Abstract
Larger-bodied species in a wide range of taxonomic groups including mammals, fishes and birds tend to decline more steeply and are at greater risk of extinction. Yet, the diversity in life histories is governed not only by body size, but also by time-related traits. A key question is whether this size-dependency of vulnerability also holds, not just locally, but globally across a wider range of environments. We test the relative importance of size- and time-related life-history traits and fishing mortality in determining population declines and current exploitation status in tunas and their relatives. We use high-quality datasets of half a century of population trajectories combined with population-level fishing mortalities and life-history traits. Time-related traits (e.g. growth rate), rather than size-related traits (e.g. maximum size), better explain the extent and rate of declines and current exploitation status across tuna assemblages, after controlling for fishing mortality. Consequently, there is strong geographical patterning in population declines, such that populations with slower life histories (found at higher cooler latitudes) have declined most and more steeply and have a higher probability of being overfished than populations with faster life histories (found at tropical latitudes). Hence, the strong, temperature-driven, latitudinal gradients in life-history traits may underlie the global patterning of population declines, fisheries collapses and local extinctions.
Keywords: vulnerability, declines, life histories, fishes, fishing mortality, scombrids
1. Introduction
Species are declining in abundance faster than ever in the history of the Earth [1,2]. Comparative studies of vulnerability to decline have contributed greatly to elucidate the underlying processes and patterns of species declines and extinction risk in a wide range of taxonomic groups [3–5]. The loss of biodiversity is not random [6,7]. Over the past decade, species vulnerability to declines and extinctions have been linked to two major factors: their exposure to a threatening process, and their intrinsic sensitivity based on their life-history traits, habitat preferences and behavioural ecology [3,8,9].
Marine fishes provide a unique opportunity to understand the intrinsic patterns and processes of decline and collapse. This is because exposure to the threatening process—fishing mortality—is routinely estimated in commercially important marine fish species. By contrast, in mammals, the variable population response of species is noted, but the local variation in hunting pressure among populations of a species is unknown and cannot be controlled for [3,10]. Once fishing mortality can be controlled for, it is apparent that life histories and demography relate to several measures of species vulnerability including declines, collapses, recoveries and threat status [9,11].
Specifically, maximum body size is a broadly reliable life-history correlate of population and species vulnerability to decline and extinction risk. Larger species tend to have declined more steeply and are under greater threat of extinction, than smaller-bodied species [12–15]. This is because larger-bodied fishes tend to have life-history strategies resulting in lower intrinsic rates of population increase and compensatory density dependence [16–18]. The performance of other life-history traits for explaining declines has been mixed [19–21]. This is because, until recently, time-related reproductive investment and allocation traits have been less available and harder to estimate accurately, especially across a wide range of populations [9,18,22].
Body size alone does not explain the full extent of species life-history diversity. With the increasing availability of high-quality life-history data, the diversity of life and variation in life histories is viewed as not only varying with maximum body size of species, but also across time and reproductive allocation dimensions [23–25]. Specifically, time- and rate-related traits, such as age at maturity, longevity, growth rate and mortality rate explain a large proportion of the variation in the life histories of mammals, birds, reptiles and fishes, ranking species along a slow–fast continuum of life histories [23,25–27].
Species biological rates and times, especially growth and production rates, scale with body size and temperature. Thus, temperature is an important determinant of life histories, mainly through its control on metabolic rates and other time-related life-history rates [28,29]. Temperature varies strongly with latitude, hence due to the temperature-dependency of ectotherm metabolic rates, we might expect tropical fishes to have faster population growth rates, as a result of faster growth, earlier maturation and shorter lifespans, than similar-sized temperate ones [30–32]. In tunas and their relatives, for example, time-related traits are biogeographical patterned such that while both large and small tunas are found across a wide range of latitudes, the tunas with faster life histories are found in warmer tropical waters, and those with slower life histories occur in temperate seas [25]. As species life-history strategies are intimately linked to their response and degree of resilience to fisheries exploitation [18,33,34], we hypothesized that temperate tunas would decline more than their similar-sized tropical counterparts, for a given level of fishing mortality.
Here, we test the relative importance of size- and time-related traits for explaining population declines and current exploitation status in tunas and their relatives, while controlling for fishing mortality. Tunas and their relatives (Family Scombridae) are great candidates for such a study as this family of fishes is widely distributed in the tropical and temperate oceans and have some of the highest quality marine population trend and life-history data known. To test for the role of life histories and fishing mortalities in determining the vulnerability of populations to decline, we geographically match high-quality datasets of half a century of population biomass trajectories and fishing mortalities [35], with population-level life-history trait data [36].
2. Material and methods
(a). Data collection
We collated time series of yearly adult biomass, yearly fishing mortality rates and the standard fisheries reference points BMSY and FMSY for 26 populations of tunas and their relatives from a scombrid fisheries stock assessment dataset [35]. We selected those populations that have experienced on average negative annual rates of change in adult biomass during their period of exploitation as we aimed to examine the interaction between life histories and fishing in determining population declines. We calculated three metrics to describe population biomass trajectories, which we refer to as metrics of vulnerability to decline: (i) average annual rate of decline in adult biomass, (ii) total extent of decline in adult biomass, and (iii) current exploitation status defined using the Bcurrent/BMSY reference point. Bcurrent/BMSY is the ratio of the current adult biomass relative to the adult biomass that would provide the maximum sustainable yield (MSY) and determines whether a population is currently overfished (Bcurrent < BMSY) or not (Bcurrent > BMSY). For each population, we also calculated the average fishing mortality across all years (Faverage) and divided it by the fishing mortality predicted to produce MSY (FMSY), which we refer to as relative fishing mortality rate (Faverage/FMSY). We use this metric to control for the different fishing mortality rates that populations have experienced within their history of exploitation or at least as they started to be assessed (electronic supplementary material, S1). The relative fishing mortality rate metric was not available for three populations (North Pacific Albacore tuna, Northeast Pacific chub mackerel and Pacific bluefin tuna), and therefore we were not able to include these populations in our analyses (electronic supplement material, S1). Furthermore, we also excluded from the statistical analyses populations with increasing population trajectories (on average positive annual rates of change in adult biomass) as this study focuses on examining the interaction between life histories and fishing in determining population declines (electronic supplement material, S1).
We extracted the following life-history traits from a comprehensive life-history database [25,36]: maximum body size (Lmax, cm), length and age-at-maturity (Lm, cm and Tm, years), longevity (Tmax, years), growth rates described with the von Bertalanffy growth coefficient k (1/year), the average absolute batch fecundity (Fabs), relative batch fecundity (Frel, number of oocytes per gram) and spawning interval (Spwint, days) and duration (Spwseason, months). We report length-based estimates as fork lengths throughout. For each life-history trait, we calculated a population-level estimate combining the life-history information from multiple studies carried out within their population distributions (electronic supplementary material, S2).
(b). Analyses
We fitted general and logistic linear models to 19 populations of scombrids to examine the relative importance of life histories and relative fishing mortality rates in determining their vulnerability to decline. These 19 populations have complete datasets including the three metrics of vulnerability to decline, a metric of relative fishing mortality and life-history traits (electronic supplementary material, S1 and S2). Specifically, we examined whether the small–large and slow–fast trait axes of life-history variation in scombrids explain their rate and extent of decline and current exploitation status, after controlling for the different relative fishing mortality rates experienced by populations. Maximum body size was used as a proxy to describe the small–large dimension of life-history variation and growth rate to describe the fast–slow dimension of life-history variation (electronic supplementary material, S2).
We assume that vulnerability to decline is a function of the exposure of a population to extrinsic threats, such as fishing mortality, coupled with the population’ intrinsic sensitivity based on their life histories. We tested the following hypotheses about intrinsic factors (life histories) while controlling for extrinsic factors (fishing)—declines will be more severe and exploitation status worse for: (H1) populations subject to higher fishing mortality rates; (H2) populations with larger body sizes after controlling for fishing mortalities; and (H3) populations with slower growth rates after controlling for fishing mortalities.
We fitted a general linear regression with normally distributed errors to model the two continuous dependent variables: the average annual rate of decline and total extent of decline in adult biomass. We fitted a logistic regression assigning a binomial error distribution and a logit link function to model the probability of the populations being overfished or not. We used an information-theoretic approach with Akaike's information criterion corrected for small sample sizes (AICc) to evaluate all the candidate models and assign them relative strengths of evidence [37]. We determined the maximized log-likelihood for each candidate model (i) and calculated the values for AICc, ΔAICc (ΔAICc = AICci—min AICc), where AICci is the AICc for model i, and min AICc is the smallest AICc value in the set of models, and the Akaike weight (wi) [37]. We selected the best models (with largest Akaike weights) and calculated the standard error and the 95% confidence intervals (CIs) for each covariate to assess the effect size, and those variables whose CIs excluded zero were deemed to have a strong effect on the predictor variables [38]. We examined model diagnostics for heteroscedasticity, normality and independence of residuals [39]. Owing to issues of non-normality and non-constancy of variance (observed within the residual analyses), all the models were linearized by taking the natural logarithms of the variables. All data management, analyses and figures were done using the R statistical software v. 3.0.2 [40], including the R packages ‘MuMIn’ [41] and ‘ggplot2’ [42].
We conducted two sensitivity analyses to test the robustness of our choice of life-history proxies to describe the first two axes of life-history variation in scombrids, and found our analyses are robust to the choice of life-history proxies (electronic supplementary material, S2). Moreover, by examining the span of variation in the life-history traits and relative fishing mortalities against the three metrics of variability to decline, we also assessed whether populations with certain life histories have been preferentially targeted with high fishing intensities (electronic supplementary material, S3). We find scombrid populations, irrespective of their life histories, have been exposed to a wide range of relative fishing mortality rates. This exposure to wide-ranging fishing intensities and the diverse intrinsic sensitivities in scombrid populations allows testing for the combined effects of exposure to fishing and life histories in determining vulnerability to population declines.
3. Results
After controlling for the different relative fishing mortality rates experienced by each population, those populations with slower growth rates (rather than populations with larger body size) declined faster, to a greater extent, and more steeply, and are more likely to be currently overfished. We find there was a broad agreement between each of the three metrics of vulnerability to decline (rate, extent and exploitation status) and the life-history trait of growth rate, once the relative fishing mortality experienced by the populations was controlled for (table 1). We found greatest support for those models including growth rate and relative fishing mortality rate (both lowest AICc and highest weights wi ranging from 0.57 to 0.75; table 1a–c). Thus, once fishing mortality was controlled for in the model, the growth rate of populations describing the slow–fast dimension of life histories better explained the variation in decline rate, extent and current exploitation status than maximum size representing the large–small life-history dimension. Moreover, we found weaker support (wi = 0.2–0.3) for the models that only included relative fishing mortality rates as an explanatory variable. Last, we found the weakest support (wi = 0.05–0.14) for the models including both maximum body size and relative fishing mortality rate as explanatory variables (table 1).
Table 1.
Summary of explanatory generalized linear models evaluating the effects of relative fishing mortality rates and life history on three measures of vulnerability to decline: (a) rates of decline in adult biomass, (b) the extents of decline in adult biomass and (c) current exploitation status (probability of being overfished) in scombrid populations. ((a,b) General linear regression with normally distributed errors and (c) logistic regression with binomial distributed errors. Models are sorted by AICc, which is the Akaike's information criterion with a correction for small sample sizes; K, number of parameters; l (θ), the value of the maximized log-likelihood function; ΔAICc = AICci—min AIC, where AICci is the AICc for model i, and min AICc is the smallest AICc value in the set of models; wi, the Akaike weights, expresses the relative likelihoods of candidate models, with the weight of any particular model varying from 0 (no support) to 1 (complete support) relative to the entire model set; R2, coefficient of determination.)
| hypotheses | K | l (θ) | AICc | ΔAICc | wi | R2 |
|---|---|---|---|---|---|---|
| (a) rate of decline in adult biomass | ||||||
| relative fishing mortality + growth rate | 4 | −13.48 | 37.82 | 0 | 0.57 | 0.31 |
| relative fishing mortality | 3 | −15.76 | 39.11 | 1.29 | 0.3 | 0.12 |
| historical fishing mortality + maximum size | 4 | −14.88 | 40.62 | 2.81 | 0.14 | 0.20 |
| (b) extent of decline in adult biomass | ||||||
| relative fishing mortality + growth rate | 4 | −5.49 | 21.80 | 0.00 | 0.69 | 0.30 |
| relative fishing mortality | 3 | −8.09 | 23.80 | 1.93 | 0.26 | 0.08 |
| relative fishing mortality + maximum size | 4 | −8.04 | 26.90 | 5.08 | 0.05 | 0.08 |
| (c) probability of being overfished | ||||||
| relative fishing mortality + growth rate | 4 | −8.88 | 25.00 | 0.00 | 0.75 | |
| relative fishing mortality | 3 | −11.56 | 27.70 | 2.69 | 0.20 | |
| relative fishing mortality + maximum size | 4 | −11.54 | 30.30 | 5.33 | 0.05 | |
Those populations with slower growth rates, rather than populations with larger body size, are four times more likely to have experienced faster population declines (evidence ratio = 0.57/0.14), are 14 times more likely to have experienced larger extents of population declines (evidence ratio = 0.69/0.05) and are 15 times more likely to be currently overfished (evidence ratio = 0.75/0.05), after controlling for the different relative fishing mortality rates experienced by each population (table 1). Indeed, we find strong evidence for an effect of growth rate, as the 95% CIs of the effect sizes do not cross zero in all three measures of vulnerability (table 2). We also find evidence that, both growth rate and relative fishing mortality, in combination, have a strong effect on the probability of populations being overfished as their 95% CIs do not cross zero (table 2).
Table 2.
Vulnerability to decline depends on somatic growth rates for scombrid populations after accounting for the relative fishing mortality rates populations have been exposed to. (Panels show selected best models (models with the largest Akaike weights from table 1) for each measure of vulnerability to decline. (a) The rates of decline in adult biomass, (b) the extent of decline in adult biomass, and (c) the current exploitation status (probability of being overfished) in scombrid populations. (a,b) General linear regression with normally distributed errors and (c) logistic regression with binomial distributed errors. The summary of the models includes the estimated coefficients, standard errors (s.e.) and the 95% CIs for each covariate.)
| parameter | estimate | s.e. | lower CI | upper CI | ||
|---|---|---|---|---|---|---|
| (a) rate of decline in adult biomass | ||||||
| relative fishing mortality | 0.18 | 0.17 | −0.16 | 0.52 | ||
| growth rate | −0.51 | 0.24 | −0.99 | −0.03 | ||
| (b) extent of decline in adult biomass | ||||||
| relative fishing mortality | 0.07 | 0.11 | −0.15 | 0.29 | ||
| growth rate | −0.36 | 0.16 | −0.68 | −0.05 | ||
| parameter | estimate | s.e. | lower CI | upper CI | odds ratio exp (coef.) | inverse odd ratio 1/exp (coef.) |
|---|---|---|---|---|---|---|
| (c) probability of being overfished | ||||||
| relative fishing mortality | 3.25 | 1.72 | 0.79 | 7.99 | 25.70 | |
| growth rate | −3.96 | 2.35 | −10.36 | −0.48 | 52.35 | |
aParameter deemed significant as CI excludes 0.
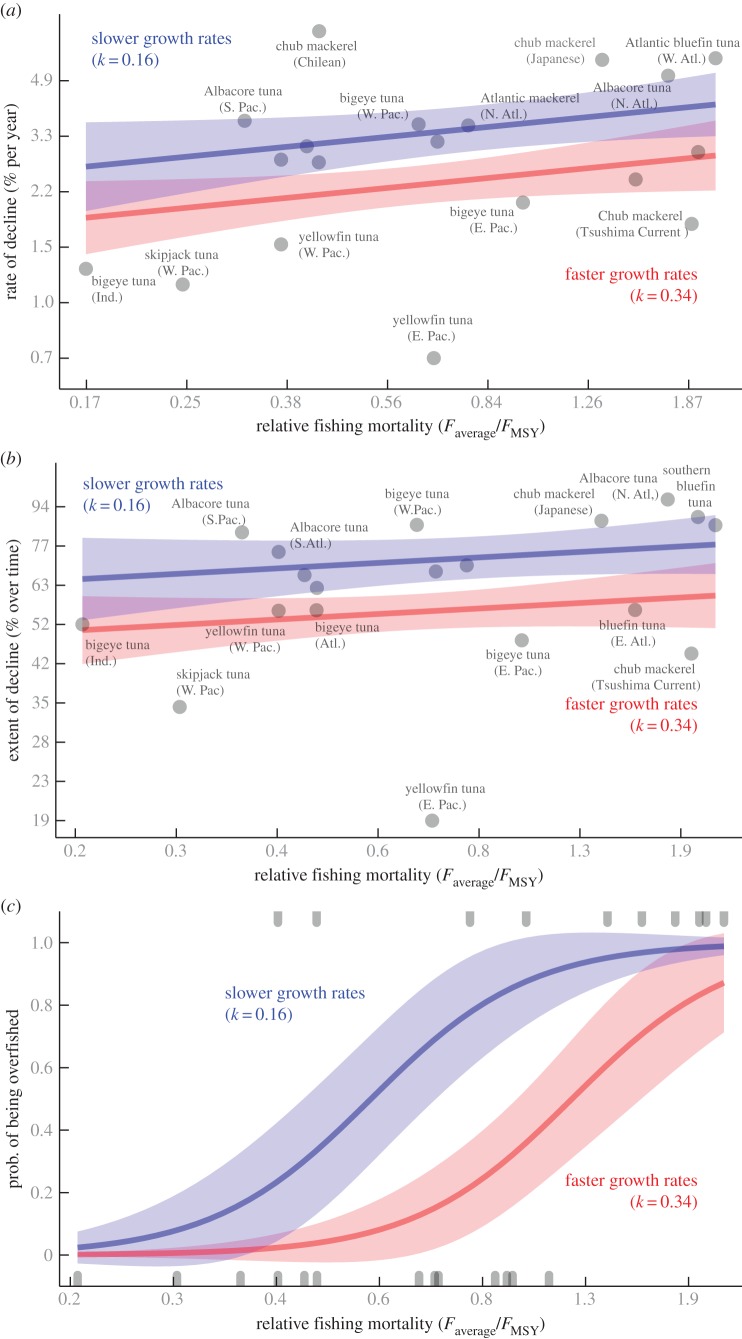
We observe that scombrid populations of temperate and subtropical species, such as chub mackerel (Scomber japonicus), Atlantic mackerel (S. scombrus), Albacore tuna (Thunnus alalunga) and Atlantic and southern bluefin tuna (Thunnus thynnus and Thunnus maccoyii), which have the slowest growth rates and greatest longevities, have suffered the fastest and greatest declines in adult biomass and have a higher probability of being overfished even after fishing pressure has been properly controlled for (figure 1). By contrast, the majority of tropical populations of scombrid species, such as skipjack tuna (Katsuwonus pelamis), yellowfin tuna (Thunnus albacares) and bigeye tuna (Thunnus obesus), which are among the fastest growers and shortest lived species of scombrids, have suffered the slowest, and least severe population declines in adult biomass, and have a lower probability of being overfished.
Figure 1.
Vulnerability to declines depends on somatic growth rates for scombrid populations after accounting for the exposure to the threatening process of fishing. (a) Predicted average annual rates of decline in adult biomass with separate lines set for two values of growth rates corresponding to the first (blue line, slow growth k = 0.16) and third (red line, fast growth k = 0.34) quartile values. (b) Predicted total extent of decline in adult biomass with separate lines set for two values of growth rates corresponding to the first (blue line) and third (red line) quartile values, k = 0.16 and k = 0.34, respectively. (c) Predicted probability of being overfished (Bcurrent/BMSY < 1) with separate lines set for two values of growth rates corresponding to the first (blue line, slow growth k = 0.16) and third (red line, fast growth k = 0.34) quartile values. Panels show regression lines and 95% CIs derived from (a,b) general linear models and (c) logistic regression models. Predictions with 95% confidence values correspond to the best models (the models with the largest Akaike weights, see table 1). Population codes are found in the electronic supplementary material, S1.
4. Discussion
By focusing on a well-studied taxonomic group of species with detailed population trends and life-history data, we have shown that time-related traits describing the speed of life, rather than size-related traits, better explained the extent and rate of declines and current exploitation status of tunas and their relatives. At a first glance, our findings contrast strongly with the large majority of previous comparative studies revealing than large-bodied fish species tend to have declined more steeply and are under greater threat of extinction, than smaller-bodied species [12–14]. Instead, it is likely that our finding complements, rather than contradicts, these studies, and we explain how next.
There are a number of reasons why maximum body size might have been most frequently identified as the best life-history correlate of vulnerability in fishes. First, maximum size of the species tends to be most commonly available trait and often the only life-history trait available for testing, by comparison time-related traits have been less frequently tested [11,22]. Second, body-size-related measures are easier to gather accurately, with minimum observation error, whereas time-related traits must be estimated from numerous samples [36,43].Third, some comparative studies were unable to control for (or only partially controlled for) the different fishing mortalities experienced by species [13,14,44]. This leads to a failure to adequately disentangle the relative importance of fishing and life histories with the result that the significance of maximum size may have been overestimated.
Aside from these quality and data availability issues, we find almost without exception that previous tests of life-history trait relationships with population trajectories while accounting for fishing mortality occurred within fish assemblages and were all conducted at local scales either in the European shelf seas [20,21,45] or the Pacific coral reefs [12,19,46]. Hence, within a single and relative homogeneous environment, maximum body size may be sufficient to rank the relative vulnerability of species to fishing exploitation within fish assemblages. Our study shows a geographical patterning in population declines with populations found at higher latitudes having declined most and more steeply than populations at lower latitudes after controlling for fishing mortality. This suggests time-related traits, such as growth rate, age at maturity and longevity, might be more suitable to rank population and species vulnerability to fishing exploitation across assemblages at larger geographical scales with larger environmental and temperature gradients. Yellowfin tuna is a good example. Although it is relatively large—up to 232 cm in length—it is a fast-growing and short-lived tropical species, and consequently it can cope with relatively high fishing mortality rates compared with the similar-sized temperate bluefin tunas [25]. We hypothesize that this is because life histories are locally adapted, most often to temperature and related environmental conditions [28,29,47].
To our knowledge, there are only four global scale tests of the link between life histories and vulnerability. First, a global comparative study across freshwater and marine fishes showed body size best explained extinction risk in marine fishes [13,13]. Second, two global comparative studies across the taxonomic class Chondrichthyes—sharks, rays and chimaeras—similarly showed that body size, along with depth, best explained their extinction risk [14,48]. Like many other studies, these studies could not control for fishing mortality, nor were the authors able to test whether time-related traits were important, nor were traits geographically matched to population status. Third, a global analysis testing for life-history links to the proportion of populations (within each fish species) that have collapsed suggested life-history traits are not good correlates of species collapses [49]. Our advance, enabling us to reveal the importance of time-related traits, was to both control for fishing mortality as well as being able to geographically match life-history traits to population trends.
Our findings suggest it may be fruitful to better measure and account for external threats and their interaction with life-history traits, particularly time-related traits in comparative analysis of vulnerability. Time-related traits such as growth rates and age at maturity are increasingly identified in empirical studies as the primary correlates of the maximum population growth rates (rmax), a strong metric of species fitness and extinction risk [16,50,51]. Hence, time-related traits may serve as the ultimate correlate, whereas body size may serve as a useful proximate correlate of species vulnerability to decline, and extinction risk [16,18]. With increasing interest in the importance of scale in understanding patterns and processes of population vulnerability [10,52], future studies should also strive to bridge the gap between local and global processes of decline to better determine what aspects of species life-history histories predisposes them to be more susceptible to fishing pressure. The potential connection between biogeography, through temperature, and life histories may play an important role in determining the spatial patterning of fisheries yield, sustainability and vulnerability [30,53].
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We gratefully acknowledge the constructive comments of Sebastian Pardo and Holly Kindsvater.
Data accessibility
The datasets supporting this article are available in the electronic supplementary material, S1 and S2.
Authors' contributions
M.J.J.J., N.K.D., I.M. and J.F. designed and conducted the research and drafted the manuscript.
Competing interests
We have no competing interests.
Funding
M.J.J.J. was supported in part by a graduate scholarship from Caja Madrid Foundation, Spain, and an EU Marie Curie International Outgoing Fellowship—PIOF-GA-2013-628116. N.K.D. was supported by the Canada Research Chairs Program and Natural Science and Engineering Research Council Discovery and Accelerator Grant.
References
- 1.Pimm SL, Russell GJ, Gittleman JL, Brooks TM. 1995. The future of biodiversity. Science 269, 347–350. ( 10.1126/science.269.5222.347) [DOI] [PubMed] [Google Scholar]
- 2.Butchart SH, et al. 2010. Global biodiversity: indicators of recent declines. Science 328, 1164–1168. ( 10.1126/science.1187512) [DOI] [PubMed] [Google Scholar]
- 3.Cardillo M, Mace GM, Gittleman JL, Jones KE, Bielby J, Purvis A. 2008. The predictability of extinction: biological and external correlates of decline in mammals. Proc. R. Soc. B 275, 1441–1448. ( 10.1098/rspb.2008.0179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collen B, McRae L, Deinet S, De Palma A, Carranza T, Cooper N, Loh J, Baillie JEM. 2011. Predicting how populations decline to extinction. Phil. Trans. R. Soc. B 366, 2577–2586. ( 10.1098/rstb.2011.0015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson SC, Farmer RG, Ferretti F, Houde ALS, Hutchings JA. 2011. Correlates of vertebrate extinction risk in Canada. Bioscience 61, 538–549. ( 10.1525/bio.2011.61.7.8) [DOI] [Google Scholar]
- 6.Balmford A. 1996. Extinction filters and current resilience: the significance of past selection pressures for conservation biology. Trends Ecol. Evol. 11, 193–196. ( 10.1016/0169-5347(96)10026-4) [DOI] [PubMed] [Google Scholar]
- 7.Purvis A, Agapow P-M, Gittleman JL, Mace GM. 2000. Nonrandom extinction and the loss of evolutionary history. Science 288, 328–330. ( 10.1126/science.288.5464.328) [DOI] [PubMed] [Google Scholar]
- 8.Dulvy NK, Ellis JR, Goodwin NB, Grant A, Reynolds JD, Jennings S. 2004. Methods of assessing extinction risk in marine fishes. Fish Fish. 5, 255–276. ( 10.1111/j.1467-2679.2004.00158.x) [DOI] [Google Scholar]
- 9.Reynolds JD, Dulvy NK, Goodwin NB, Hutchings JA. 2005. Biology of extinction risk in marine fishes. Proc. R. Soc. B 272, 2337–2344. ( 10.1098/rspb.2005.3281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cowlishaw G, Pettifor RA, Isaac NJB. 2014. High variability in patterns of population decline: the importance of local processes in species extinctions. Proc. R. Soc. B 276, 63–69. ( 10.1098/rspb.2008.0767) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juan-Jordá MJ, Mosqueira I, Freire J, Dulvy NK. 2013. Life history correlates of marine fisheries vulnerability: a review and a test with tunas and mackerel species. In CIESM workshop monograph no. 45—marine extinctions—patterns and processes, 188 p (ed. Briand F.), pp. 113–128. Monaco: CIESM Publisher. [Google Scholar]
- 12.Jennings S, Reynolds JD, Polunin NVC. 1999. Predicting the vulnerability of tropical reef fishes to exploitation with phylogenies and life histories. Conserv. Biol. 13, 1466–1475. ( 10.1046/j.1523-1739.1999.98324.x) [DOI] [Google Scholar]
- 13.Olden JD, Hogan ZS, Zanden MJV. 2007. Small fish, big fish, red fish, blue fish: size-biased extinction risk of the world's freshwater and marine fishes. Glob. Ecol. Biogeogr. 16, 694–701. ( 10.1111/j.1466-8238.2007.00337.x) [DOI] [Google Scholar]
- 14.Field IC, Meekan MG, Buckworth RC, Bradshaw CJA. 2009. Susceptibility of sharks, rays and chimaeras to global extinction. Adv. Mar. Biol. 56, 275–363. [DOI] [PubMed] [Google Scholar]
- 15.Collette BB, et al. 2011. High value and long life—double jeopardy for tunas and billfishes. Science 333, 291–292. ( 10.1126/science.1208730) [DOI] [PubMed] [Google Scholar]
- 16.Hutchings JA, Myers RA, Garcia VB, Lucifora LO, Kuparinen A. 2012. Life-history correlates of extinction risk and recovery potential. Ecol. Appl. 22, 1061–1067. ( 10.1890/11-1313.1) [DOI] [PubMed] [Google Scholar]
- 17.Goodwin NB, Grant A, Perry AL, Dulvy NK, Reynolds JD. 2006. Life history correlates of density-dependent recruitment in marine fishes. Can. J. Fish. Aquat. Sci. 63, 494–509. ( 10.1139/f05-234) [DOI] [Google Scholar]
- 18.Andersen KH, Beyer JE. 2013. Size structure, not metabolic scaling rules, determines fisheries reference points. Fish Fish. 16, 1–22. ( 10.1111/faf.12042) [DOI] [Google Scholar]
- 19.Russ GR, Alcala AC. 1998. Natural fishing experiments in marine reserves 1983–1993: roles of life history and fishing intensity in family responses. Coral Reefs 17, 399–416. ( 10.1007/s003380050146) [DOI] [Google Scholar]
- 20.Jennings S, Reynolds JD, Mills SC. 1998. Life history correlates of responses to fisheries exploitation. Proc. R. Soc. Lond. B 265, 333–339. ( 10.1098/rspb.1998.0300) [DOI] [Google Scholar]
- 21.Dulvy NK, Metcalfe JD, Glanville J, Pawson MG, Reynolds JD. 2000. Fishery stability, local extinctions, and shifts in community structure in skates. Conserv. Biol 14, 283–293. ( 10.1046/j.1523-1739.2000.98540.x) [DOI] [Google Scholar]
- 22.Reynolds JD. 2003. Life histories and extinction risk. In Macroecology (eds Blackburn TM, Gaston KJ.), pp. 195–217. Oxford, UK: Blackwell. [Google Scholar]
- 23.Bielby J, Mace G, Bininda-Emonds O, Cardillo M, Gittleman J, Jones K, Orme C, Purvis A. 2007. The fast-slow continuum in mammalian life history: an empirical reevaluation. Am. Nat. 169, 748–757. ( 10.1086/516847) [DOI] [PubMed] [Google Scholar]
- 24.Rochet MJ, Cornillon PA, Sabatier R, Pontier D. 2000. Comparative analysis of phylogenetic and fishing effects in life history patterns of teleost fishes. Oikos 91, 255–270. ( 10.1034/j.1600-0706.2000.910206.x) [DOI] [Google Scholar]
- 25.Juan-Jordá MJ, Mosqueira I, Freire J, Dulvy NK. 2013. Life in 3-D: life history strategies in tunas, mackerels and bonitos. Rev. Fish Biol. Fish. 23, 135–155. ( 10.1007/s11160-012-9284-4) [DOI] [Google Scholar]
- 26.Gaillard JM, Pontier D, Allaine D, Lebreton JD, Trouvilliez J, Clobert J. 1989. An analysis of demographic tactics in birds and mammals. Oikos 56, 59–76. ( 10.2307/3566088) [DOI] [Google Scholar]
- 27.Saether BE, Engen S, Matthysen E. 2002. Demographic characteristics and population dynamical patterns of solitary birds. Science 295, 2070–2073. ( 10.1126/science.1068766) [DOI] [PubMed] [Google Scholar]
- 28.Fry FEJ. 1971. The effect of environmental factors on the physiology of fish. In Fish physiology: environmental relations and behaviour (eds Hoar WS, Randall DJ.), pp. 1–98. New York, NY: Academic Press. [Google Scholar]
- 29.Charnov EL, Gillooly JF. 2004. Size and temperature in the evolution of fish life histories. Integr. Comp. Biol. 44, 494–497. ( 10.1093/icb/44.6.494) [DOI] [PubMed] [Google Scholar]
- 30.Savage VM, Gilloly JF, Brown JH, Charnov EL. 2004. Effects of body size and temperature on population growth. Am. Nat. 163, 429–441. ( 10.1086/381872) [DOI] [PubMed] [Google Scholar]
- 31.Munch SB, Salinas S. 2009. Latitudinal variation in lifespan within species is explained by the metabolic theory of ecology. Proc. Natl Acad. Sci. USA 106, 13860–13864. ( 10.1073/pnas.0900300106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pauly D. 2010. Gasping fish and panting squids: oxygen, temperature and the growth of water-breathing animals. In Excellence in ecology, book 22, International Ecology Institute (ed. Kinne O.). Oldendorf/Luhe, Germany. [Google Scholar]
- 33.Fromentin JM, Fonteneau A. 2001. Fishing effects and life history traits: a case study comparing tropical versus temperate tunas. Fish. Res. 53, 133–150. ( 10.1016/S0165-7836(00)00299-X) [DOI] [Google Scholar]
- 34.Burgess M, Polasky S, Tilman D. 2013. Predicting overfishing and extinction threats in multispecies fisheries. Proc. Natl Acad. Sci. USA 110, 15 943–15 948. ( 10.1073/pnas.1314472110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Juan-Jordá MJ, Mosqueira I, Cooper AB, Dulvy NK. 2011. Global population trajectories of tunas and their relatives. Proc. Natl Acad. Sci. USA 51, 20 650–20 655. ( 10.1073/pnas.1107743108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Juan-Jordá MJ, Mosqueira I, Freire J, Dulvy NK. 2013. The conservation and management of tunas and their relatives: setting life history research priorities. PLoS ONE 8, e70405 ( 10.1371/journal.pone.0070405) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burnham KP, Anderson DR. 2002. Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn New York, NY: Springer. [Google Scholar]
- 38.Mazerolle MJ. 2006. Improving data analysis in herpetology: using Akaike's information criterion (AIC) to assess the strength of biological hypotheses. Amphib. Reptilia 27, 169–180. ( 10.1163/156853806777239922) [DOI] [Google Scholar]
- 39.Zuur A, Ieno EN, Walker N, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. New York, NY: Springer. [Google Scholar]
- 40.R Development Core Team. 2013. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 41.Bartón K. 2009. MuMIn: multi-model inference. R package, v. 0.12.2. See http://r-forge.r-project.org/projects/mumin/.
- 42.Wickham H. 2009. ggplot2: elegant graphics for data analysis. New York, NY: Springer. [Google Scholar]
- 43.Pardo SA, Cooper AB, Dulvy NK. 2013. Avoiding fishy growth curves. Methods Ecol. Evol. 4, 353–360. ( 10.1111/2041-210x.12020) [DOI] [Google Scholar]
- 44.Dulvy NK, Reynolds JD. 2002. Predicting extinction vulnerability in skates. Conserv. Biol. 16, 440–450. ( 10.1046/j.1523-1739.2002.00416.x) [DOI] [Google Scholar]
- 45.Jennings S, Greenstreet SPR, Reynolds JD. 1999. Structural change in an exploited fish community: a consequence of differential fishing effects on species with contrasting life histories. J. Anim. Ecol. 68, 617–627. ( 10.1046/j.1365-2656.1999.00312.x) [DOI] [Google Scholar]
- 46.Taylor B, Houk P, Russ G, Choat J. 2014. Life histories predict vulnerability to overexploitation in parrotfishes. Coral Reefs 33, 869–878. ( 10.1007/s00338-014-1187-5) [DOI] [Google Scholar]
- 47.Andersen KH, Pedersen M. 2010. Damped trophic cascades driven by fishing in model marine ecosystems. Proc. R. Soc. B 277, 795–802. ( 10.1098/rspb.2009.1512) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dulvy NK, et al. 2014. Extinction risk and conservation of the world's sharks and rays. eLife 3, e00590 ( 10.7554/eLife.00590) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pinsky ML, Jensen OP, Ricard D, Palumbi SR. 2011. Unexpected patterns of fisheries collapse in the world's oceans. Proc. Natl Acad. Sci. USA 108, 8317–8322. ( 10.1073/pnas.1015313108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Myers RA, Fowlow PS. 1997. Maximum population growth rates and recovery times for Atlantic cod, Gadus morhua. Fish. Bull. 95, 762–772. [Google Scholar]
- 51.Denney NH, Jennings S, Reynolds JD. 2002. Life-history correlates of maximum population growth rates in marine fishes. Proc. R. Soc. Lond. B 269, 2229–2237. ( 10.1098/rspb.2002.2138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cardillo M, Meijaard E. 2011. Are comparative studies of extinction risk useful for conservation? Trends Ecol. Evol. 27, 167–171. ( 10.1016/j.tree.2011.09.013) [DOI] [PubMed] [Google Scholar]
- 53.Jennings S, Mélin F, Blanchard JL, Forster RM, Dulvy NK, Wilson RW. 2008. Global-scale predictions of community and ecosystem properties from simple ecological theory. Proc. R. Soc. B 275, 1375–1383. ( 10.1098/rspb.2008.0192) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article are available in the electronic supplementary material, S1 and S2.