnows [28,33]. Data collection was performed blindly with respect to the background level of risk of the model and the experience/naiveté of the model. One hour after the end of each trial, the model was removed, the shelter was added to the tank, and a full water flush was conducted. Observations did not occur for all conditionings. Sample sizes were 46–50 per group.
(iii). Phase 3: observer testing
Observers acclimated alone for at least 16 h before testing. During this phase, observers were tested for their responses to 20 ml of the conditioning cue (i.e. the odour they experienced in the presence of the model) or to a control (NO). As in conditioning, we recorded behaviour for 8 min before and after the injection of the cue. For this phase, we recorded the number of lines crossed by the observer and the time spent under shelter. Again, data collection was performed blindly. Sample sizes were 22–27 per experimental group.
(e). Statistical analysis
(i). Conditioning trials
We first tested for differences in pre-stimulus lines crossed using a four-way repeated-measures ANOVA, testing for the effects of risk (low versus high), conditioning cue (PO versus NO) and fish role (model versus observer, as repeated-measures in the same tank replicate) while also introducing species (pike versus sturgeon) as a factor to test for potential bias. For pre-stimulus shoaling index, only one value was obtained from each pair of fish (observer + model). Hence, a three-way ANOVA was performed, testing the effects of risk, conditioning cue and species. Because the treatments had no effects on pre-stimulus behaviour (p > 0.1 for all terms and interactions for both response variables), we computed a change in behaviour (post-pre) for each response variable. We then performed a four-way repeated-measures ANOVA investigating the effects of risk, cue, fish role and species on the change in lines crossed and a three-way ANOVA on the change in shoaling index. Post-hoc tests were ANOVAs and targeted t-tests. When performing multiple post-hoc tests on the same experimental group, we adjusted alpha (α = 0.05), dividing by the number of comparisons.
(ii). Testing
For testing, the response variables (activity and shelter use) could be analysed together with a multivariate approach. Again, we tested for differences in pre-stimulus behaviour with a four-way MANOVA, testing the effect of risk (low versus high), conditioning cue (PO versus NO), testing cue (conditioning cue versus novel cue) and species as factors. Again, pre-stimulus values did not differ significantly among treatments (p > 0.1 for all terms), and we computed the change in behaviour and performed a four-way MANOVA followed by post-hoc three-way MANOVAs and t-tests.
3. Results
(a). Social conditioning
(i). Lines crossed
The four-way repeated-measures ANOVA revealed a significant interaction between risk and cue (p < 0.001) and risk and fish role (p = 0.034; table 1a; figure 1). There was no effect of species (p = 0.62), indicating that minnows had no bias towards the pike or sturgeon cues. When looking at the response pattern for low-risk models, we found a significant effect of cue (p < 0.001), no effect of fish role (p = 0.07) and no interaction between the two factors (p = 0.14; table 1b; figure 1). Both observers and models responded strongly to the PO while not responding to the NO. However, in the high-risk treatment, models responded more strongly than observers when exposed to both odours (p < 0.001), but there was no effect of cue (p = 0.26) and no interaction between the two factors (p = 0.38; table 1c; figure 1). High-risk models responded equally to the PO and the novel odour, while observers failed to respond to either of the cues. Additionally, the response to PO was weaker among high-risk models than the low-risk models (t95 = 2.4, p = 0.018; figure 1).
Table 1.
Conditioning: (a) results of the four-way repeated-measures ANOVA on the change in lines crossed, testing for the effects of risk (low versus high), the conditioning cue (PO versus NO), the fish role (model versus observer, as repeated measures) and the species used for the cue (pike versus sturgeon). (b) Results of a three-way repeated measures ANOVA for social conditionings involving low-risk models and for (c) those involving high-risk models. Significant differences are in italics.
| F | d.f. | p-value | |
|---|---|---|---|
| (a) overall RM ANOVA | |||
| within subjects | |||
| role | 22.38 | 1, 187 | <0.001 |
| role × risk | 4.58 | 1, 187 | 0.034 |
| role × cue | 0.28 | 1, 187 | 0.60 |
| role × species | 0.01 | 1, 187 | 0.93 |
| role × risk × cue | 2.85 | 1, 187 | 0.09 |
| between subjects | |||
| risk | <0.01 | 1, 187 | 0.96 |
| cue | 12.62 | 1, 187 | <0.001 |
| species | 0.24 | 1, 187 | 0.62 |
| risk × cue | 26.67 | 1, 187 | <0.001 |
| (b) low-risk RM ANOVA | |||
| within subjects | |||
| role | 3.40 | 1, 90 | 0.07 |
| role × cue | 2.23 | 1, 90 | 0.14 |
| role × species | 0.59 | 1, 90 | 0.44 |
| between subjects | |||
| cue | 37.78 | 1, 90 | <0.001 |
| species | 0.69 | 1, 90 | 0.41 |
| (c) high-risk RM ANOVA | |||
| within subjects | |||
| role | 25.92 | 1, 96 | <0.001 |
| role × cue | 0.78 | 1, 96 | 0.38 |
| role × species | 0.46 | 1, 96 | 0.50 |
| between subjects | |||
| cue | 1.29 | 1, 96 | 0.26 |
| species | 0.01 | 1, 96 | 0.92 |
Figure 1.
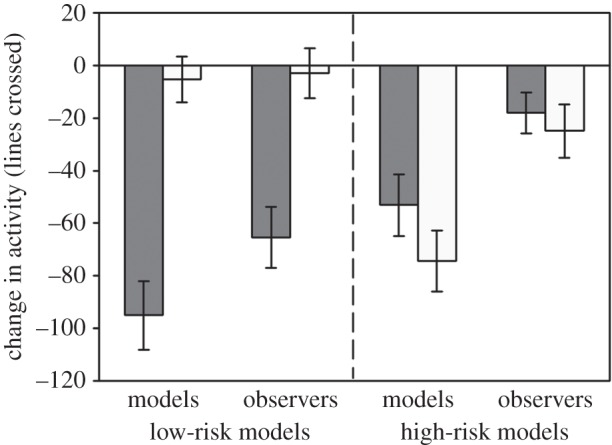
Conditioning: mean (±s.e.) change in lines crossed by low- and high-risk models and their naive observers when exposed to either PO (dark bars) or NO (light bars).
(ii). Shoaling index
The three-way ANOVA revealed a significant interaction between risk and conditioning (p < 0.001; table 2; figure 2). When models came from a low-risk background, the model and observer shoaled more in response to the PO compared to NO (t93 = 4.4, p < 0.001, α = 0.025). By contrast, when models came from a high-risk background, shoaling did not differ in response to PO and NO (t97 = 1.4, p = 0.16, α = 0.025). In addition, the increased shoaling in response to PO was greater for low-risk individuals compared with those from high-risk (t95 = 2.8, p = 0.006, α = 0.025), but this pattern was reversed in response to NO (t93 = −3.0, p = 0.003, α = 0.025; figure 2). Again, species did not affect responses (p = 0.17, α = 0.025), indicating no bias.
Table 2.
Conditioning: results of the three-way ANOVA on the change in shoaling index, testing for the effects of risk (low versus high), the conditioning cue (PO versus NO), and the species used for the cue (pike versus sturgeon). Significant differences are in italics.
| F | d.f. | p-value | |
|---|---|---|---|
| risk | 0.04 | 1, 187 | 0.84 |
| cue | 4.66 | 1, 187 | 0.034 |
| species | 1.89 | 1, 187 | 0.17 |
| risk × cue | 16.77 | 1, 187 | <0.001 |
Figure 2.
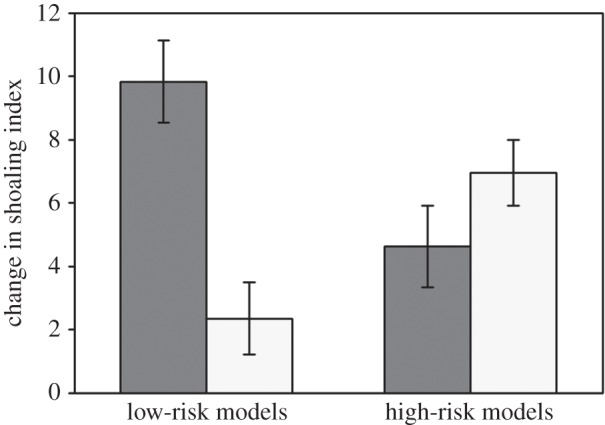
Conditioning: mean (±s.e.) change in shoaling index for pairs of minnows (experienced model + naive observer) exposed to either PO (dark bars) or NO (light bars). Models were from low or high background risk.
(b). Observer testing
The behaviour of observers during testing was significantly correlated with their responses during conditioning, although the association was weak (lines crossed: r2 = 0.05, p = 0.003). When assessing treatment effects, the four-way MANOVA revealed a significant interaction between risk, conditioning cue and testing cue (p = 0.047; table 3a; figure 3), and again, species had no effect (p = 0.77). For observers learning from low-risk models, we found a significant interaction between the conditioning and testing cues (p < 0.001; table 3b). The observers that were conditioned with models exposed to PO showed a strong antipredator response to the PO and had no response towards the NO. In contrast, observers conditioned with models that were exposed to the NO did not display an antipredator response towards either odour, indicating they did not acquire any information about risk from their models.
Table 3.
Testing: (a) results of the four-way MANOVA on the change in lines crossed and the change in shelter use, testing for the effects of risk (low versus high), the conditioning cue (PO versus NO), the testing cue (conditioning odour versus NO), and the species used for the conditioning cue (pike versus sturgeon). (b) Results of a three-way MANOVA for observers conditioned with low-risk models, and for (c) observers conditioned with high-risk models. Significant differences are in italics.
| F | d.f. | p-value | |
|---|---|---|---|
| (a) overall MANOVA | |||
| risk | 1.37 | 2, 189 | 0.26 |
| conditioning cue | 1.63 | 2, 189 | 0.20 |
| testing cue | 2.36 | 2, 189 | 0.10 |
| species | 0.26 | 2, 189 | 0.77 |
| risk × conditioning cue | 5.56 | 2, 189 | 0.005 |
| risk × testing cue | 3.01 | 2, 189 | 0.052 |
| conditioning cue × testing cue | 3.87 | 2, 189 | 0.023 |
| risk × conditioning cue × testing cue | 3.11 | 2, 189 | 0.047 |
| (b) low-risk MANOVA | |||
| conditioning cue | 7.6 | 2, 93 | 0.001 |
| testing cue | 6.11 | 2, 93 | 0.003 |
| species | 0.85 | 2, 93 | 0.43 |
| conditioning cue × testing cue | 8.7 | 2, 93 | <0.001 |
| (c) high-risk MANOVA | |||
| conditioning cue | 0.51 | 2, 94 | 0.60 |
| testing cue | 1.1 | 2, 94 | 0.34 |
| species | 0.06 | 2, 94 | 0.94 |
| conditioning cue × testing cue | 0.13 | 2, 94 | 0.88 |
Figure 3.
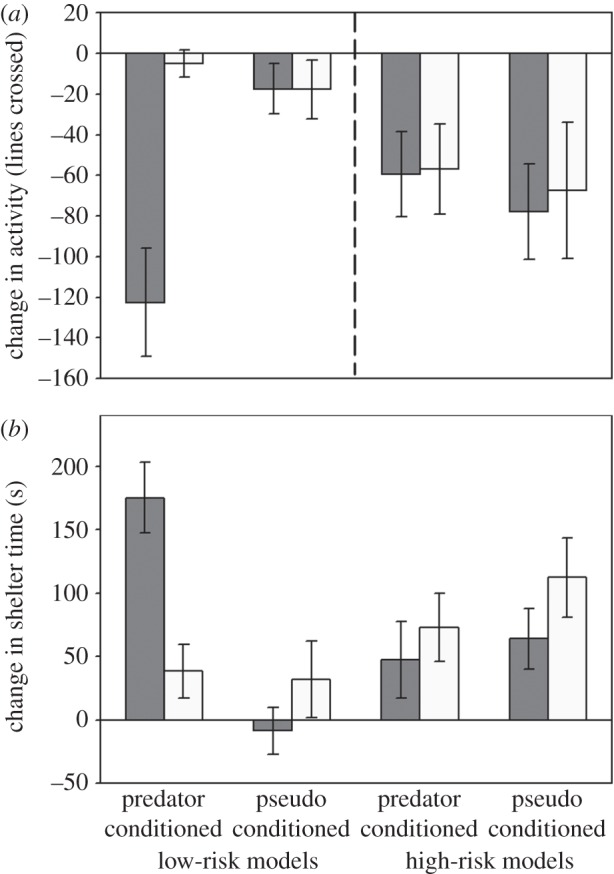
Testing observers: mean (±s.e.) change in lines crossed (a) and time under shelter (b) for observers exposed to either their conditioning odour (dark bars) or NO (light bars). A prior social conditioning consisted of interacting with a model from either low or high background risk while being exposed to either the odour known as a predator by the model (predator conditioned) or NO (pseudo conditioned).
For observers learning from high-risk models, however, the response pattern was quite different. We found no effect of the conditioning cue (p = 0.60), testing cue (p = 0.34) or an interaction between the two factors (p = 0.88; table 3c; figure 3), indicating observers responded similarly to the testing cues regardless of whether their high-risk model was exposed to a known predator or NO. A simple comparison between the response of high-risk versus low-risk observers to NO revealed that the high-risk observers responded more strongly than the low-risk observers (F2,92 = 3.68, p = 0.029; figure 3).
4. Discussion
Fundamental to our results is the finding that exposing fathead minnows to high background risk induced neophobia, a phenomenon previously documented in a few other species. Minnows from a high-risk background exhibited decreased activity and increased shoaling behaviour when exposed to NO, compared to low-risk minnows. The neophobic responses of these individuals were similar in intensity to their predator-experienced responses, at least according to our behavioural endpoints. However, it is still possible that these responses appear different to conspecifics. In addition, high-risk models did not respond to the PO with an intensity as high as that displayed by low-risk models. This finding is consistent with a key prediction of the risk allocation hypothesis; prey faced with high-frequency risk will decrease threat responses to fulfil other necessary activities such as foraging [34,35].
During conditioning, observers tended to respond more weakly than models, a phenomenon previously described in studies on cultural transmission [36–38]. The weaker responses may reflect the short lag-time between the model's response and the observer's assimilation of that information, or it may reflect an initial uncertainty about socially transmitted information. However, this ‘dilution’ pattern was more pronounced among observers learning from high-risk models, suggesting that uncertainty mediates the weaker response of the observers. More surprisingly, the observers paired with high-risk models showed weaker and similar antipredator responses to the cues (predator and novel), and thus, our data do not support the hypothesis that observers could distinguish between neophobic and experienced responses. These weaker responses could have been due to habituation to the hyper-vigilant phenotype exhibited by high-risk models. In qualitative observations, high-risk models, and not low-risk models, appeared agitated and easily startled, and presumably behaved as such throughout their acclimation period with observers. It is possible that observers began to devaluate the fright responses of their models.
The testing phase in this experiment allowed us to determine whether the observer learned via their interaction with the model. The pattern of responses by observers paired with low-risk models matched previous studies where a naive observer learned to recognize a predator cue from models displaying an observable antipredator response (e.g. [29,39]). By contrast, the learned responses from observers paired with high-risk models were weaker which was unsurprising given that high-risk models showed a weaker response to the PO. Therefore, it appears that social learning of predator information via high-risk models leads to the recognition of the predator as a lower threat. While the lower response of high-risk models may be due to an immediate cost–benefit trade-off between antipredator responses and foraging, observers came from a low-risk environment and had no such trade-off.
One unpredicted outcome of our experiment was the emergence of neophobic responses by observers that were paired earlier with neophobic tutors. Indeed, these observers had a weak response to both the PO and NO, indicating that observers were unable to discriminate between the responses of high-risk models and that the weak response of observers to PO was driven by neophobia, rather than true recognition. How could this happen? Observers were paired with models for 24 h, during which they were not exposed to any other stimuli that would cause the emergence of neophobic responses. However, as mentioned earlier, high-risk models appeared to display a hyper-vigilant phenotype. We have quantified aspects of this behavioural change in an ongoing experiment; minnows from high-risk backgrounds, compared to low risk, reduce foraging activity, spend more time freezing, and exhibit pacing behaviour, all in the absence of odour cues, while exhibiting neophobia when odours are presented (A.L.C. and M.C.O.F., 2015, unpublished data). Taken together, one plausible explanation for our results is that the high-risk phenotype exhibited by models indicated to observers that the environment was in fact highly dangerous, hence providing similar information to the ACs used to create the high-risk environment of the models. This hypothesis is supported by the fact that some symptoms of stress in other species are culturally transmitted (e.g. [40,41]). Moreover, in our experiment, simply observing a fright response did not cause neophobia for observers that were paired with low-risk models. Regardless of the specific mechanism behind culturally transmitted neophobia in minnows, our study clearly demonstrates neophobia was acquired via social interaction and suggests high-risk environments promote culturally transmitted neophobia more than social learning.
Across animal taxa, exposure to high risk can have remarkably similar effects (e.g. avoidance behaviour, reduced activity, aggression, hypervigilance, neophobia, etc.) [42], and these outcomes have been highly conserved throughout evolutionary history [43]. Because of this, animal models for studying the symptoms associated with exposure to high risk have been valuable in the framework of understanding post-traumatic stress disorder (PTSD). Experimental studies reveal that the symptoms of PTSD (e.g. emotional distress, restlessness, depression and fear of the unexpected) are best simulated by predation risk, compared to other types of disturbance [42]. While the acquisition of neophobic behaviour via social cues has not yet been reported, a similar phenomenon has been documented in humans. There is a small but growing body of evidence for what is often referred to as ‘secondary traumatization’ in human psychology (for a review of similar terms, see [44]), a phenomenon where PTSD symptoms are indirectly acquired from individuals that experienced trauma first hand. Family members of war veterans and Holocaust survivors, for instance, have been found to exhibit these symptoms at levels significantly greater than controls, but we know little about how they compare to those of the victims of the direct trauma, or when, and for how long, the acquired symptoms occur [45,46]. These studies are correlation-based, and thus, assortative mating may also be a plausible explanation (i.e. individuals prone to these symptoms mate together) [45]. More evidence for secondary traumatization comes from professionals working with mental health patients (reviewed in: [47]). A few studies have found positive correlations between caseload and the occurrence of PTSD symptoms, but others have found no association as it is often difficult to determine whether the trauma symptoms resulted from interacting with patients or simply from the stressful nature of the occupation [47].
A recently proposed framework conceptualizes the critical nature of the social environment for the susceptibility and recovery of PTSD [48], but with social interaction comes secondary traumatization. This is a fairly new field of study, and we do not know of any non-human research. Our work appears to be the first experimental (causal) evidence for this type of phenomenon and suggests that non-human research might prove valuable in furthering our understanding of the ‘social ecology’ of risk disorders.
Acknowledgements
Thanks goes to Brandon Demuth, Zach Hoover, Iain Phillips and Natasha Kreitals for help obtaining pike and sturgeon cues, and to Van Wishingrad for discussions about the manuscript's title.
Ethics
Minnow collection was conducted under a Special Collecting Permit from Saskatchewan's Ministry of Environment, and all work was approved by the University of Saskatchewan's Animal Care and Use Committee (protocol no. 20130079).
Authors' contributions
A.L.C. and M.C.O.F. developed this experiment and wrote the manuscript. A.L.C. conducted the observations and analyses. A.G.E.M. performed background exposures and critically reviewed the manuscript.
Competing interests
We have no competing interests.
Funding
This work was funded by the Natural Sciences and Engineering Research Council of Canada.
References
- 1.Miner BG, Sultan SE, Morgan SG, Padilla DK, Relyea RA. 2005. Ecological consequences of phenotypic plasticity. Trends Ecol. Evol. 20, 685–692. ( 10.1016/j.tree.2005.08.002) [DOI] [PubMed] [Google Scholar]
- 2.DeWitt TJ, Sih A, Wilson DS. 1998. Costs and limits of phenotypic plasticity. Trends Ecol. Evol. 13, 77–81. ( 10.1016/S0169-5347(97)01274-3) [DOI] [PubMed] [Google Scholar]
- 3.Relyea RA. 2001. Morphological and behavioral plasticity of larval anurans in response to different predators. Ecology 82, 523–540. ( 10.1890/0012-9658%282001%29082%5B0523%3AMABPOL%5D2.0.CO%3B2) [DOI] [Google Scholar]
- 4.Trussell GC. 1996. Phenotypic plasticity in an intertidal snail: the role of a common crab predator. Evolution 50, 448–454. ( 10.2307/2410815) [DOI] [PubMed] [Google Scholar]
- 5.Abrams PA, Rowe L. 1996. The effects of predation on the age and size of maturity of prey. Evolution 50, 1052–1061. ( 10.2307/2410646) [DOI] [PubMed] [Google Scholar]
- 6.Sih A, Moore RD. 1993. Delayed hatching of salamander eggs in response to enhanced larval predation risk. Am. Nat. 142, 947–960. ( 10.1086/285583) [DOI] [PubMed] [Google Scholar]
- 7.Chivers DP, Kiesecker JM, Marco A, DeVito J, Anderson MT, Blaustein AR. 2001. Predator-induced life history changes in amphibians: egg predation induces hatching. Oikos 92, 135–142. ( 10.1034/j.1600-0706.2001.920116.x) [DOI] [Google Scholar]
- 8.Lima SL, Dill LM. 1990. Behavioral decisions made under the risk of predation—a review and prospectus. Can. J. Zool. 68, 619–640. ( 10.1139/z90-092) [DOI] [Google Scholar]
- 9.Bishop TD, Brown JA. 1992. Threat-sensitive foraging by larval threespine sticklebacks (Gasterosteus aculeatus). Behav. Ecol. Sociobiol. 31, 133–138. ( 10.1007/BF00166346) [DOI] [Google Scholar]
- 10.Chivers DP, Mirza RS, Bryer PJ, Kiesecker JM. 2001. Threat-sensitive predator avoidance by slimy sculpins: understanding the importance of visual versus chemical information. Can. J. Zool. 79, 867–873. ( 10.1139/z01-049) [DOI] [Google Scholar]
- 11.Dall SR, Johnstone RA. 2002. Managing uncertainty: information and insurance under the risk of starvation. Phil. Trans. R. Soc. Lond. B 357, 1519–1526. ( 10.1098/rstb.2002.1061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouskila A, Blumstein DT. 1992. Rules of thumb for predation hazard assessment—predictions from a dynamic model. Am. Nat. 139, 161–176. ( 10.1086/285318) [DOI] [Google Scholar]
- 13.Ferrari MCO, Wisenden BD, Chivers DP. 2010. Chemical ecology of predator–prey interactions in aquatic ecosystems: a review and prospectus. Can. J. Zool. 88, 698–724. ( 10.1139/Z10-029) [DOI] [Google Scholar]
- 14.Crane AL, Ferrari MCO. 2013. Social learning of predation risk: a review and prospectus. In Social learning theory: phylogenetic considerations across animal, plant, and microbial taxa (ed. Clark K.), pp. 53–82. New York, NY: Nova Science Publisher. [Google Scholar]
- 15.Ferrari MCO, Messier F, Chivers DP. 2008. Larval amphibians learn to match antipredator response intensity to temporal patterns of risk. Behav. Ecol. 19, 980–983. ( 10.1093/beheco/arn056) [DOI] [Google Scholar]
- 16.Mathis A, Unger S. 2012. Learning to avoid dangerous habitat types by aquatic salamanders, Eurycea tynerensis. Ethology 118, 57–62. ( 10.1111/j.1439-0310.2011.01987.x) [DOI] [Google Scholar]
- 17.Rieucau G, Giraldeau L-A. 2011. Exploring the costs and benefits of social information use: an appraisal of current experimental evidence. Phil. Trans. R. Soc. B 366, 949–957. ( 10.1098/rstb.2010.0325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rendell L, et al. 2010. Why copy others? Insights from the social learning strategies tournament. Science 328, 208–213. ( 10.1126/science.1184719) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galef BG, Giraldeau LA. 2001. Social influences on foraging in vertebrates: causal mechanisms and adaptive functions. Anim. Behav. 61, 3–15. ( 10.1006/anbe.2000.1557) [DOI] [PubMed] [Google Scholar]
- 20.White DJ. 2004. Influences of social learning on mate-choice decisions. Anim. Learn. Behav. 32, 105–113. ( 10.3758/BF03196011) [DOI] [PubMed] [Google Scholar]
- 21.Greenberg R, Mettke-hofmann C. 2001. Ecological aspects of neophobia and neophilia in birds. Curr. Ornithol. 16, 119–178. [Google Scholar]
- 22.Voelkl B, Schrauf C, Huber L. 2006. Social contact influences the response of infant marmosets towards novel food. Anim. Behav. 72, 365–372. ( 10.1016/j.anbehav.2005.10.013) [DOI] [Google Scholar]
- 23.Gentsch C, Lichtsteiner M, Feer H. 1981. Taste neophobia in individually and socially reared male rats. Physiol. Behav. 27, 199–202. ( 10.1016/0031-9384(81)90257-2) [DOI] [PubMed] [Google Scholar]
- 24.Brown GE, Ferrari MC, Elvidge CK, Ramnarine I, Chivers DP. 2013. Phenotypically plastic neophobia: a response to variable predation risk. Proc. R. Soc. B 280, 20122712 ( 10.1098/rspb.2012.2712) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrari MCO, McCormick MI, Meekan MG, Chivers DP. 2015. Background level of risk and the survival of predator-naive prey: can neophobia compensate for predator naivety in juvenile coral reef fishes? Proc. R. Soc. B 282, 20142197 ( 10.1098/rspb.2014.2197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown GE, Chivers DP, Elvidge CK, Jackson CD, Ferrari MC. 2014. Background level of risk determines the intensity of predator neophobia in juvenile convict cichlids. Behav. Ecol. Sociobiol. 68, 127–133. ( 10.1007/s00265-013-1629-z) [DOI] [Google Scholar]
- 27.Chivers DP, McCormick MI, Mitchell MD, Ramasamy RA, Ferrari MC. 2014. Background level of risk determines how prey categorize predators and non-predators. Proc. R. Soc. B 281, 20140355 ( 10.1098/rspb.2014.0355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith RJF. 1992. Alarm signals in fishes. Rev. Fish Biol. Fish. 2, 33–63. ( 10.1007/BF00042916) [DOI] [Google Scholar]
- 29.Mathis A, Chivers DP, Smith RJF. 1996. Cultural transmission of predator recognition in fishes: intraspecific and interspecific learning. Anim. Behav. 51, 185–201. ( 10.1006/anbe.1996.0016) [DOI] [Google Scholar]
- 30.Ferrari MCO, Trowell JJ, Brown GE, Chivers DP. 2005. The role of learning in the development of threat-sensitive predator avoidance by fathead minnows. Anim. Behav. 70, 777–784. ( 10.1016/j.anbehav.2005.01.009) [DOI] [Google Scholar]
- 31.Chivers DP, Smith RJF. 1994. The role of experience and chemical alarm signaling in predator recognition by fathead minnows, Pimephales promelas. J. Fish Biol. 44, 273–285. ( 10.1111/j.1095-8649.1994.tb01205.x) [DOI] [Google Scholar]
- 32.Mathis A, Chivers DP, Smith RJF. 1993. Population differences in responses of fathead minnows (Pimephales promelas) to visual and chemical stimuli from predators. Ethology 93, 31–40. ( 10.1111/j.1439-0310.1993.tb00976.x) [DOI] [Google Scholar]
- 33.Pfeiffer W. 1962. The fright reaction of fish. Biol. Rev. 37, 495–511. ( 10.1111/j.1469-185X.1962.tb01333.x) [DOI] [PubMed] [Google Scholar]
- 34.Ferrari MCO, Sih A, Chivers DP. 2009. The paradox of risk allocation: a review and prospectus. Anim. Behav. 78, 579–585. ( 10.1016/j.anbehav.2009.05.034) [DOI] [Google Scholar]
- 35.Lima SL, Bednekoff PA. 1999. Temporal variation in danger drives antipredator behavior: the predation risk allocation hypothesis. Am. Nat. 153, 649–659. ( 10.1086/303202) [DOI] [PubMed] [Google Scholar]
- 36.Cook M, Mineka S, Wolkenstein B, Laitsch K. 1985. Observational conditioning of snake fear in unrelated rhesus monkeys. J. Abnormal Psychol. 94, 591–610. ( 10.1037/0021-843X.94.4.591) [DOI] [PubMed] [Google Scholar]
- 37.Curio E. 1988. Cultural transmission of enemy recognition by birds. In Social learning in animals: the roots of culture (eds Heyes CM, Galef BG), pp. 75–97. San Diego, CA: Academic Press. [Google Scholar]
- 38.Suboski MD, Bain S, Carty AE, McQuoid LM, Seelen MI, Seifert M. 1990. Alarm reaction in acquisition and social transmission of simulated-predator recognition by zebra danio fish (Brachydanio rerio). J. Comp. Psychol. 104, 101–112. ( 10.1037/0735-7036.104.1.101) [DOI] [Google Scholar]
- 39.Mineka S, Cook M. 1993. Mechanisms involved in the observational conditioning of fear. J. Exp. Psychol. Gen. 122, 23–38. ( 10.1037/0096-3445.122.1.23) [DOI] [PubMed] [Google Scholar]
- 40.Dietz DM, LaPlant Q, Watts EL, Hodes GE, Russo SJ, Feng J, Oosting RS, Vialou V, Nestler EJ. 2011. Paternal transmission of stress-induced pathologies. Biol. Psychiatry 70, 408–414. ( 10.1016/j.biopsych.2011.05.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McAdie TM, Keeling L. 2002. The social transmission of feather pecking in laying hens: effects of environment and age. Appl. Anim. Behav. Sci. 75, 147–159. ( 10.1016/S0168-1591(01)00182-4) [DOI] [Google Scholar]
- 42.Goswami S, Rodríguez-Sierra O, Cascardi M, Paré D. 2013. Animal models of post-traumatic stress disorder: face validity. Front. Neurosci. 7, 89 ( 10.3389/fnins.2013.00089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cantor C. 2009. Post-traumatic stress disorder: evolutionary perspectives. Aust. NZ J. Psychiatry 43, 1038–1048. ( 10.1080/00048670903270407) [DOI] [PubMed] [Google Scholar]
- 44.Canfield J. 2005. Secondary traumatization, burnout, and vicarious traumatization: a review of the literature as it relates to therapists who treat trauma. Smith College Stud. Social Work 75, 81–101. ( 10.1300/J497v75n02_06) [DOI] [Google Scholar]
- 45.Arzi NB, Solomon Z, Dekel R. 2000. Secondary traumatization among wives of PTSD and post-concussion casualties: distress, caregiver burden and psychological separation. Brain Injury 14, 725–736. ( 10.1080/026990500413759) [DOI] [PubMed] [Google Scholar]
- 46.Baranowsky AB, Young M, Johnson-Douglas S, Williams-Keeler L, McCarrey M. 1998. PTSD transmission: a review of secondary traumatization in Holocaust survivor families. Can. Psychol. 39, 247–256. ( 10.1037/h0086816) [DOI] [Google Scholar]
- 47.Sabin-Farrell R, Turpin G. 2003. Vicarious traumatization: implications for the mental health of health workers? Clin. Psychol. Rev. 23, 449–480. ( 10.1016/S0272-7358(03)00030-8) [DOI] [PubMed] [Google Scholar]
- 48.Charuvastra A, Cloitre M. 2008. Social bonds and posttraumatic stress disorder. Annu. Rev. Psychol. 59, 301–328. ( 10.1146/annurev.psych.58.110405.085650) [DOI] [PMC free article] [PubMed] [Google Scholar]


