Abstract
Natural and anthropogenic static electric fields are commonly found in the environment and can have both beneficial and harmful effects on many animals. Here, we asked how the fruitfly responds to these fields and what the consequences of exposure are on the levels of biogenic amines in the brain. When given a choice in a Y-tube bioassay Drosophila avoided electric fields, and the greater the field strength the more likely Drosophila were to avoid it. By comparing wild-type flies, flies with wings surgically removed and vestigial winged flies we found that the presence of intact wings was necessary to produce avoidance behaviour. We also show that Coulomb forces produced by electric fields physically lift excised wings, with the smaller wings of males being raised by lower field strengths than larger female wings. An analysis of neurochemical changes in the brains showed that a suite of changes in biogenic amine levels occurs following chronic exposure. Taken together we conclude that physical movements of the wings are used by Drosophila in generating avoidance behaviour and are accompanied by changes in the levels of amines in the brain, which in turn impact on behaviour.
Keywords: static electric fields, high voltage, locomotion, amines, fly
1. Introduction
Electric fields in the environment can have profound effects on the behaviour of plants and animals. Considerable attention has focused on insects, and we now know that static electric fields can have both beneficial and detrimental effects, as well as affecting their behavioural responses to these fields. Bees, for example, produce static electric fields during flying and walking caused by friction charging. The fields are sufficiently large to induce movements of the antennae of conspecifics nearby, which are thought to act as a biologically relevant stimulus that may play a role in social communication [1]. These fields may also alter the behaviour of the bee parasite Varroa jacobsoni, and can assist their attack by attraction of the parasite to the charged bee [2]. Other studies have suggested that accumulation of charge during flight could assist foraging and pollination through the transfer of pollen grains onto plant stigmata [3–5]. Static electric fields can also lead to changes in walking [6,7] and avoidance behaviour [6,8], and influence locomotion and agitation [9–11].
Recent studies have suggested that while insects have not evolved a specialized sense for detecting static electric fields, they can do so by virtue of the displacement of long slender structures such as the antennae or mechanosensory hairs caused by Coulomb forces [7]. Such displacements are sufficient to evoke neural activity and thus lead to avoidance behaviour, as has also been demonstrated in bees [1]. The attractive and repulsive forces generated by static electric fields have proportionately more influence on smaller insects than larger insects [9], and this has led to the development of electric screens to prevent entry of small insects into glasshouses [12].
Electromagnetic fields (EMFs) have been shown to alter the neurochemistry of the brains of rats, raising the levels of amine metabolites and dopamine in specific regions of the brain [13,14]. EMFs have also been shown to increase the levels of heat shock proteins, which often increase in response to stress [15]. Taken together these results raise the question as to whether static electric fields could also alter the brain's neurochemistry in insects. Amines play a major role in the behaviour of insects, from underpinning extreme changes in phenotypic plasticity in locusts where dopamine and serotonin have a major role in driving the swarming form of migratory and desert locusts [16–18], to social interactions between nest-mates influencing brain biogenic amine homeostasis in stressed ants [19,20]. They also play a crucial role in aggressive encounters between dominant and submissive animals [21].
For the model organism Drosophila, an understanding of the effect of electric fields on physiology and behaviour is crucially important given the wide-scale use of plastics in the rearing of these insects. Plastics are well known for their ability to both charge and retain a charge for long periods through triboelectrification [8,22], and chronic exposure to these fields during rearing is inevitable. Moreover, understanding whether static electric fields have meaning to Drosophila as an environmental stimulus in the context of spontaneous choice and learning is important to establish. This study therefore asks if static electric fields generated through induction charging affect Drosophila behaviour, how they might be detected and how they change the levels of biogenic amines in the brain.
2. Material and methods
Wild-type Drosophila melanogaster (Oregon-R) were obtained from Blades Biological Supplies Ltd (UK) and reared on a yeast/sugar medium with added live baker's yeast [23] in 50 ml glass bottles at 20 ± 1°C and under a 16 L : 8 D cycle.
(a). Responses to electric fields
A Y-tube consisting of three cylindrical glass chambers (2 mm thick, 150 mm in length × 30 mm inner diameter) fused together at 120° (figure 1a) was used to quantify the responses of Drosophila to induced static electric fields. Two copper rings, 5 × 28 mm (width × diameter), were positioned inside each arm 14 mm from the intersection with the vertical arm and attached to an insulated socket through a 7 mm hole in the surface (figure 1a). One copper ring was connected to a Brandenburg Alpha III power supply (Brandenburg, UK) and the other to ground. The ends of each arm were covered by two further tubes to capture insects as they passed through the Y-tube. One arm of a dual gooseneck cold light source (Schott KL 1500 LCD) was focused through each arm of the Y-tube to encourage upward, phototactic movement of the flies. The vertical arm (C) was used as a release chamber and covered with an aluminium mesh (1 mm2) connected to ground to prevent electric fields in this area. The Y-tube was secured by a metallic holder and placed inside a dark aluminium cage [7] in a laboratory in which the relative humidity ranged 35–45%.
Figure 1.
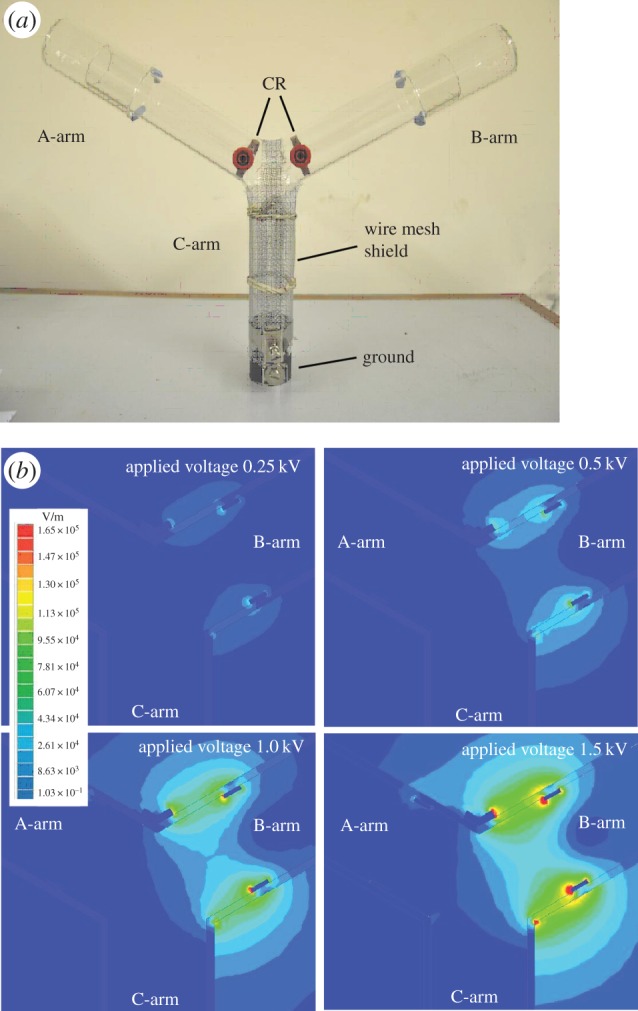
The Y-bioassay and static electric field distribution. (a) Photograph showing the Y-tube and the copper ring electrodes (CR). (b) Maxwell FEA models of the electric field distribution around the Y-tube at 0.25, 0.5, 1.0 and 1.5 kV. (Online version in colour.)
Groups of 20 flies (males and females) were placed into eight new tubes (50 ml), each containing a small piece of wet tissue to prevent desiccation. Flies were starved for 24 h prior to experimentation to encourage locomotory behaviour. The stopper of the tube containing the flies was removed, and the tube was presented to the base of the Y-tube and the flies allowed to move freely. Different voltages (0, 0.12, 0.25, 0.5, 1, 1.5, 2 and 3 kV) were applied to one arm of the Y-tube to test their effect on each group of flies. The responses of flies to each voltage were tested with eight different tubes of flies (n = 8). After 5 min, the distribution of the flies within each arm of the Y-tube was assessed, and the number of flies remaining in the release arm was analysed using one-way ANOVA (SPSS v. 17 software). Flies were exposed to one voltage only. Preliminary studies showed that there were a constant number of flies remaining in the release arm at all voltages, suggesting that the release arm was not affected by electric fields. The charged arm of the Y-tube was randomly switched between experiments to reduce the effects of experimental bias, and after each experiment the apparatus was washed and dried.
(b). Electric field modelling within the Y-tube apparatus
Maxwell SV v. 7 two-dimensional software (Ansoft Corporation, Pittsburgh, USA) was used to model electric fields within the Y-tube, based on the physical properties of the materials, their sizes and the applied voltages. This modelling provides accurate estimates of the magnitude of the electric fields within the Y-tube that can be correlated with the response of the flies at different field strengths [7]. In Maxwell SV, the Y-tube was drawn as a simple two-dimensional x–y model with a cross-section taken from each part of the Y-tube (figure 1b). The electric fields generated by different applied voltages (0, 0.25, 0.5, 1, 1.5, 2 and 3 kV) were modelled.
(c). The role of the wings in detection and avoidance of static fields
To assess the role of the wings in the detection and avoidance of electric fields, three groups of flies were exposed to static electric fields at different voltages (0, 0.5, 1, 2 and 3 kV) using the Y-tube apparatus. The flies included wild-type flies, cut wing flies in which the wings of wild-type flies were physically removed and vestigial winged mutants (Blades Biological Ltd, UK). For all flies, the halteres remained intact. Flies were grouped into five tubes (50 ml), each group consisting of 20 flies (males and females randomly selected). Flies were maintained in these tubes for 24 h to recover, with the addition of a small piece of wet tissue in the tube to prevent desiccation. Flies were allowed to move freely, and the number in each arm of the Y-tube was counted after 5 min and the experiment repeated six times for each fly group.
(d). Wing displacement
To measure the direct effects of electric fields on the wings, flies were placed under a glass chamber (2 mm thick, 100 × 30 mm, L × D) fixed in a metallic holder. A copper ring electrode (4 × 28 mm, W × D) was fixed at the entrance of the chamber and attached to a DC power supply (Brandenburg Alpha III, Brandenburg, UK) to generate a static field. Eight male and eight female flies were tested individually. First, each living fly was fixed on a glass slide underneath the charged electrode using sticky paper (EasiStick’ Traps, Fargro Ltd) and then exposed to electric fields at different voltages. The fly was then killed using CO2 and tested again. To measure wing displacement, flies were photographed at each voltage using a Nikon digital camera (D80) and photographs analysed using Canvas X (ACD Systems Inc., USA) to calculate wing angle (the angle between a line that extended from the mid-point of the head to the wing hinge and another between the tip of the wing and the wing hinge). No difference was found between live and dead flies (data not shown).
In addition, the wings of individual flies were excised and placed on a glass slide to measure the field strength required to raise the wing towards the charged electrode. After each trial, images of the wing were collected using a compound microscope (Zeiss Axiophot) with a digital camera (Roper Scientific RTE/CCD-1300-y). The length and width of the wings were measured using MetaMorph v. 6 software (Universal Imaging Corporation, PA, USA).
(e). Statistical analysis
During all experiments, the numbers of flies in the uncharged and charged arms of the Y-tube were counted, and a response index (RI) was calculated based on the number of flies in the uncharged arm minus the number of flies in the charged arm, divided by the total number of flies [24,25]. An RI value above zero indicated that the flies avoided the electric fields. Data were tested for normality and homogeneity, and the statistical significance assessed using one-way ANOVA.
(f). Measurement of biogenic amines
The levels of biogenic amines in entire heads of Drosophila exposed to static electric fields of 70 kV m−1 were measured by collecting the heads of 10 flies following freezing in liquid nitrogen. The heads were homogenized in 50 µl of ice-cold 0.1 M perchloric acid containing 5 ng of 3, 4-dihydroxybenzylamine. After centrifugation of the homogenate (0°C, 15 000 r.p.m., 30 min), 40 µl of the supernatant was collected. Amines in the brain and heads were measured using high-performance liquid chromatography with electrochemical detection as described elsewhere [18,19].
3. Results
(a). Avoidance behaviour of flies
Static electric fields were applied to the electrode of one arm of the Y-tube to determine the behavioural responses of flies. Separate gender groups of wild-type flies were exposed to static electric fields generated at 0–3 kV for 5 min (n = 8 trials at each voltage with 20 flies per trial), and the flies in the uncharged and charged arms recoded. The results showed that both male and female wild-type flies significantly avoided electric fields (ANOVA, F4,25 = 6.64, p = 0.0009 and F4,25 = 20.41, p < 0.0001) after 5 min of exposure (figure 2a,b). Post hoc analysis showed that the threshold for avoidance occurred at 1 kV (field strength of 34–43 kV m−1, Maxwell SV modelling). The mean RI at this voltage was 0.59 ± 0.06 in males and 0.52 ± 0.05 in females, compared with 0.07 ± 0.14 in males and −0.10 ± 0.10 in females at 0 kV. The avoidance behaviour was also exhibited at higher voltages (1.5 and 2 kV; figure 2a,b). No statistically significant difference in avoidance behaviour was found between male and female flies (ANOVA, F1,60 = 0.808, p = 0.37). It should be noted that not all flies avoided the static electric fields even at higher applied voltages, and not all flies moved out of the release tube (see Material and methods).
Figure 2.
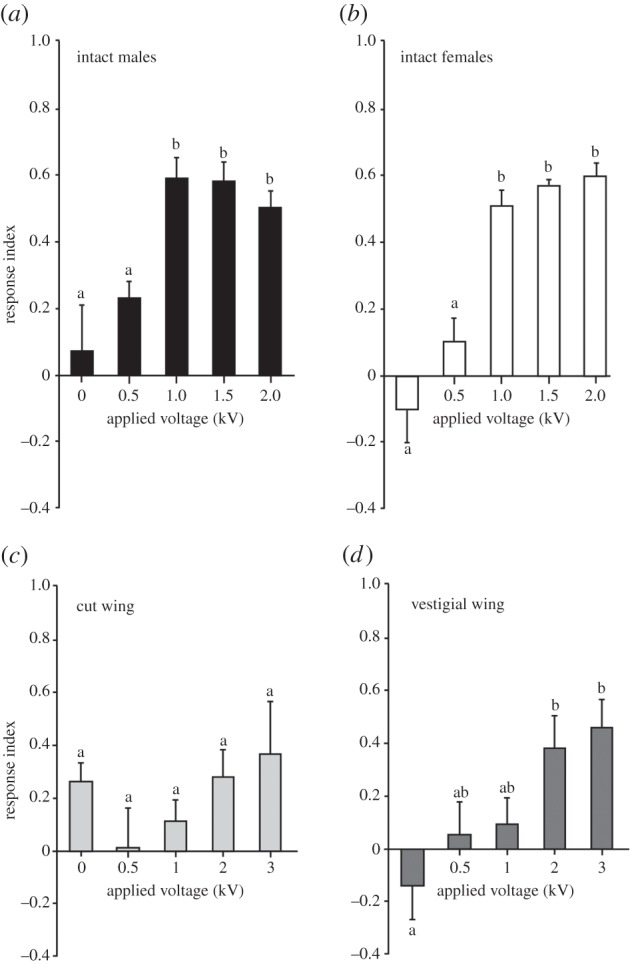
Avoidance behaviour of wild-type male and female flies to static electric fields at different voltages. (a) Males and (b) females showed significant avoidance behaviour to electric fields at 1 kV and above (p < 0.05 in both cases). There were no significant differences between male and female flies (p > 0.05 in all cases). (c) With wings removed, there was no significant difference in avoidance of static electric fields at any applied voltage (one-tailed t-test, p > 0.05 in all cases). (d) Vestigial wing flies significantly avoided the electric field at 2 kV and above. Bars on graphs represent mean ± s.e.m.
Given that the wings have been shown to vibrate during exposure to fields [26], we asked whether the wings play a role in detection of electric fields. To determine their role in generating avoidance, the wings of wild-type flies were cut close to the wing hinge. Analysis showed that the flies with cut wings (n = 6 trials with 20 flies for each trial) did not avoid electric fields even after application of 3 kV (ANOVA, F4,25 = 1.21, p = 0.33; figure 2c). By contrast, vestigial winged flies (n = 6 trials with 20 flies for each trial) showed significant avoidance (figure 2d) when electric fields of 2 kV (field strength 52–104 kV m−1) and 3 kV (95–164 kV m−1) were applied (ANOVA, F4,25 = 4.42, p = 0.004). The mean responses after 5 min at 0 and 2 kV were −0.14 ± 0.13 and 0.38 ± 0.12, respectively. Raising the applied voltage to 3 kV also led to greater avoidance compared with control (RI of −0.14 ± 0.13 and 0.46 ± 0.08).
(b). The role of the wings in the detection and avoidance of static electric fields
The movements of the wings were determined from flies fixed below a copper electrode. The wings of female and male flies (n = 8 for both groups) were displaced by static electric fields (ANOVA, F6,49 = 73.56, p < 0.0001 and F6,49 = 55.01, p < 0.0001), with greater field strengths causing greater angular displacements of the wing (figure 3). Post hoc tests showed significant wing elevation at 2 kV (57–96 kV m−1) and above in males compared to controls (Student's t-test, p < 0.0001 at 2, 3, 4 and 5 kV), and at 3 kV (96–115 kV m−1) and above in females (Student's t-test, p < 0.0001 at 3, 4 and 5 kV). There was also significantly greater elevation in males (14.48° ± 1.90°) than females (20.44° ± 4.20°) when 5 kV was applied (field strengths of 163–183 kV m−1, p = 0.0095).
Figure 3.
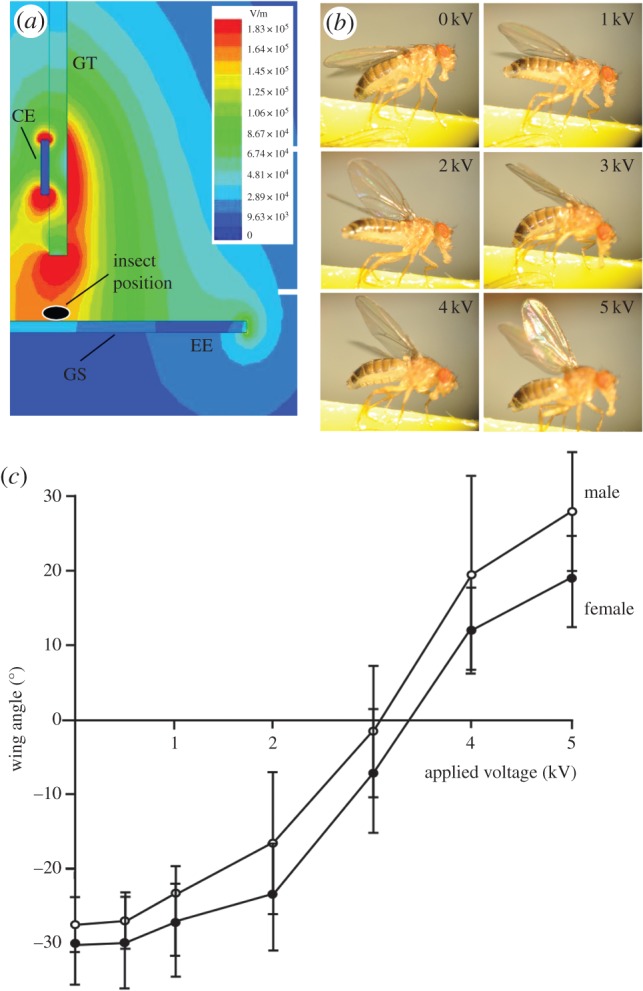
The effect of static electric fields on intact wings. (a) The magnitude and distribution of static electric fields around the charged electrode (CE) at 2 kV were modelled using Maxwell SV software. The 2 kV voltage applied to the electrode generated a field strength between 57 and 96 kV m–1 around the fly's position. GT, glass tube; GS, glass slide; EE, earth electrode. (b) Photographs of the effect of static electric fields at different voltages on wing deflection on a live Drosophila. (c) The elevation of Drosophila wings in response to fields at different voltages. Symbols represent mean ± s.e.m. Different letters indicate significant differences between applied voltages. (Online version in colour.)
To determine whether active movements contributed to wing elevation during exposure to electric fields, the wings of dead male and female flies were also analysed. There were no statistical differences in the elevation angles between the dead and live flies. For example, the mean (±s.e.m.) deflections of the wings of live male flies at 0, 2 and 5 kV were, respectively, −26.4 ± 0.99°, −16.55 ± 3.3° and 32.9 ± 4.55°, compared with −27.8 ± 1.55°, −18.54 ± 2.48 and 25.55 ± 3.05° for dead flies (Student's t-test, p = 0.438, 0.559 and 0.21).
(c). The effect of static electric fields on excised wings
Excised wings were exposed individually to electric fields at different voltages to determine the threshold required to raise the wings. The results demonstrated that male excised wings (n = 8) were raised by application of 0.68 ± 0.05 kV (mean ± s.e.m.), corresponding to a modelled electric field strength of 28–38 kV m−1 compared with 1.06 ± 0.09 kV (38–48 kV m−1) for excised wings of females. The differences between males and females were statistically significant (Student's t-test, p = 0.004).
To determine whether the difference between the sexes may be related, in part, to the morphology of the wings, the length and width of excised wings of both male and female flies were measured. The length and width of the wings in males were significantly smaller than those of females, with a mean length in males of 1.29 ± 0.036 mm compared with 1.49 ± 0.036 mm in females, and a mean width of 0.89 ± 0.024 mm in males compared with 0.98 ± 0.022 mm in females (Student's t-test, p = 0.0019 and 0.012, respectively).
(d). Neurochemical changes in brain amines during exposure to static electric fields
The levels of four key amines in the brains of Drosophila exposed to static electric fields at 70 kV m−1 for 4, 24 and 72 h were measured and compared with time-matched controls (figure 4). Serotonin levels decreased only slightly following 4 h exposure from 1.14 ± 0.21 (mean ± s.e.m.) to 0.93 ± 0.15 pmol brain−1, but to significantly lower levels following 24 h exposure from 0.8 ± 0.05 to 0.173 ± 0.021 pmol brain−1 (Student's t-test, p < 0.0001, d.f. = 17). After 72 h exposure, the levels of serotonin returned to control levels (0.89 ± 0.15 and 0.93 ± 0.015 pmol brain−1, respectively, p = 0.834, d.f. = 17).
Figure 4.
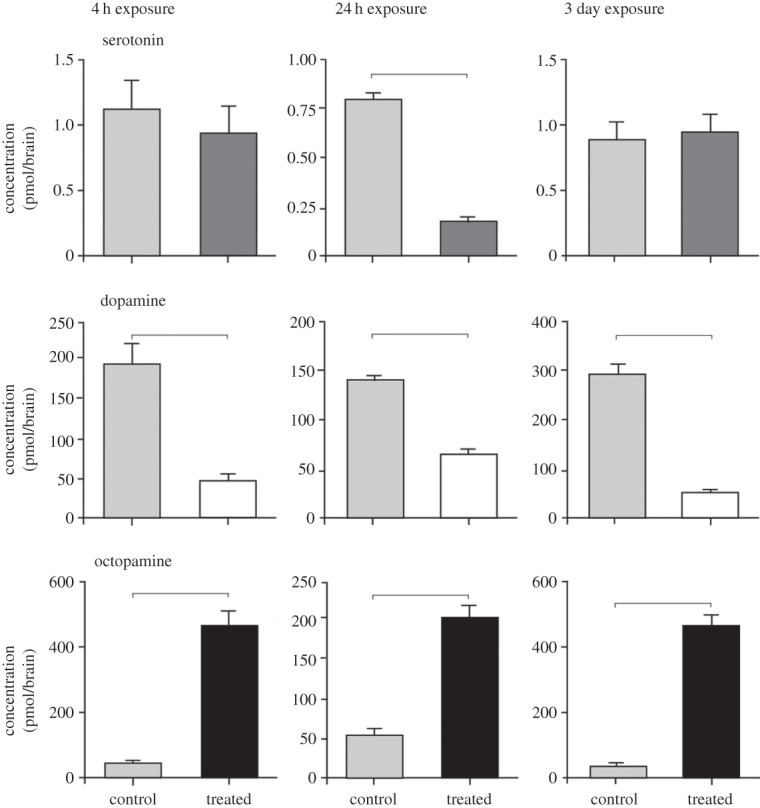
Analysis of three biogenic amines isolated from Drosophila heads. Serotonin levels significantly decreased only following exposure to a static electric field for 24 h. Dopamine levels were significantly reduced compared with controls at all exposure times and octopamine levels increased markedly in the isolated heads at all exposure times. Results are based on n = 9–10 heads and tested at each time point against time-matched controls using Student's t-tests. Significant differences are indicated by a line above each graph. Bars on graphs represent mean ± s.e.m.
Dopamine levels decreased significantly in treated flies at all exposure times. After 4 h exposure, dopamine levels decreased from 198.5 ± 26.24 to 48.85 ± 5.54 pmol brain−1. After 24 h exposure, dopamine levels declined from 142.7 ± 6.58 pmol brain−1 to 66.58 ± 3.97 pmol brain−1, while after 72 h exposure, dopamine levels fell from 293.5 ± 19.88 to 48.85 ± 5.54 pmol brain−1 (Student's t-test, p < 0.0001, d.f. = 17, for all exposure times).
By contrast, octopamine levels increased following exposure to static electric fields. After 4 h exposure, octopamine levels increased from 38.86 ± 9.97 to 467.9 ± 40.25 pmol brain−1. After 24 h exposure, octopamine levels increased from 53.5 ± 6.61 pmol brain−1 to 207.3 ± 14.16 pmol brain−1, while after 72 h exposure, octopamine levels increased from 29.65 ± 4.24 to 467.9 ± 40.25 pmol brain−1 (Student's t-test, p < 0.0001, d.f. = 17, for all exposure times). The levels of tyramine at all exposure times were low and below detection levels.
4. Discussion
Here we show that Drosophila avoid static electric fields and that exposure to static fields of 26 kV m−1 and above led to displacement of the wings. Removal of the wings reduced avoidance, suggesting that the wings were involved in the detection of static electric fields. We also found that exposure to levels of static electric fields that can be encountered in the environment led to changes in the neurochemistry of the brain.
(a). Are the levels of static fields used in the laboratory representative of those in the natural environment?
Natural electric fields in the environment range between 0.1 and 0.3 kV m−1 [27], so that the fields experienced by insects in their environment are normally less than those used in the experiments described here to elicit avoidance behaviour and, as such, one might argue about the relevance of such stimuli in the normal behavioural repertoire of insects. Static fields of far greater magnitude occur around man-made structures such as electricity transmission lines [28] or through friction charging [29]. Dezelak et al. [30] calculated the field at ground level underneath 400 kV transmission lines and found that it was 9–11 kV m−1, which is of the same order of magnitude as the fields that evoke avoidance of Drosophila used here. Maxwell SV models of the electric fields around transmission lines show that forces are much higher near the operating wires (up to 95 kV within 1.5 m of the wires), suggesting that such fields are likely to have a far greater impact on flying insects.
(b). Avoidance movements
Drosophila, like cockroaches [6] and other Diptera [31], show clear avoidance of static electric fields [32]. What is notable about Drosophila is that the wings had a major role to play in avoidance, while the antennae are involved in cockroaches [7]. While intact wild-type flies avoided static fields at relatively low levels, wild-type flies with excised wings could not, even at much higher applied voltages. By contrast, vestigial winged mutant Drosophila showed avoidance, but at field levels higher than for intact wild-type flies. Thus, the surface area of the wings appears to be a major determinant of avoidance rather than the antennae or other structure such as the halteres.
Previously, we suggested that electric fields could be detected via electrical forces causing deflection of sensory appendages resulting in mechanical stimulation [7]. Any electrically neutral object has a random distribution of negative and positive charges over the surface, and when that object enters an electric field it will experience forces on the electrons that cause an uneven distribution (polarization) of charges. The electric forces can generate physical movement of the object towards or away from the electric field region as a result of interaction between charges [33,34]. We found that the wings could be moved by electric fields of the same magnitude as those found to evoke avoidance behaviour. When a fly was placed underneath a negatively charged electrode, the static electric field forces caused polarization leading to passive elevation of the wing towards the electrode, as unlike charges were attracted.
The wings of intact male flies and excised male wings required significantly lower electric fields to lift them compared with female wings. This is arguably counterintuitive given that avoidance in general appears to be related to the surface area of the wing. However, it should be noted that the wings of females, while larger, are also likely to be heavier, meaning that there may be a trade-off between force to raise the wing and the mass of the wing opposing lift.
(c). Detection of static electric fields
Movements of the wings are detected by three key mechanoreceptors: campaniform sensilla, the tegula and stretch receptors [35–37] located in and on the wings. Sensory neurons from these sensors transmit information about the wings' position and deformation to the thoracic ganglia. Locally, responses are processed by interneurons and motor neurons that control the wing muscles [38], while intersegmental interneurons may also receive sensory inputs from the wing, which ultimately lead to limb movements [39]. Thus, deflection of the wings by Coulomb forces generated by static electric fields has the potential to generate or modify limb movements that could form the basis of avoidance.
(d). Aminergic control of behaviour
The levels of serotonin and dopamine in the heads of flies found in this study were of the same order of magnitude found in previous studies [40]. The levels of dopamine, while being an order of magnitude higher than that described by Watson et al. [40], were lower than the levels found by others [41]. Monoamines are known to underpin behavioural states, often caused by social interaction between conspecifics [20] or by environmental conditions [16]. For example, the levels of octopamine in insects have been correlated with active and stressful states [42–45], and aggression [21]. Octopamine is also associated with flight and can both increase the likelihood of flight [46] and induce adipokinetic hormone, which mobilizes lipids as fuel for flight [47]. Moreover, amines play a key role as reinforcement signals during learning and memory in insects [48]. That octopamine levels increase in flies exposed to static fields could be related to both active and stressful states caused by Coulomb forces acting on the flies and increased activity required to overcome those forces, either by enhanced locomotion or flight.
Dopamine is often associated with motor control and arousal in insects [49], and has a dual role in learning in Drosophila, being involved in both appetitive and aversive learning [50]. We found that the exposure to static electric fields led to decreased dopamine levels. In Drosophila dopamine signalling can act via cryptochrome to increase arousal [51]. Interestingly, cryptochrome also plays a role in the light-dependent magnetosensitive responses in Drosophila [52], raising the possibility that static electric fields might also directly impact on cryptochrome.
Changes in serotonin levels in flies exposed to static electric fields were not consistent over all time periods of exposure and were not significantly lower except following 24 h exposure. Such a pattern of expression may suggest a reduced role of serotonin in electric field-induced behaviour, as in aggression [53,54]. For example, depletion of serotonin has no effect on aggression in crickets [54].
Taken together, it is clear that a suite of changes in amine levels occurs during exposure to electric fields at levels present in the environment, many of which can act to modulate changes in behaviour. It is clear, therefore, that electric fields present in the environment have the potential to cause changes in insect behaviour and neurochemistry. Moreover, given that changes in amine levels produced in response to electric fields persist over time, the use of Drosophila from cultures maintained in plastic rearing tubes, as commonly occurs in many research laboratories, which can charge through triboelectrification, could provide flies with an altered neurochemical background, and could be inappropriate for behavioural analysis.
Supplementary Material
Authors' contributions
P.L.N. and C.W.J. conceived, designed and coordinated the study, and assisted H.A. in collecting and analysing the data for biogenic amines; P.L.N. drafted the manuscript; S.S. advised and coordinated electric field modelling studies; and M.S.A.G. collected data for the avoidance of Drosophila to electric fields. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
M.S.A.G. was sponsored by Al Baha University, Saudi Arabia and would like to thank the Saudi Arabia Cultural Bureau, London. H.A. was supported by grants-in-aid for Scientific Research (KAKENHI) from MEXT, Scientific Research Priority Areas (area no. 454, grant no. 17075001) and from the Japan Society for the Promotion of Science (no. 23300113).
References
- 1.Greggers U, Koch G, Schmidt V, Dürr A, Floriou-Servou A, Piepenbrock D, Göpfert MC, Menzel R. 2013. Reception and learning of electric fields in bees. Proc. R. Soc. B 280, 20130528 ( 10.1098/rspb.2013.0528) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colin ME, Richard D, Fourcassie V, Belzunces LP. 1992. Attraction of Varroa jacobsoni, parasite of Apis mellifera by electrical charges. J. Insect Physiol. 38, 111–117. ( 10.1016/0022-1910(92)90039-G) [DOI] [Google Scholar]
- 3.Corbet SA, Beament J, Eisikowitch D. 1982. Are electrostatic forces involved in pollen transfer? Plant Cell Environ. 5, 125–129. ( 10.1111/1365-3040.ep11571488) [DOI] [Google Scholar]
- 4.Colin ME, Richard D, Chauzy S. 1991. Measurement of electric charges carried by bees: evidence of biological variations. J. Bioelect. 10, 17–32. ( 10.3109/15368379109031397) [DOI] [Google Scholar]
- 5.Gan-Mor S, Schwartz Y, Bechar A, Eisikowitch D. 1995. Relevance of electrostatic forces in natural and artificial pollination. Can. Agri. Eng. 37, 189–195. [Google Scholar]
- 6.Jackson CW, Hunt E, Sharkh S, Newland PL. 2011. Static electric fields modify the locomotory behaviour of cockroaches. J. Exp. Biol. 214, 2020–2026. ( 10.1242/jeb.053470) [DOI] [PubMed] [Google Scholar]
- 7.Newland PL, Hunt E, Sharkh SM, Hama N, Takahata M, Jackson CW. 2008. Static electric field detection and behavioural avoidance in cockroaches. J. Exp. Biol. 211, 3682–3690. ( 10.1242/jeb.019901) [DOI] [PubMed] [Google Scholar]
- 8.Hunt EP, Jackson CW, Newland PL. 2005. ‘Electropellancy’ behaviour of Periplaneta americana exposed to friction charged dielectric surfaces. J. Electrostatics 63, 853–859. ( 10.1016/j.elstat.2005.03.081) [DOI] [Google Scholar]
- 9.Edwards DK. 1960. Effects of artificially produced atmospheric electrical fields upon the activity of some adult Diptera. Can. J. Zool. 38, 899–912. ( 10.1139/z60-096) [DOI] [Google Scholar]
- 10.Watson DB. 1984. Effect of an electric field on insects. New Z. J. Sci. 27, 139–140. [Google Scholar]
- 11.Maw MG. 1961. Behaviour of an insect on an electrically charged surface. Can. Entomol. 93, 391–393. ( 10.4039/Ent93391-5) [DOI] [Google Scholar]
- 12.Tanaka N, et al. 2005. An electric dipolar screen with oppositely polarized insulators for excluding whiteflies from greenhouses. Crop Protection 27, 215–221. ( 10.1016/j.cropro.2007.05.009) [DOI] [Google Scholar]
- 13.Vasquez BJ, Anderson LE, Lowery CI, Adey WR. 1988. Diurnal patterns in brain biogenic amines of rats exposed to 60-Hz electric fields. Bioelectromagnetics 9, 229–236. ( 10.1002/bem.2250090304) [DOI] [PubMed] [Google Scholar]
- 14.Merkulova LM. 1990. The effect on an impulse electromagnetic field on the bioamine allowance of the spinal ganglion in rats under whole-body exposure. Radiobiologiia 30, 252–255. [PubMed] [Google Scholar]
- 15.Goodman R, Blank M. 1998. Magnetic field stress induces expression of hsp70. Cell Stress Chaperone 3, 79–88. () [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anstey ML, Rogers SM, Ott SR, Burrows M, Simpson SJ. 2009. Serotonin mediates behavioral gregarization underlying swarm formation in desert locusts. Science 323, 627–630. ( 10.1126/science.1165939) [DOI] [PubMed] [Google Scholar]
- 17.Ma Z, Guo W, Guo X, Wang X, Kang L. 2011. Modulation of behavioral phase changes of the migratory locust by the catecholamine metabolic pathway. Proc. Natl Acad. Sci. USA 108, 3882–3887. ( 10.1073/pnas.1015098108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alessi A, O'Connor V, Aonuma H, Newland PL. 2014. Dopaminergic modulation of phase reversal in desert locusts. Front. Behav. Neurosci. 8, 371 ( 10.3389/fnbeh.2014.00371) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aonuma H, Watanabe T. 2012. Changes in the content of brain biogenic amine associated with early colony establishment in the queen of the ant, Formica japonica. PLoS ONE 7, e43377 ( 10.1371/journal.pone.0043377) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Penick CA, Brent CS, Dolezal K, Liebig J. 2014. Neurohormonal changes associated with ritualized combat and the formation of a reproductive hierarchy in the ant Harpegnathos saltator. J. Exp. Biol. 217, 1496–1503. ( 10.1242/jeb.098301) [DOI] [PubMed] [Google Scholar]
- 21.Stevenson PA, Dyakonova V, Rillich J, Schildberger K. 2005. Octopamine and experience-dependent modulation of aggression in crickets. J. Neurosci. 25, 1431–1441. ( 10.1523/JNEUROSCI.4258-04.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson CW, McGonigle D. 2005. Direct monitoring of the electrostatic charge of house flies (Musca domestica L.) as they walk on a dielectric surface. J. Electrostatics 63, 803–808. ( 10.1016/j.elstat.2005.03.075) [DOI] [Google Scholar]
- 23.Kraaijeveld AR, Elrayes NP, Hansjürgen Schuppe H, Newland PL. 2011. L-arginine enhances immunity to parasitoids in Drosophila melanogaster and increases NO production in lamellocytes. Dev. Comp. Immunol. 35, 857–864. ( 10.1016/j.dci.2011.03.019) [DOI] [PubMed] [Google Scholar]
- 24.Stortkuhl KF, Kettler S and Hovemann T. 2005. An increase receptive field of olfactory receptor Or43a in the antennal lobe of Drosophila reduces benzaldehyde-driven avoidance behaviour. Chem. Senses 30, 81–87. ( 10.1093/chemse/bji003) [DOI] [PubMed] [Google Scholar]
- 25.Turner SL, Ray A. 2009. Modification of CO2 avoidance behaviour in Drosophila by inhibitory odorants. Nature 461, 277–281. ( 10.1038/nature08295) [DOI] [PubMed] [Google Scholar]
- 26.Watson DB, Sedcole NP, Chan E, Smart KG. 1997. The movement of insects in an electric field. In 10th Int. Conf. Electromagn. Compatability, Coventry, UK, 1–3 September 1997, pp. 54–58. Stevenage, UK: IET Digital Library; ( 10.1049/cp:19971118) [DOI] [Google Scholar]
- 27.Adlerman EJ, Williams ER. 1996. Seasonal variation of the global electrical circuit. J. Geophys. Res. Atmos. 101, 29 679–29 688. ( 10.1029/96JD01547) [DOI] [Google Scholar]
- 28.Orlov VM. 1990. Invertebrates and high voltage power lines. J. Bioelectricity 9, 121–131. [Google Scholar]
- 29.Chubb J. 2003. Introductory workshop in electrostatics. In Electrostatics. 2003 International Conference. [Google Scholar]
- 30.Dezelak K, Stumberger G, Jakl F. 2010. Reduction of electric and magnetic field emissions caused by overhead power line. Int. Conf. Renew. Energies Power Quality 342, 1–4. [Google Scholar]
- 31.Perumpral JV, Earp UF, Stanley JM. 1978. Effects of electrostatic fields on locational preference of house flies and flight activities of cabbage loopers. Environ. Entomol. 7, 482–486. ( 10.1093/ee/7.3.482) [DOI] [Google Scholar]
- 32.Matsuda Y, Nonomura T, Kakutani K, Takikawa Y, Kimbara J, Kasaishi Y, Osamura K, Kusakari S, Toyoda H. 2011. A newly devised electric field screen for avoidance and capture of cigarette beetles and vinegar flies. Crop Prot. 30, 155–162. ( 10.1016/j.cropro.2010.09.001) [DOI] [Google Scholar]
- 33.Bhatnagar VP. 1993. A complete course in certificate physics. New Delhi, India: Pitambar Publishing Company. [Google Scholar]
- 34.Ellse M, Honeywill C. 1998. Fields, forces and synthesis. London, UK: Thomas and Sons Ltd. [Google Scholar]
- 35.Daly HV, Doyen JT, Purcell AH. 1998. Introduction to Insect Biology and Diversity. Oxford, UK: Oxford University. [Google Scholar]
- 36.Elson RC. 1987. Integration of wing proprioceptive and descending exteroceptive sensory inputs by thoracic interneurones of the locust. J. Exp. Biol. 128, 193–217. [Google Scholar]
- 37.Wolf H, Pearson KG. 1988. Proprioceptive input patterns elevator activity in the locust flight system. J. Neurophysiol. 59, 1831–1853. [DOI] [PubMed] [Google Scholar]
- 38.Burrows M. 1996. The neurobiology of an insect brain. Oxford, UK: Oxford University Press. [Google Scholar]
- 39.Matheson T. 2002. Metathoracic neurons integrating intersegmental sensory information in the locust. J. Comp. Neurol. 44, 95–114. ( 10.1002/cne.10140) [DOI] [PubMed] [Google Scholar]
- 40.Watson DG, Zhou P, Midgley JM, Milligan CD, Kaiser K. 1993. The determination of biogenic amines in four strains of the fruitfly Drosophila melanogaster. J. Pharm. Biomed. Anal. 11, 1145–1149. ( 10.1016/0731-7085(93)80096-J) [DOI] [PubMed] [Google Scholar]
- 41.Hardie SL, Hirsch J. 2006. An improved method for separation and detection of biogenic amines in adult Drosophila brain extracts by high performance liquid chromatography. J. Neurosci. Meth. 153, 243–249. ( 10.1016/j.jneumeth.2005.11.001) [DOI] [PubMed] [Google Scholar]
- 42.Bailey BA, Martin RJ, Downer RGH. 1984. Haemolymph octopamine levels during and following flight in the American cockroach, Periplaneta americana L. Can. J. Zool. 62, 19–22. ( 10.1139/z84-004) [DOI] [Google Scholar]
- 43.Davenport AP, Evans PD. 2008. Changes in haemolymph octopamine levels associated with food deprivation in the locust, Schistocerca gregaria. Physiol. Entomol. 9, 269–274. ( 10.1111/j.1365-3032.1984.tb00709.x) [DOI] [Google Scholar]
- 44.Corbet SA. 1991. A fresh look at the arousal syndrome of insects. Adv. Insect Physiol. 23, 81–116. ( 10.1016/s0065-2806(08)60092-2) [DOI] [Google Scholar]
- 45.Orchard I, Ramirez JM, Lange AB. 1993. A multifunctional role for octopamine in locust flight. Annu. Rev. Entomol. 38, 227–249. ( 10.1146/annurev.en.38.010193.001303) [DOI] [Google Scholar]
- 46.Buhl E, Schildberger K, Stevenson PA. 2008. A muscarinic cholinergic mechanism underlies activation of the central pattern generator for locust flight. J. Exp. Biol. 211, 2346–2357. ( 10.1242/jeb.017384) [DOI] [PubMed] [Google Scholar]
- 47.Orchard I. 1987. Adipokinetic hormones-an update. J. Insect Physiol. 33, 451–463. ( 10.1016/0022-1910(87)90108-9) [DOI] [Google Scholar]
- 48.Schwaerzel M, Monastirioti M, Scholz H, Friggi-Grelin F, Birman S, Heisenberg M. 2003. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J. Neurosci. 23, 10 495–10 502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kume K, Kume S, Park SK, Hirsh J, Jackson FR. 2005. Dopamine is a regulator of arousal in the fruit fly. J. Neurosci. 25, 7377–7384. ( 10.1523/JNEUROSCI.2048-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Waddell S. 2013. Reinforcement signalling in Drosophila; dopamine does it all after all. Curr. Opinion Neurobiol. 23, 324–329. ( 10.1016/j.conb.2013.01.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kumar S, Chen D, Sehgal A. 2012. Dopamine acts through Cryptochrome to promote acute arousal in Drosophila. Genes Dev. 26, 1224–1234. ( 10.1101/gad.186338.111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gegear RJ, Casselman A, Waddell S, Reppert SM. 2008. CRYPTOCHROME mediates light-dependent magnetosensitivity in Drosophila. Nature 454, 1014–1018. ( 10.1038/nature07183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baier A, Wittek B, Brembs B. 2002. Drosophila as a new model organism for the neurobiology of aggression? J. Exp. Biol. 205, 1233–1240. [DOI] [PubMed] [Google Scholar]
- 54.Stevenson PA, Hofmann HA, Schoch K, Schildberger K. 2000. The fight and flight responses of crickets depleted of biogenic amines. J. Neurobiol. 43, 107–120. () [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


