Abstract
Developmental plasticity describes situations where a specific input during an individual's development produces a lasting alteration in phenotype. Some instances of developmental plasticity may be adaptive, meaning that the tendency to produce the phenotype conditional on having experienced the developmental input has been under positive selection. We discuss the necessary assumptions and predictions of hypotheses concerning adaptive developmental plasticity (ADP) and develop guidelines for how to test empirically whether a particular example is adaptive. Central to our analysis is the distinction between two kinds of ADP: informational, where the developmental input provides information about the future environment, and somatic state-based, where the developmental input enduringly alters some aspect of the individual's somatic state. Both types are likely to exist in nature, but evolve under different conditions. In all cases of ADP, the expected fitness of individuals who experience the input and develop the phenotype should be higher than that of those who experience the input and do not develop the phenotype, while the expected fitness of those who do not experience the input and do not develop the phenotype should be higher than those who do not experience the input and do develop the phenotype. We describe ancillary predictions that are specific to just one of the two types of ADP and thus distinguish between them.
Keywords: adaptive developmental plasticity, predictive adaptive response, evolution
1. Introduction
Field fall crickets whose gravid mothers were exposed to a wolf spider show a heightened immobility response to spider cues, and survive better in an environment containing predatory spiders than crickets whose mothers were not exposed to spider cues [1]. Bulb mites that had rich diets as juveniles develop an aggressive ‘fighter’ adult phenotype, whereas those that had poorer nutrition as juveniles become non-aggressive ‘scramblers’ [2]. Starlings that were disadvantaged in competition in the nest go on to fly less well as adults [3], and women who were separated from their parents before the age of seven show worse health in their forties [4].
These are all examples of developmental plasticity: the capacity of the same genotype to produce different phenotypic outcomes depending upon inputs during development [5]. For the crickets responding to spiders and the bulb mites developing the aggressive phenotype, the plasticity may plausibly be adaptive. That is, it may be advantageous in fitness terms for individuals to show those particular phenotypic traits given those particular developmental histories. On the other hand, for impaired starling flight following developmental disadvantage, or poorer human health following parental separation, there is no evidence of any advantage to the phenotypic outcome. It is, presumably, always disadvantageous to fly less well or experience poorer health. The plasticity therefore appears deleterious: the adult phenotype simply represents the cost of getting a bad start in life.
The four cases of developmental plasticity discussed above seem easy to classify. However, there is often debate about whether a given case of plasticity is adaptive or not. The apparent increase in children's symptoms of attention deficit hyperactivity disorder following exposure to maternal stress [6] could represent damage (stress makes brains work less well), or it could represent a shift in behavioural phenotype that was advantageous in ancestral environments [7]. Similarly, poor fetal growth in humans has a range of effects on adult phenotype, some of which have been argued to be adaptive and some of which appear entirely detrimental [8]. Successfully resolving debates about the boundaries of adaptation requires conceptual clarity: what are the necessary entailments and minimal empirical predictions that follow from the claim that an instance of plasticity is adaptive? [9]. These are questions we will address in this review.
A further complexity is that, as we will outline below, there are two different classes of hypothesis for why developmental plasticity could be adaptive. We will call these informational and somatic state-based. Each class relies on different assumptions about what relationships have held over evolutionary time, and also makes different predictions about the relative fitness of different classes of individuals. When researchers suggest adaptive hypotheses about developmental plasticity in particular systems, they are not always explicit about which type of explanation they envisage; indeed, the two classes of hypothesis do not have universally established names.
Our aim in this article is to provide a conceptual review of what adaptive developmental plasticity (henceforth ADP) is, when it can evolve and how its signature might be detected empirically. We are concerned here exclusively with the ultimate level of analysis; we do not discuss the proximate mechanisms by which developmental plasticity might be delivered. We also do not explicitly consider when selection will favour ADP over alternative phenotype-varying mechanisms such as bet-hedging or adult plasticity (on this question, see [10]). In §2, we consider terminology, defining ADP and relating it to other terms in the literature. In §3, we describe the two classes of adaptive hypothesis for developmental plasticity. This leads to a discussion of the differences in their assumptions and claims, and hence when each might be applicable (§4). We then turn to the empirical predictions of the two classes of hypothesis, and how they could be tested (§5). In §6, we revisit the distinction between adaptive and deleterious developmental plasticity, leading us to consider what we term semi-adaptive explanations.
2. Terminology
All putative cases of ADP involve a developmental input I, and a later phenotype P. We assume that both the input and the phenotype can take two discrete states (I and ⌝I (not I), P and ⌝P): for example, nutrition either good or poor, adult phenotype either aggressive or non-aggressive. Our framework generalizes easily to cases where input and phenotype can adopt more than two discrete states or are continuous, but we restrict ourselves to the dichotomous case for ease of discussion. Informational hypotheses involve a third variable, the adult environment, which may not be the same as the developmental environment; again we define two possibilities, E and ⌝E. Somatic state-based hypotheses invoke a different third variable, the individual's somatic state (defined below) in adulthood. We denote the possible somatic states of the individual S and ⌝S. We denote probabilities with p and expected fitness with w. These are defined over evolutionary time.
We distinguish developmental plasticity from other types of plasticity by stipulating that in developmental plasticity, responsiveness to I must occur during the period of the organism's initial growth, and P must persist into later life-stages (this is similar to Snell-Rood's [11] distinction between developmental and activational plasticity). In many cases, I is experienced directly by the individual developing P. In other cases, I is transduced by the parent, for example, through changes to the in utero or in ovo milieu. These latter cases represent maternal/paternal [12] or transgenerational [13] effects. Such cases would still qualify as (potentially adaptive) developmental plasticity in our terms.
Various terms are used in the literature on developmental plasticity, including predictive adaptive response (PAR), hormesis and stress inoculation. PAR has been used primarily in the human literature to describe (ex hypothesi adaptive) alterations in metabolism and life-history parameters following restricted nutrition in utero [14]. PARs so defined are instances of ADP. However, the PAR literature invokes adaptive hypotheses specifically of the informational type (as defined in §3) rather than the somatic state-based type. Thus, although all cases of PAR qualify as ADP, not all phenomena that qualify as ADP are PARs. Hormesis refers to improvements in the ability of organisms to cope with some challenge if, earlier in their development, they have experienced that challenge in small doses [15]. Stress inoculation [16] is a related concept. Hormesis and stress inoculation qualify as ADP, but, again, not all ADP is encompassed by these terms.
3. Different hypotheses for adaptive developmental plasticity
(a). Informational hypotheses
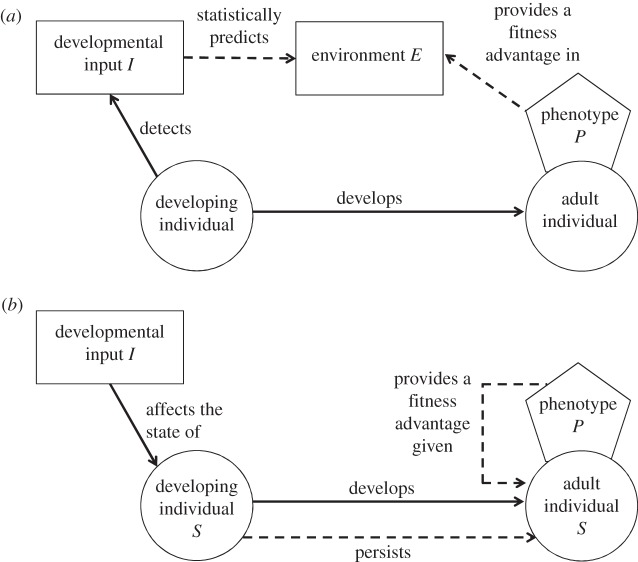
Informational ADP hypotheses make claims about the inter-relationships of three entities, the developmental input I, the adult environment E and the adult phenotype P, over evolutionary time. P is a phenotype adapted to E. Exposure to I is hypothesized to carry information about the likelihood of experiencing E in the future. I and E are not the same thing; I is often referred to as the cue, token or inducing stimulus [17]. I acts in effect as a ‘weather forecast’ of the future environment [18]. The logic of informational hypotheses is set out in figure 1a. The developing individual detects that it is experiencing I; I has over evolutionary time predicted the subsequent adult experience of E, and P is a better phenotype to have in E than ⌝P is. Thus, the adaptive part of informational ADP is that, by developing P in response to experiencing I, the individual ends up with an improved probability of having a suitable phenotype for its adult environment.
Figure 1.
The logical structure of (a) informational and (b) somatic state-based ADP hypotheses. Processes occurring in developmental time are shown with solid arrows, while relationships that hold over evolutionary time are shown as dashed arrows.
Examples of cases for which such informational explanations are well supported include that of field fall crickets described at the beginning of this article [1], and the induction of defensive body structures through exposure to predator kairomones in Daphnia spp. [19–21]. It is plausible in these cases that the cues I have a purely informational role. For example, the kairomones detected by juvenile Daphnia have no nutritional value and are not toxic. Thus, there is no evidence that they affect individuals other than by serving as input to an evolved predator-defence-triggering adaptation. An alternative way of stating this is that it is easy to imagine a single loss-of-function mutation that completely abolishes the responsiveness of developing Daphnia to predator kairomones and leaves the individuals otherwise unaffected.
More formally, the assumptions of informational hypotheses are that, over evolutionary time:
-
(I1)
p(E|I) > p(E|⌝I)
-
(I2)
w(P|E) > w(⌝P|E) and w(⌝P|⌝E) > w(P|⌝E)
-
(I3)
Because of (I1) and (I2), w(P|I) > w(⌝P|I) and w(⌝P|⌝I) > w(P|⌝I).
Note that (I1) is simply the formal statement of the requirement that I must carry information about E [22]. Assumption (I2) states that the fitness of individuals with phenotype P must be higher than those without it in adult environment E, but lower than those without it in adult environment ⌝E. Both lemmas of (I2) are necessary. If the first were true but not the second, we should expect the fixation of P rather than any plasticity, and if the second were true but not the first, we should expect the fixation of ⌝P. It is thus a necessary claim of informational models that the expected fitnesses of the two phenotype classes crossover depending upon the adult environment. Finally, (I3) simply follows from (I1) and (I2): if the optimal phenotype is reversed between environments E and ⌝E, then as long as developmental input I predicts E strongly enough, then the phenotype with the highest expected fitness following I will be P, and following ⌝I will be ⌝P.
(b). Somatic state-based hypotheses
The logic of somatic state-based ADP hypotheses is laid out in figure 1b. In such hypotheses, I is an input that has a general effect on the developing individual, such that some somatic state variable S is enduringly altered. By somatic state variable, we mean a parameter with broad fitness implications such as size, muscular strength, organ capacity or extent of DNA damage. In the example of the bulb mite [2], I is the food available and S is size; the less good the food is, the smaller the size the mite can grow to. Importantly, the influence of I on S cannot be merely transient: a small mite will remain small through adulthood. The other component of somatic state-based models is a phenotypic trait P that is potentially independent of S. P in the bulb mite case is aggression. It would be possible for a small individual to be aggressive, or a large one non-aggressive, so S and P are potentially independent. However, what has evolved is a mechanism linking the two together: the switching rule ‘if you find yourself large, become aggressive; if you find yourself small, become non-aggressive’. For this rule to be adaptive, the optimal phenotype must be dependent on somatic state. That is, expected fitness for a large mite must be higher if it becomes aggressive than if it does not, while for a small mite, the opposite must be true.
Formally, then, the following are the assumptions of somatic state-based models:
-
(S1)
p(S|I) > p(S|⌝I)
-
(S2)
w(P|S) > w(⌝P|S) and w(⌝P|⌝S) > w(P|⌝S)
-
(S3)
Because of (S1) and (S2), w(P|I) > w(⌝P|I) and w(⌝P|⌝I) > w(P|⌝I).
Assumption (S1) states that the developmental input I must be related to adult state variable S, such that individuals experiencing I during development are more likely to end up with S than those who do not experience I. Assumption (S2) specifies a fitness crossover, and formally states the requirement that the optimal phenotype must be state-dependent. Expected fitness must be higher by developing P than ⌝P if your state is S, but the opposite must be true if your state is ⌝S. Finally, (S3) is entailed by the first two assumptions. Since I is related to S, and S means that it is advantageous to develop P, then on average, fitness will be higher for individuals who experience I and develop P than for those who experience I and do not, and, symmetrically, higher for those who experience ⌝I and develop ⌝P than for those who experience ⌝I and develop P.
4. Comparing informational and somatic state-based hypotheses
From the discussion above, it should be clear that there are considerable similarities between informational and somatic state-based models, but also some key differences. Both types of hypothesis require the same crossover pattern in the expected fitness of the different possible phenotypes given the possible developmental inputs ((I3) and (S3), respectively). This is because (I3) and (S3) are just the definition of adaptiveness restated: in adaptive evolution, the trait, which in this case is the development of the phenotype P conditional on experiencing developmental input I, must be positively associated with expected fitness [23]. Although obvious, this is important enough to constitute the unifying principle of ADP: ‘in cases of ADP, individuals who experience the developmental input and develop the phenotype must have higher expected fitness than those who experience the developmental input and do not develop the phenotype; whilst individuals who do not experience the developmental input and do not develop the phenotype must have higher expected fitness than those who do not experience the developmental input but do develop the phenotype’. This principle holds as long as the environments in which fitness is measured are representative of those in which selection has acted over evolutionary time. Where the unifying principle is met, there is selection for developmental plasticity; this is a necessary but not sufficient condition for its evolution. Whether plasticity results will depend on the evolvability and costs of proximate mechanisms to deliver it [24].
The unifying principle provides a clear way to distinguish ADP from non-adaptive (i.e. deleterious or neutral) plasticity. If the pattern described by the unifying principle is not present for a particular I–P pairing, then the plasticity is not adaptive. For example, if there were a class of starlings that could experience disadvantage in the nest (I) but still fly just as vigorously as their peers (⌝P), those starlings would have a fitness advantage over the starlings that actually exist (i.e. w(⌝P|I) > w(P|I)). Since this violates the unifying principle, the plasticity in this case is not adaptive. Bateson et al. [25] suggest that where plasticity exists, it is mostly beneficial, presumably because natural selection would be expected to eliminate deleterious forms of plasticity. However, certain patterns of early-life input may drive development against immutable mechanistic or energetic constraints, meaning that, for example, starlings which experience early adversity and yet fly as vigorously as their peers are simply not biologically achievable. Thus, deleterious plasticity may well be widespread, and adaptiveness needs to be demonstrated rather than assumed.
While informational and somatic state-based ADP hypotheses concur in predicting the pattern described by the unifying principle, the reasons that pattern comes about are different in the two cases. In each case, there is an association between I and something that comes later (assumptions (I1) and (S1)), and a fitness crossover (assumptions (I2) and (S2)), but they are not the same associations and crossovers. In informational models, the association is between I and the adult environment E and its nature is statistical: over evolutionary time, I has served as a cue that E is likely to follow. Thus, a plausibility test for informational hypotheses in particular cases is consideration of cue validity. In the organism's natural environment, does the proposed developmental input I statistically predict E with sufficient fidelity? This is something that can be tested in systems where long-term, individual-based ecological datasets are available [26].
The likelihood that the cue reliability of I is high enough for ADP to evolve via the informational route will depend on a number of factors. First, informational hypotheses are most convincing when there is a tight linkage between the proposed I and E. For example, kairomones produced by aquatic predators of Daphnia have no natural source other than those predators, nor can the predators be present and not produce them [27]. By contrast, some ADP claims rely on hypothesized relationships that are likely to be much less strong, such as parental behaviour towards the child being a cue of the prevailing extrinsic mortality rate [28–30]. While parents might on average behave differently in environments of high than low danger [31], there will be other sources of variation in parental behaviour, so the claim that the relationship between I and E could be strong enough for natural selection to build adaptations on is problematic [32].
A second factor that might affect the evolvability of informational ADP is the length of the lifetime relative to speed of environmental change. Informational ADP hypotheses are most straightforward to make in cases where the organism is short-lived, or where the phenotype P is visible to selection soon after I is received. Where the organism is long-lived and there is considerable delay between the experience of I and the fitness advantage of P, within-lifetime environmental change can undermine the informational value of I [33–35]. For example, even a modest amount of year-to-year variation is sufficient to destroy the informational content of birth-year conditions for predicting adulthood conditions in humans [33,35]. Thus, information ADP claims for long-lived organisms entail assumptions about the temporal structure of environments E over evolutionary time. Such assumptions need to be tested, or, where that is not possible, a plausibility case made, for each case.
Under somatic state-based hypotheses, the relationship between I and S is causal: I has a lasting effect on S, which in turn determines the relative fitness of P and ⌝P. Thus, somatic state-based hypotheses need make no assumptions about the temporal stability of the external environment through the individual's life (indeed, they do not invoke the adult environment as an explanatory construct at all). For these hypotheses, it is not particularly useful to conceptualize I as being a cue or providing information. The relationship of I to S is that of cause to consequence, and it would be an unusual usage of the term information to describe events as conveying information about their consequences. Thus, issues of cue reliability do not arise.
In the view of the above, somatic state-based models are most plausible where I is something that has unavoidable general consequences for development. Another way of stating this is that mutation cannot readily produce an alternate type of individual that is developmentally unaffected by I. It is hard to imagine a mutant mammal whose growth was unaffected by the amount of food available, given that growing tissues requires calories. However, like informational models, somatic state-based models rely on assumptions that are seldom tested in practice. In particular, somatic state-based hypotheses claim that since I causes S, the organism's optimal strategy is to adopt P as a best response. It is seldom explained why the individual would not do better to invest its energy instead in changing S in adulthood. For example, rather than becoming non-aggressive adults, bulb mites could evolve a second growth period that they enter only if small after the first. If the payoffs for being large and aggressive are large enough, this would seem an equally plausible adaptive strategy as the one actually observed.
To take another example, Bateson et al. [36] showed that starlings which experienced greater telomere attrition during development were more impulsive as adults. Telomere attrition is a marker of cellular ageing and is accelerated by developmental stress. Birds that experience greater attrition are likely to live less long as adults [37]. Thus, Bateson et al. argue, birds finding themselves with greater attrition have an adaptive payoff from adopting an impulsive, ‘live for the short-term’ behavioural strategy appropriate to their poor survival prospects. However, it is not clear why a better strategy would not be to repair their telomeres. Such repair is mechanistically possible, at least in principle.
When faced with difficulties such as these, somatic state-based hypotheses have to invoke some kind of constraint. It may be more difficult to evolve a completely changed pattern of growth than it is to evolve an alternate aggression morph. It may be that cellular repair of the kind required in the starling case is uneconomic or impossible in an adult individual, so birds do better by shifting their behaviour. Indeed, behavioural phenotypes may be particularly amenable to somatic state-based ADP explanations, since conditionally shifting the ‘software’ of behavioural strategy may be generally easier to achieve than overhauling the ‘hardware’ of morphology or physiology. While these kinds of constraints may exist, they are hard to demonstrate empirically. Moreover, as with constraints-based explanations in evolution more generally, they feel intellectually unsatisfactory, since they are somewhat ad hoc. At the very least, advocates of somatic state-based hypotheses need to explain why the effect of I on S cannot be undone in adulthood, but instead has to be mitigated by developing P.
To summarize, the principal differences between informational and somatic state-based hypotheses concern their first and second assumptions (as listed in §3). Informational hypotheses need to justify their assumption that over evolutionary time, I carried information about E reliably enough for plasticity to evolve. Somatic state-based hypotheses need to justify their assumption that over evolutionary time, I caused variation in S that could not be avoided or reversed in adulthood. In both cases, assumptions will depend on the biology and evolutionary history of the organism involved.
5. Empirical tests of adaptive developmental plasticity
We are now in a position to examine how ADP hypotheses can be tested. This can be decomposed into two sub-questions; first, how to test whether the plasticity is adaptive, and second, how to test whether the plasticity is best thought of in informational or state-based terms. We will assume that we are dealing with systems where some measure of, or proxy for, fitness is available. For the first sub-question, the unifying principle makes the criteria clear: amongst those experiencing I, those who develop P should have higher average fitness than those who do develop ⌝P, while among those who experience ⌝I, those who do develop ⌝P should have higher average fitness than those who develop P (figure 2). This crossover pattern should exist for ADP of any type. Note that average fitness does not need to be the same across individuals who experience I and those who experience ⌝I; whether ADP is informational or somatic state-based, this is unlikely to be the case. This is not important for the evolutionary stability of ADP, since selection is hypothesized to be acting on the conditional development of P|I, not on the probability of experiencing I, which is assumed to be extrinsic.
Figure 2.
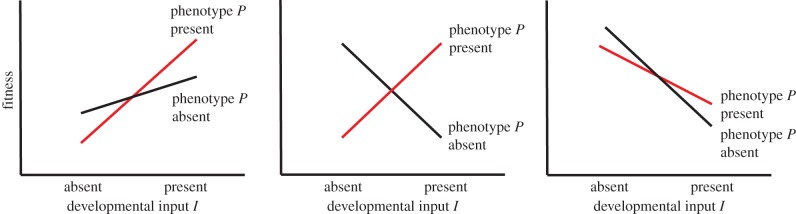
The pattern of expected fitness that should be seen in all cases of ADP. The slopes of the lines are unimportant provided that they cross, and hence patterns resembling any of the three panels would represent ADP. (Online version in colour.)
The basic test of whether plasticity is adaptive, then, involves measuring the fitness of four classes of individuals: those who experience the input and develop the phenotype, those who do not experience the input and do not develop the phenotype, those who experience the input but do not develop the phenotype, and those who do not experience the input but do develop the phenotype. A potential difficulty is that if the conditional adaptation ‘if I, develop P’ has gone to fixation, then individuals of the last two classes are likely to be rare or non-existent. However, variation in evolved traits can usually be found: even at equilibrium, it will persist at some level through mutation and stochastic developmental variation. For example, in bulb mites, a rich diet during development produces 75% fighters and 25% scramblers, while a poor diet produces 10% fighters and 90% scramblers [2]. In induction experiments with Daphnia, the presence of predator cues raises the frequency of defended morphs to only around 80%, and moreover there is genetic variation in cue responsiveness [20]. Thus, the required variation should be identifiable.
A number of recent empirical studies have attempted to establish whether there is a fitness crossover [26,38–42]. However, the fitness crossover sought was not the one shown in figure 2. The crossover they investigated compared w(E|I) with w(⌝E|I) and w(⌝E|⌝I) with w(E|⌝I). This relates to the prediction that individuals for whom the adult environment is congruent with early experience should be advantaged relative to those for whom the adult environment is incongruent. There are a number of points to be made about this ‘mismatch’ crossover prediction. First, it is a prediction made only by informational ADP hypotheses. It arose particularly in discussion of the PAR concept, which is an informational hypothesis. Thus, although the presence of the ‘mismatch’ crossover is suggestive of ADP, its absence is not evidence against ADP. If the ADP is somatic state-based, there is no reason to expect a fitness crossover based on congruence of adult and developmental environment. Second, even under informational ADP hypotheses, the ‘mismatch’ crossover prediction only applies to individuals that developed the phenotype P. A strong test of the mismatch crossover prediction should thus measure the phenotypic trait P, and show that any crossover was detectable only in the subset of individuals showing it. Empirical studies have not typically done this. Indeed, the empirical tests cited above do not even specify what the putatively adaptive phenotype is. This may reflect an assumption that if the plasticity has evolved, the phenotypic response to I will be at fixation, and so it can simply be assumed that all individuals who experienced I will develop P. However, as already mentioned, there will always be variation in the conditional expression of the phenotype. It would thus be better for the phenotype P of the adults who experienced I to be measured independently of I and E. The presence of P should moderate the interaction of I and E in predicting fitness.
Demonstrating the pattern shown in figure 2 is a necessary step in any empirical test of ADP, but researchers also need to specify what type of ADP hypothesis they are advancing, and justify the relevant assumptions as discussed in §4. Table 1 provides a checklist of the steps necessary to confirm an ADP hypothesis. Four steps are required in all cases; after that, the steps vary according to type of hypothesis proposed. In well-understood cases, many of the steps have been addressed (e.g. in Daphnia, [19–21]). Many studies fulfil just one or two of the steps [41,43]. This is still useful, not least because it stimulates further studies that will fill in the gaps.
Table 1.
The definitions that must be made and empirical patterns demonstrated to document a case of ADP. (See text for definitions. In keeping with the text, the empirical patterns have been stated in terms of dichotomous variables. They can be straightforwardly translated into general linear model form for continuous empirical cases. For example, the second requirement translates as the need for a significant regression coefficient of P on I, and the third line as the need for a significant interaction between P and I in predicting w.)
| general | |
|---|---|
| define I and P | |
| show that I leads to the development of P | |
| demonstrate that w(P|I) > w(⌝P|I) and w(⌝P|⌝I) > w(P|⌝I) | |
| specify an informational or somatic state-based hypothesis | |
| information-based | somatic state-based |
| define relevant adult environment E | define a relevant adult somatic state variable S |
| demonstrate that w(P|E) > w(⌝P|E) and w(⌝P|⌝E) > w(P|⌝E); or, relatedly, demonstrate that w(E|I) > w(⌝E|I) and w(⌝E|⌝I) > w(E|⌝I) | demonstrate that w(P|S) > w(⌝P|S) and w(⌝P|⌝S) > w(P|⌝S) |
| show that under evolutionarily relevant conditions, I carries information about E | show that I causes lasting variation in S |
6. Adaptive and non-adaptive plasticity revisited
So far, we have simplified the possible typology of developmental plasticity into non-adaptive, adaptive (informational) and adaptive (somatic state-based). While this classification is heuristically useful, cases of developmental plasticity typically involve a mixture of adaptive and non-adaptive components. In fact, all ADP hypotheses contain non-adaptive elements. In many somatic state-based hypotheses, the input I leaves the individual in a poorer state S than she otherwise would be; she adopts P because this is her best strategy given S; for this reason, state-based ADP is often characterized as ‘coping’, ‘mitigation’ or ‘making the best of a bad job’ [9,25]. The impact of I on S, therefore, is deleterious; individuals would have higher fitness if they could manage not to end up with S despite I. Thus, there is a non-adaptive branch of the hypothesis (I affects S), and an adaptive response branch (S triggers P). The adaptive branch is assumed to represent the outcome of selection, whereas the non-adaptive branch is assumed to persist because there are some things selection cannot do.
Less obviously, there are non-adaptive or constraint ideas implicit in informational hypotheses too. In these hypotheses, individuals use early experience to gain information about the best phenotype to develop, and commit to this. However, this strategy leaves them at risk of ending up mismatched to the adult environment [18,44]. It is seldom explored whether it is optimal to use early-life experience to commit to a phenotype in this way; it seems obvious that, in general terms, a better strategy would be to remain open to environmental input, and be able to shift phenotype during adult life. There are plenty of mechanisms that enable organisms to do this, associative learning and tanning being two examples. To the extent that informational ADP hypotheses discuss these issues, they invoke some kind of constraint. There are some aspects of phenotype to which an early commitment is necessary simply because of the way that bodies and brains develop (or else there is an unspecified cost to remaining plastic, as assumed by Botero et al. [10]). While this may be correct, it is a non-adaptive explanation; there are some things that selection cannot build. Thus, even informational ADP hypotheses typically invoke adaptation only within an envelope of developmental constraints.
These complexities mean that all cases of developmental plasticity will contain both non-adaptive and adaptive components: the non-adaptive components are the constraints that cannot readily be modified by selection, and the adaptive components are the variable responses available to selection within the envelope defined by those constraints. This is why it is important to be precise about exactly what P is in any putative example of ADP.
To further complicate the picture, adaptive responses may entrain non-adaptive by-products. For example, we recently found that European starlings which had experienced greater food scarcity in the nest were more food motivated than their peers at 1 year old [45]. This was despite the fact that their energy reserves at time of testing were no different. The persistence of this ‘hungry phenotype’ is hard to explain in adaptive terms. There is no reason to believe that nestling food scarcity is a cue to the future environment—if anything, parents have large broods where the environment is good, so food scarcity in the nest should be a negative predictor of later scarcity [9]. There may have been some lasting somatic difference as a result of early scarcity, such as raised metabolic rate [46], but we were not able to demonstrate this. One possibility is that the adult ‘hungry phenotype’ is simply a non-adaptive carryover of a response that was immediately adaptive at fledging. If birds fledge with low energy reserves, they need to initiate independent foraging quickly, and so their food motivation needs to be upregulated. The fitness benefit of successfully initiating the hungry phenotype conditional on body weight at 20 days will be much stronger than the fitness cost of failing to turn it off when it is no longer advantageous. Thus, when we examine the long-term effects of early experience, we may often be studying phenotypes whose initiation by a developmental input was adaptive at the time, but whose persistence much later in life is a by-product not removed, because its costs later in life are relatively low. This in effect a ‘semi-adaptive’ explanation; the phenotype measured by the researcher is the non-adaptive echo of something that did have a function earlier in life. Semi-adaptive and truly adaptive ADP can in principle be distinguished empirically, but doing so in practice will not be easy.
7. Discussion and conclusion
Developmental plasticity is an important phenomenon. It is consequential for public health, because variation in health and behavioural outcomes may be related to variation in developmental inputs years earlier. It is important for behavioural ecologists, because there is always variation in the response of adult individuals to current situations, some of which may be owing to different developmental histories. From both an evolutionary and a practical perspective, it is important to be able to identify which aspects of developmental plasticity are adaptive and which not. We have presented a framework within which this distinction can be made, at least in principle.
It is important to distinguish different types of adaptive explanation for developmental plasticity, specifically informational ADP hypotheses from somatic state-based ones. Although there has been extensive discussion of ADP in recent years, the informational versus somatic state-based distinction is not always clearly presented. Even where it has been, the term adaptive has most often been used in the context of informational effects, with other terms such as mitigation, coping and ‘making the best of a bad job’ attaching to somatic state-based possibilities. However, somatic state-based hypotheses are properly adaptive hypotheses.
Both informational and somatic state-based ADP is likely to exist in nature. However, the selective scenarios under which each can emerge are different. Thus, why, and as well as whether, a case of developmental plasticity turns out to be adaptive will depend on the details of the biology of the organism. It is not a surprise, for example, that the ‘mismatch’ fitness crossover predicted by informational ADP models is not empirically ubiquitous [38,42]. Moreover, it is our impression that somatic state-based hypotheses have been neglected. They have been neglected in general theoretical discussions, which focus heavily on the role of developmental inputs in providing information about the environment [47,48], and less on developmental inputs as determinants of adult somatic state. This is puzzling, because there is a long tradition of theory in behavioural ecology showing that animal decisions should be state-dependent [49–51], to which somatic state-based ADP hypotheses can be connected. The literature on whether perinatal influences on adult phenotype in humans might be adaptive has also focused heavily on informational ideas [14,28,30,44]. The developmental inputs discussed in these contexts, such as early-life nutrition and the level of parental investment, are not only separated from the putatively adaptive response by a long time delay, but are also of the kind likely to have very general impacts on systemic functioning and survival prospects [9,52,53]. Thus, to the extent that ADP explanations are relevant, somatic state-based hypotheses seem to be promising candidates [54–56]. By carefully investigating the assumptions of both informational and state-based hypotheses, and by filling in as many as possible of the steps shown in table 1, it may be possible to adjudicate between the competing possibilities in such cases.
Acknowledgements
We thank Isabel Smallegange for providing additional data, and Patrick Bateson, Willem Frankenhuis and Ian Rickard for discussions of these issues.
Authors' contributions
D.N. and M.B. developed the ideas in this review together. D.N. drafted the manuscript with help from M.B. Both authors gave final approval for submission.
Competing interests
We delcare we have no competing interests.
Funding
This work was supported by the BBSRC under grant no. BB/J016446/1 and the NC3Rs under grant no. NC/K000802/1.
References
- 1.Storm JJ, Lima SL. 2010. Mothers forewarn offspring about predators: a transgenerational maternal effect on behavior. Am. Nat. 175, 382–390. ( 10.1086/650443) [DOI] [PubMed] [Google Scholar]
- 2.Smallegange IM. 2011. Complex environmental effects on the expression of alternative reproductive phenotypes in the bulb mite. Evol. Ecol. 25, 857–873. ( 10.1007/s10682-010-9446-6) [DOI] [Google Scholar]
- 3.O'Hagan D, Andrews CP, Bedford T, Bateson M, Nettle D. 2015. Early life disadvantage strengthens flight performance trade-offs in European starlings, Sturnus vulgaris. Anim. Behav. 102, 141–148. ( 10.1016/j.anbehav.2015.01.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nettle D. 2014. What the future held: childhood psychosocial adversity is associated with health deterioration through adulthood in a cohort of British women. Evol. Hum. Behav. 35, 519–525. ( 10.1016/j.evolhumbehav.2014.07.002) [DOI] [Google Scholar]
- 5.Stearns SC. 1989. The evolutionary significance of phenotypic plasticity. Bioscience 39, 436–445. ( 10.2307/1311135) [DOI] [Google Scholar]
- 6.Rodriguez A, Bohlin G. 2005. Are maternal smoking and stress during pregnancy related to ADHD symptoms in children? J. Child Psychol. Psychiatry 46, 246–254. ( 10.1111/j.1469-7610.2004.00359.x) [DOI] [PubMed] [Google Scholar]
- 7.Nederhof E, Schmidt MV. 2012. Mismatch or cumulative stress: toward an integrated hypothesis of programming effects. Physiol. Behav. 106, 691–700. ( 10.1016/j.physbeh.2011.12.008) [DOI] [PubMed] [Google Scholar]
- 8.McMillen IC, Robinson JS. 2005. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol. Rev. 85, 571–633. ( 10.1152/physrev.00053.2003) [DOI] [PubMed] [Google Scholar]
- 9.Monaghan P. 2008. Early growth conditions, phenotypic development and environmental change. Phil. Trans. R. Soc. B 363, 1635–1645. ( 10.1098/rstb.2007.0011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Botero CA, Weissing FJ, Wright J, Rubenstein DR. 2014. Evolutionary tipping points in the capacity to adapt to environmental change. Proc. Natl Acad. Sci. USA 112, 184–189. ( 10.1073/pnas.1408589111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snell-Rood EC. 2013. An overview of the evolutionary causes and consequences of behavioural plasticity. Anim. Behav. 85, 1004–1011. ( 10.1016/j.anbehav.2012.12.031) [DOI] [Google Scholar]
- 12.Uller T. 2008. Developmental plasticity and the evolution of parental effects. Trends Ecol. Evol. 23, 432–438. ( 10.1016/j.tree.2008.04.005) [DOI] [PubMed] [Google Scholar]
- 13.Burton T, Metcalfe NB. 2014. Can environmental conditions experienced in early life influence future generations? Proc. R. Soc. B 281, 20140311 ( 10.1098/rspb.2014.0311) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gluckman PD, Hanson MA, Spencer HG. 2005. Predictive adaptive responses and human evolution. Trends Ecol. Evol. 20, 527–533. ( 10.1016/j.tree.2005.08.001) [DOI] [PubMed] [Google Scholar]
- 15.Costantini D, Metcalfe NB, Monaghan P. 2010. Ecological processes in a hormetic framework. Ecol. Lett. 13, 1435–1447. ( 10.1111/j.1461-0248.2010.01531.x) [DOI] [PubMed] [Google Scholar]
- 16.Parker KJ, Buckmaster CL, Sundlass K, Schatzberg AF, Lyons DM. 2006. Maternal mediation, stress inoculation, and the development of neuroendocrine stress resistance in primates. Proc. Natl Acad. Sci. USA 103, 3000–3005. ( 10.1073/pnas.0506571103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nijhout HF. 2003. Development and evolution of adaptive polyphenisms. Evol. Dev. 5, 9–18. ( 10.1046/j.1525-142X.2003.03003.x) [DOI] [PubMed] [Google Scholar]
- 18.Bateson P, et al. 2004. Developmental plasticity and human health. Nature 430, 419–421. ( 10.1038/nature02725) [DOI] [PubMed] [Google Scholar]
- 19.Laforsch C, Tollrian R. 2014. Inducible defenses in multipredator environments: cyclomorphosis in Daphnia cucullata. Ecology 85, 2302–2311. ( 10.1890/03-0286) [DOI] [Google Scholar]
- 20.Havel JE. 1985. Cyclomorphosis of Daphnia pulex spined morphs. Limnol. Oceanogr. 30, 853–861. ( 10.4319/lo.1985.30.4.0853) [DOI] [Google Scholar]
- 21.Havel JE, Dodson SI. 1987. Reproductive costs of Chaoboras-induced polymorphism in Daphia pulex. Hydrobiologia 150, 273–281. ( 10.1007/BF00008708) [DOI] [Google Scholar]
- 22.Shannon CE. 1948. A mathematical theory of communication. Bell Syst. Tech. J. 27, 379–423. ( 10.1145/584091.584093) [DOI] [Google Scholar]
- 23.Price GR. 1970. Selection and covariance. Nature 227, 520–521. ( 10.1038/227520a0) [DOI] [PubMed] [Google Scholar]
- 24.DeWitt TJ, Sih A, Wilson DS. 1998. Costs and limits of phenotypic plasticity. Trends Ecol. Evol. 13, 77–81. ( 10.1016/S0169-5347(97)01274-3) [DOI] [PubMed] [Google Scholar]
- 25.Bateson P, Gluckman P, Hanson M. 2014. The biology of developmental plasticity and the predictive adaptive response hypothesis. J. Physiol. 592, 2357–2368. ( 10.1113/jphysiol.2014.271460) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Douhard M, Plard F, Gaillard J, Capron G, Delorme D, Duncan P, Loe LE, Bonenfant C. 2014. Fitness consequences of environmental conditions at different life stages in a long-lived vertebrate. Proc. R. Soc. B 281, 20140276 ( 10.1098/rspb.2014.0276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dicke M, Sabelis MW. 1988. Infochemical terminology: based on cost–benefit analysis rather than origin of compounds? Funct. Ecol. 2, 131–139. ( 10.2307/2389687) [DOI] [Google Scholar]
- 28.Brumbach BH, Figueredo AJ, Ellis BJ. 2009. Effects of harsh and unpredictable environments in adolescence on development of life history strategies: a longitudinal test of an evolutionary model. Hum. Nat. 20, 25–51. ( 10.1007/s12110-009-9059-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chisholm JS, Quinlivan JA, Petersen RW, Coall DA. 2005. Early stress predicts age at menarche and first birth, adult attachment, and expected lifespan. Hum. Nat. 16, 233–265. ( 10.1007/s12110-005-1009-0) [DOI] [PubMed] [Google Scholar]
- 30.Del Giudice M. 2009. Sex, attachment, and the development of reproductive strategies. Behav. Brain Sci. 32, 1–21; discussion 21–67 ( 10.1017/S0140525X09000016) [DOI] [PubMed] [Google Scholar]
- 31.Quinlan RJ. 2007. Human parental effort and environmental risk. Proc. R. Soc. B 274, 121–125. ( 10.1098/rspb.2006.3690) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hönekopp J. 2009. Pre-adjustment of adult attachment style to extrinsic risk levels via early attachment style is neither specific, nor reliable, nor effective, and is thus not an adaptation. Behav. Brain Sci. 32, 31 ( 10.1017/S0140525X09000120) [DOI] [Google Scholar]
- 33.Wells JCK. 2007. Flaws in the theory of predictive adaptive responses. Trends Endocrinol. Metab. 18, 331–337. ( 10.1016/j.tem.2007.07.006) [DOI] [PubMed] [Google Scholar]
- 34.Baig U, Belsare P, Watve M, Jog M. 2011. Can thrifty gene(s) or predictive fetal programming for thriftiness lead to obesity? J. Obes. 2011, 861049 ( 10.1155/2011/861049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nettle D, Frankenhuis WE, Rickard IJ. 2013. The evolution of predictive adaptive responses in human life history. Proc. R. Soc. B 280, 20131343 ( 10.1098/rspb.2013.1343) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bateson M, Brilot BO, Gillespie R, Monaghan P, Nettle D. 2015. Developmental telomere attrition predicts impulsive decision making in adult starlings. Proc. R. Soc. B 282, 20142140 ( 10.1098/rspb.2014.2140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heidinger BJ, Blount JD, Boner W, Griffiths K, Metcalfe NB, Monaghan P. 2012. Telomere length in early life predicts lifespan. Proc. Natl Acad. Sci. USA 109, 1743–1748. ( 10.1073/pnas.1113306109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Willführ KP, Myrskylä M. 2013. Phenotype-environment mismatch due to epigenetic inheritance? Programming the offspring's epigenome and the consequences of migration. Am. J. Hum. Biol. 25, 318–328. ( 10.1002/ajhb.22362) [DOI] [PubMed] [Google Scholar]
- 39.Hayward AD, Rickard IJ, Lummaa V. 2013. Influence of early-life nutrition on mortality and reproductive success during a subsequent famine in a preindustrial population. Proc. Natl Acad. Sci. USA 110, 13 886–13 891. ( 10.1073/pnas.1301817110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hayward AD, Lummaa V. 2013. Testing the evolutionary basis of the predictive adaptive response hypothesis in a preindustrial human population. Evol. Med. Public Heal. 2013, 106–117. ( 10.1093/emph/eot007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Costantini D, Monaghan P, Metcalfe NB. 2014. Prior hormetic priming is costly under environmental mismatch. Biol. Lett. 10, 20131010 ( 10.1098/rsbl.2013.1010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uller T, Nakagawa S, English S. 2013. Weak evidence for anticipatory parental effects in plants and animals. J. Evol. Biol. 26, 2161–2170. ( 10.1111/jeb.12212) [DOI] [PubMed] [Google Scholar]
- 43.Chin EH, Love OP, Verspoor JJ, Williams TD, Rowley K, Burness G. 2009. Juveniles exposed to embryonic corticosterone have enhanced flight performance. Proc. R. Soc. B 276, 499–505. ( 10.1098/rspb.2008.1294) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gluckman PD, Hanson MA, Beedle AS. 2007. Early life events and their consequences for later disease: a life history and evolutionary perspective. Am. J. Hum. Biol. 19, 1–19. ( 10.1002/ajhb) [DOI] [PubMed] [Google Scholar]
- 45.Bloxham L, Bateson M, Bedford T, Brilot B, Nettle D. 2014. The memory of hunger: developmental plasticity of dietary selectivity in the European starling, Sturnus vulgaris. Anim. Behav. 91, 33–40. ( 10.1016/j.anbehav.2014.02.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verhulst S, Holveck M-J, Riebel K. 2006. Long-term effects of manipulated natal brood size on metabolic rate in zebra finches. Biol. Lett. 2, 478–480. ( 10.1098/rsbl.2006.0496) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stamps JA, Krishnan VV. 2014. Combining information from ancestors and personal experiences to predict individual differences in developmental trajectories. Am. Nat. 184, 647–657. ( 10.1086/678116) [DOI] [PubMed] [Google Scholar]
- 48.Leimar O, McNamara JM. 2015. The evolution of transgenerational integration of information in heterogenous environments. Am. Nat. 185, 55–69. ( 10.1086/679575) [DOI] [PubMed] [Google Scholar]
- 49.Maynard Smith J, Parker GA. 1976. The logic of asymmetric contests. Anim. Behav. 24, 159–175. ( 10.1016/S0003-3472(76)80110-8) [DOI] [Google Scholar]
- 50.McNamara J, Houston A. 1996. State-dependent life histories. Nature 380, 215–221. ( 10.1038/380215a0) [DOI] [PubMed] [Google Scholar]
- 51.Mangel M, Clark CW, Clark CW. 1986. Towards a unified foraging theory. Ecology 67, 1127–1138. ( 10.2307/1938669) [DOI] [Google Scholar]
- 52.Lindström J. 1999. Early development and fitness in birds and mammals. Trends Ecol. Evol. 14, 343–348. ( 10.1016/S0169-5347(99)01639-0) [DOI] [PubMed] [Google Scholar]
- 53.Rickard IJ, Holopainen J, Helama S, Helle S, Russell AF, Lummaa V. 2010. Food availability at birth limited reproductive success in historical humans. Ecology 91, 3515–3525. ( 10.1890/10-0019.1) [DOI] [PubMed] [Google Scholar]
- 54.Jones JH. 2005. Fetal programming: adaptive life-history tactics or making the best of a bad start? Am. J. Hum. Biol. 17, 22–33. ( 10.1002/ajhb.20099) [DOI] [PubMed] [Google Scholar]
- 55.Rickard IJ, Frankenhuis WE, Nettle D. 2014. Why are childhood family factors associated with timing of maturation? A role for internal prediction. Perspect. Psychol. Sci. 9, 3–15. ( 10.1177/1745691613513467) [DOI] [PubMed] [Google Scholar]
- 56.Wells JCK. 2012. Obesity as malnutrition: the role of capitalism in the obesity global epidemic. Am. J. Hum. Biol. 24, 261–276. ( 10.1002/ajhb.22253) [DOI] [PubMed] [Google Scholar]