Abstract
Although there is mounting evidence that biodiversity is an important and widespread driver of ecosystem multifunctionality, much of this research has focused on small-scale biodiversity manipulations. Hence, which mechanisms maintain patches of enhanced biodiversity in natural systems and if these patches elevate ecosystem multifunctionality at both local and landscape scales remain outstanding questions. In a 17 month experiment conducted within southeastern United States salt marshes, we found that patches of enhanced biodiversity and multifunctionality arise only where habitat-forming foundation species overlap—i.e. where aggregations of ribbed mussels (Geukensia demissa) form around cordgrass (Spartina alterniflora) stems. By empirically scaling up our experimental results to the marsh platform at 12 sites, we further show that mussels—despite covering only approximately 1% of the marsh surface—strongly enhance five distinct ecosystem functions, including decomposition, primary production and water infiltration rate, at the landscape scale. Thus, mussels create conditions that support the co-occurrence of high densities of functionally distinct organisms within cordgrass and, in doing so, elevate salt marsh multifunctionality from the patch to landscape scale. Collectively, these findings suggest that patterns in foundation species' overlap drive variation in biodiversity and ecosystem functioning within and across natural ecosystems. We therefore argue that foundation species should be integrated in our conceptual understanding of forces that moderate biodiversity–ecosystem functioning relationships, approaches for conserving species diversity and strategies to improve the multifunctionality of degraded ecosystems.
Keywords: decomposition, ecosystem function, facilitation, primary production, salt marsh, Spartina alterniflora
1. Background
Humans depend on the environment for food, clean water, protection from natural disasters, climate buffering and other valuable services and, therefore, seek to maintain ecosystems that perform many functions at high levels simultaneously [1,2]. Variation in the ability of ecosystems to meet these burgeoning demands has fuelled a surge of research focused on identifying the principle drivers of ecosystem ‘multifunctionality’ [3–9]. A growing number of studies have shown that biodiversity enhances ecosystem multifunctionality on local, or patch, scales as the presence of many, functionally distinct species ensures complementary and efficient resource use [3,7,10–13]. However, much of this research has focused on artificially or theoretically assembled communities [3,4,9–11] and has thus been unable to evaluate the relative importance of naturally occurring ‘biodiversity patches’, i.e. resource-rich habitats with more species than their surroundings (see [14] for description), in regulating ecosystem functioning at either the local patch or larger spatial scale. Hence, what factors maintain patches of elevated biodiversity within landscapes, and if these patches enhance the overall multifunctionality of ecosystems, are questions of significant scientific and management importance that thus far remain unanswered.
Foundation species are habitat-modifying organisms that structure many ecosystems and, as a result of their dominance, play a central role in regulating carbon fluxes, nutrient cycling, soil accretion and other ecosystem functions [15–17]. The degree to which foundation species can sustain high levels of multiple functions may depend upon the composition of species that reside within their structures however—organisms whose foraging, feeding and burrowing activities may enhance or suppress the performance of certain functions [18]. Secondary foundation species such as oysters cemented to mangrove roots and epiphytes layered on trees are often among the organisms facilitated by these foundation species [15,19–22] and have the potential to modify an ecosystem's multifunctionality through their own engineering activities and through their further enhancement of biodiversity by increasing niche space [21]. Indeed, recent research in cobble beach plant communities [20], mangroves [22], mudflats [23,24], savannahs [21], tropical forests [25] and temperate woodlands [26] has shown that 20–80% of the species richness and abundance maintained by foundation species in these ecosystems can be attributed to the presence of secondary foundation species. Despite their well-documented, positive effects on community structure [15,19], studies have yet to experimentally investigate the importance of foundation species' overlap in driving ecosystem functioning and multifunctionality. To address this knowledge gap, studies that manipulate secondary foundation species' densities (and, hence, the amount of habitat they create for other, resident species) are needed to identify where they are likely to maximize ecosystem functions and multifunctionality on local, patch scales. Likewise, quantitative measures of how much the total yield of ecosystem functions may vary because of differences in secondary foundation species' distribution are key to assessing whether conserving—or enhancing—their cover might offer an effective strategy for maintaining specific, targeted functions and multifunctionality on landscape scales.
Salt marshes form along temperate, wave-protected shorelines around the globe where they provide many valuable ecosystem services including shoreline protection, carbon storage, nutrient filtration, and nursery habitat provision [27–29]. In being structured by dominant grasses and, often, bivalve and macroalgae secondary foundation species [20,23,30], salt marshes are a suitable system for investigating the relationships between foundation species' overlap, biodiversity and ecosystem multifunctionality. In the southeastern USA, Spartina alterniflora (hereafter, cordgrass) generates much of the three-dimensional structure of salt marsh habitats. Embedded in the mud around cordgrass stems, Geukensia demissa (ribbed mussels, hereafter mussels) occur in distinct, clumped aggregations dispersed throughout higher elevation marsh platforms. This distribution is thought to be maintained by predation which limits mussel survival outside of the protective structure of conspecifics, by competition for filtrate food which prevents aggregations from getting too large, and by larval recruitment which regulates the size of mussel populations within marsh landscapes [31–33]. On marsh platforms, the temperature of exposed mud can exceed 46°C, a level well above the thermal limit of mussels [34], and, thus, long-term mussel survival is dependent on shade provided by the cordgrass canopy (see the electronic supplementary material, Methods S1 and figure S1). Within cordgrass monocultures, mussels function as secondary foundation species as they are dependent on facilitation by another foundation species and independently facilitate a number of resident invertebrate functional groups [20], including predatory mud crabs, an omnivorous marsh crab, and juvenile life stages of a mud fiddler crab that feed on benthic algae and bacteria [35]. In this ecosystem, adult mud fiddler crabs that excavate far larger burrows than juvenile fiddler crabs but maintain a similar diet [36], and snails that graze on benthic algae, fungi, and both live and dead cordgrass tissue [37] are also common. As polychaetes are only occasionally observed [38], these five functional groups—mud crabs, marsh crabs, adult and juvenile fiddler crabs, and snails—comprise the majority of the resident (i.e. not migrating with the tides) macrofauna in southeastern United States salt marshes, a community that is relatively low in species richness, but high in functional diversity as each group uniquely affects the local environment [7].
Here we test the hypotheses that, as the number of mussels within an aggregation increases, salt marsh invertebrate functional group richness, diversity, and abundance (H1), ecosystem functions (H2) and multifunctionality (H3) increase at the local, patch scale. We define the patch scale as 0.25 m2 because our observations of natural mussel aggregations (as well as the results from this study) indicate that the size of the largest aggregations and their effects on the ecosystem are confined within this spatial scale in marsh platforms that occur between northeastern Florida and central North Carolina, our study region. Additionally, we test whether mussel aggregations, at their natural distribution within marsh platforms, increase the total number of marsh invertebrates (H4) and total yield of ecosystem functions (H5) at the landscape scale, which we define as 500 m2, an area representative of a small marsh platform in this region [39].
To test the first three hypotheses, we experimentally created replicate mussel aggregations spanning the size range observed in marsh platforms (0, 1, 3, 5, 10, 20, 40 and 80 mussels per aggregation, n = 3 replicates per size, see the electronic supplementary material, Methods S2 and figure S2). After 17 months, we quantified the number of mud crab, marsh crab, juvenile and adult fiddler crab, and snail functional groups and calculated two measures of biodiversity, functional group richness and inverse Simpson's diversity index, from these counts [40]. We further quantified responses of six mechanism-based proxies of ecosystem functions (hereafter, simply ‘ecosystem functions’) that are related to the marsh ecosystem services of nursery provision (invertebrate biomass), nutrient processing (infiltration, decomposition), shoreline stabilization (soil accretion), and carbon storage (benthic algae biomass, aboveground cordgrass biomass), and calculated two measures of ‘multifunctionality’, i.e. the average and threshold multifunctionality indices [4,5]. Using model selection techniques, we identified the functional form of relationships between aggregation size and each response metric to distinguish which aggregations (e.g. those with few or many mussels) maximize invertebrate abundance and richness, ecosystem functions and multifunctionality at the patch scale [10,41]. We then mapped the distribution of every mussel aggregation found within 500 m2 marsh platform ‘landscapes’ at each of 12 salt marsh sites along 850 km of coastline and calculated the number of mussels in each aggregation. Using the functional relationships between mussel aggregation size and each response metric measured in the experiment, we estimated the number of invertebrates and level of ecosystem functions supported by each 0.25 m2 patch containing a surveyed mussel aggregation as well as those supported by all remaining 0.25 m2 patches containing no mussels. We then summed the values calculated for the 2000, 0.25 m2 mussel and no mussel patches that comprised each surveyed 500 m2 landscape to scale from the patch to this landscape scale and test our fourth and fifth hypotheses. These calculations allowed us to approximate how much variation in the distribution of this secondary foundation species affects the total number of resident invertebrates and total yield of the six ecosystem functions maintained by marsh landscapes across this region.
2. Results and discussion
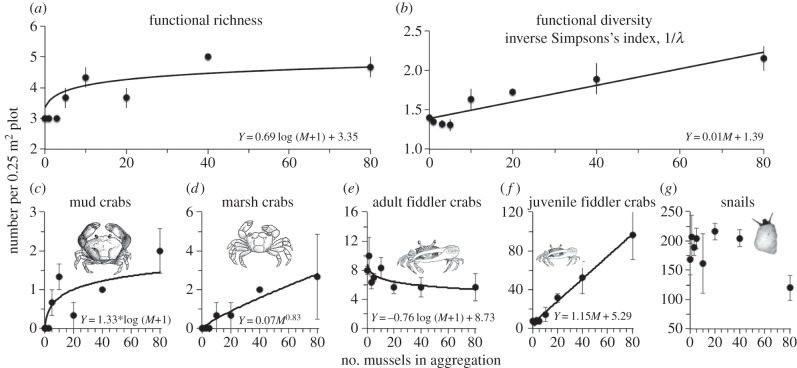
Our experiment revealed that mussel aggregations powerfully enhance both invertebrate functional group diversity and ecosystem multifunctionality at the patch scale. As mussel aggregations increased in size, functional group richness increased as a power function and the inverse Simpson's index (1/λ, where λ is the probability of two individuals taken at random from the local community represent the same functional group and, therefore, 1/λ increases with increasing functional group evenness and richness) as a linear function, such that both measures indicate that the most diverse communities were found in plots containing large aggregations of 40 and 80 mussels (figure 1a,b; see the electronic supplementary material, table S1 for model fit comparisons and summary of statistical analyses). This increasing trend in invertebrate functional richness and diversity was because of the response of mud and marsh crabs, functional groups that were absent in plots with 0 or only a few mussels, and that increased in abundance in a decelerating manner as power and log functions, respectively, as aggregations increased in size (figure 1c,d). Juvenile fiddler crabs were present in all experimental plots, but increased linearly in abundance as the number of mussels in aggregations increased, reaching maximum average densities in the largest mussel aggregations (96 ± 25 versus 8 ± 1 individuals per 0.25 m2, mean ± s.e.m., in 80 versus 0 mussel plots, respectively, figure 1e). In contrast to these functional groups that responded positively to the addition of mussels, adult fiddler crabs decreased as a log function of aggregation size and snails occurred at a similar density across the marsh, regardless of the presence of mussels (figure 1f,g). Combined, these results reveal that, although overlap of cordgrass and mussel foundation species does not universally promote all functional groups (figure 1), it does provide the conditions within which enhanced densities of several ecologically diverse functional groups of crabs occur, and all five resident macro-invertebrates consistently coexist within marsh platforms.
Figure 1.
Patch-scale effects of mussel aggregations on invertebrate functional group richness, inverse Simpson's diversity index (1/λ), and abundance. Points and error bars denote the mean ± s.e.m. of three plots per aggregation size treatment. Lines are fitted and equations are shown for statistically significant (p ≤ 0.05) best-fit models for each response.
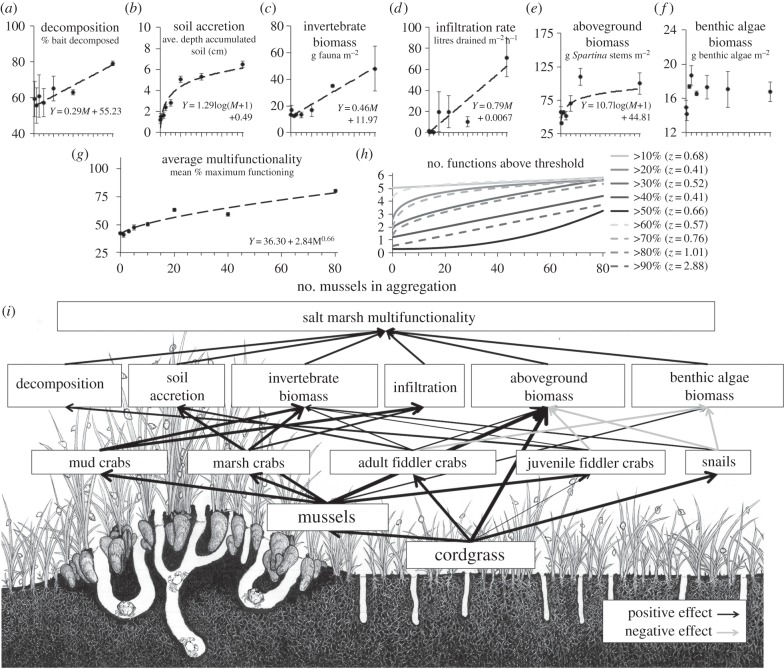
In concert with their positive effects on marsh invertebrate richness, diversity and abundance, mussel aggregations increased the level of most ecosystem functions at the patch scale (figure 2a–f). Invertebrate biomass, aboveground cordgrass biomass, decomposition, water infiltration and soil accretion functions were all stimulated by the presence of mussels, and achieved maximum average levels in larger aggregations. The type of relationship between aggregation size and these five ecosystem functions was variable, however, with invertebrate biomass, decomposition and infiltration increasing as linear functions, and soil accretion and aboveground cordgrass biomass increasing in a decelerating manner as log functions (figure 2; see the electronic supplementary material, table S2 for model fit comparisons and summary of statistical analyses). Benthic algae biomass, a function that reflects the difference between production and consumption rates, both of which are generally high in marsh platforms because of ample light and nutrient availability and persistent foraging by fiddler crabs [36,42], did not vary in response to experimental treatments (figure 2f).
Figure 2.
Links between salt marsh foundation species, invertebrates, and ecosystem functioning. The patch-scale effects of mussel aggregations on individual ecosystem functions (a–f), average multifunctionality (g), the number of functions performed above a series of per cent of maximum functioning thresholds (h) and a conceptual model of the effects of cordgrass and mussel foundation species on invertebrate functional groups and direct and indirect effects on ecosystem functioning (i). In (a–g), units of measurement for each panel are shown in small font below panel titles, points and error bars denote the mean ± s.e.m. of three plots per aggregation size treatment, and lines are fitted and equations shown for statistically significant (p ≤ 0.05) best-fit models for each response variable. Lines denote the power fit for the number of functions performed above each threshold level in (h), and the value of the power exponent, Z, for each line is provided in the legend. (i) The strength of effects is denoted by line weight and direction of effects by line colour (positive, black; negative, grey). Illustration credit: Joseph P. Morton.
Coincident with their diverse, though generally positive, effects on individual ecosystem functions, mussel aggregations also strongly enhanced multifunctionality at the patch scale. Average multifunctionality, the geometric mean of the six functions that were rescaled to be a per cent of average maximum functioning [4,5], more than doubled from 0 to 80 mussel experimental patches (37% versus 89%, respectively) and increased as a power function of aggregation size (figure 2g, see the electronic supplementary material, table S3 for model fit comparisons and summary of statistical analyses). Thus, average multifunctionality was maximized in patches containing the largest mussel aggregations. To examine whether the positive relationship between aggregation size and average multifunctionality was driven by an increase in many or just a few particularly responsive functions, we tallied the number of functions that exceeded more than 10, 20, … and 90% of the average maximum functioning performance thresholds in each experimental patch [5]. Increasing mussel aggregation size increased the number of functions maintained above each performance threshold, revealing that mussels enhance multifunctionality through their broad, positive effects on individual functions (figure 2h). Moreover, by comparing the exponent of the power function fit for each mussel aggregation size—threshold relationship [10], we find that adding mussels to an aggregation increases the number of functions exceeding performance thresholds in a decelerating manner at lower thresholds (Z < 1 at 10–70% thresholds), in a linear manner at high thresholds (Z ≈ 1 at 80% threshold) and in an exponential manner at extremely high performance thresholds (Z > 1 at 90% threshold). From these results, we conclude that, by any measure, mussels enhance salt marsh ecosystem multifunctionality and that aggregation size becomes an increasingly important factor in maintaining multiple functions as higher and higher performance thresholds are considered.
Overall, the differences in the form of these ecosystem function and multifunctionality relationships indicate that mussels boost individual ecosystem functions through distinct direct and indirect mechanisms. More specifically, from extensive experimental investigations [7,20,31,43–45], we infer that mussels directly contribute to augmenting three ecosystem functions—invertebrate biomass, soil accretion and aboveground primary production—through their own engineering of habitat and deposition of soil- and nutrient-rich pseudofaeces [31,35,45]. Conversely, mussels are very likely stimulating infiltration and decomposition functions indirectly through their facilitation of mud and marsh crabs, species that excavate wide and deep burrows, respectively, through which water can readily infiltrate [7], and facilitation of juvenile fiddler crabs that promote microbial activity as a result of their bioturbation and aeration of marsh soils [36,43]. Consequently, patches colonized by largest aggregations of mussels and, thus, particularly high densities of mud, marsh and juvenile fiddler crabs, are capable of supporting the highest performance of ecosystem functions driven by each of these distinct functional groups (figures 1 and 2).
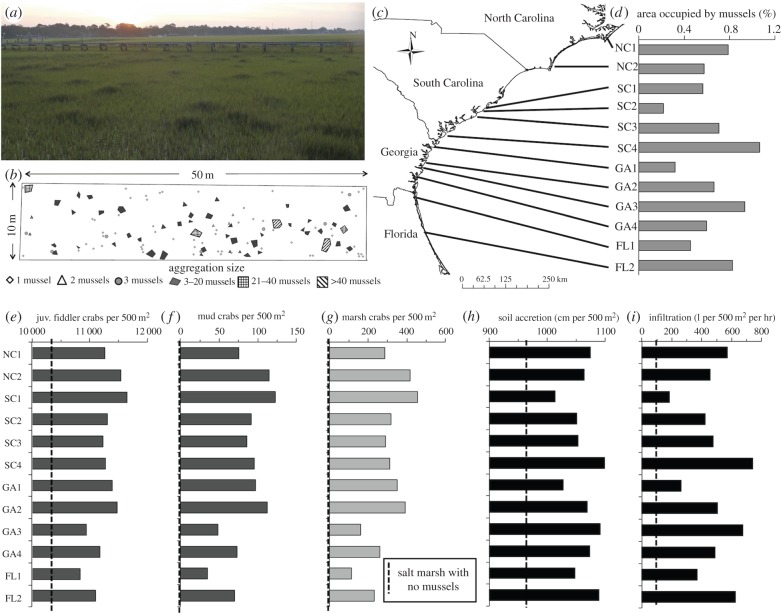
Based on the functional relationships between aggregation size and each invertebrate and ecosystem function response metric derived from the experiment (figures 1 and 2) and the number and size distribution of aggregations mapped at the 12 sites along the southeastern United States coastline (electronic supplementary material, figure S3), we estimate that mussels drive significant variation in the total number of resident invertebrates that are supported and the total yield of ecosystem functions that are generated across marsh platforms at the landscape scale (figure 3, see the electronic supplementary material, figure S4 for site information). Despite occupying only 0.2–1.1% of marsh area (figure 3d), our calculations suggest that mussel aggregations enhance the number of juvenile fiddler crabs from approximately 10 580 in salt marshes with no mussels to up to 11 640 individuals per 500 m2, a 13% increase, in marshes with the highest cover of mussels (figure 3e). Likewise, we estimate that between 34 and 122 mud crabs and 113–455 marsh crabs reside in 500 m2 marsh platforms across the 12 sites, species that are rare to absent in marsh platforms outside of mussel aggregations in this region (figures 1 and 3f,g). By contrast, mussel aggregations appear to slightly decrease (less than 1% reduction) the number of adult fiddler crabs and have little effect on the number of snails relative to salt marshes with no mussels at the larger, landscape scale (electronic supplementary material, figure S5).
Figure 3.
Estimated marsh platform-scale effects of mussel aggregations on invertebrate communities and multiple ecosystem functions. In salt marsh platforms like this one in West Ashley, SC (a), the distribution of mussel aggregations were mapped (b) and measured at each of 12 sites (c). From these maps, the per cent of marsh platform area (d), as well as the total number of resident invertebrates (juvenile fiddler crabs (e), mud crabs (f) and marsh crabs (g)) supported and total yield of ecosystem functions (soil accretion (h) and infiltration (i)) generated on 500 m2, marsh platform scale were calculated. Dashed lines show the calculated level of each invertebrate functional group and ecosystem function in a 500 m2 area of salt marsh with no mussel aggregations. Photo credit: Christine Angelini. (Online version in colour.)
In scaling from patch to landscape, we also estimate that mussel aggregations increase soil accretion by 3.4–12.1% and infiltration by 1384–5538% relative to marsh platforms with no mussels, respectively (figure 3h,i). Given that soil accretion contributes to the ability of salt marshes to gain elevation via vertical accretion and through infiltration their ability to process nutrients [45,46], the marked variation in the total yield of these functions across sites (driven by differences in the distribution of mussels) that we calculated may have important consequences for the levels of shoreline stabilization and water quality enhancement services that different salt marshes ultimately provide. While our estimates for the effects of mussels on infiltration on the landscape scale are especially high because of the steep, linear relationship between mussel aggregation size and this response metric on the patch scale (figure 1), we anticipate that they are not necessarily a gross overestimate of the true effects of mussels on infiltration, as one might suspect. This is because the many large channels that are created by the mussels (and the large crabs they facilitate) dramatically alter the amount of pore space that is open for water exchange relative to the dense, clay-rich soils that characterize the surrounding marsh (see the diagram in figure 2 for reference). In addition, our calculations indicate that mussels elevate invertebrate biomass, aboveground biomass and decomposition functions at the landscape scale, increasing their total yield by 1.9–6.5%, 1.8–6.0% and 1.5–4.7%, respectively, relative to marsh platforms with no mussels (electronic supplementary material, figure S5). As mussel aggregation size had a negligible effect on benthic algae biomass at the patch scale (figure 2f), variation in the distribution of mussel aggregations among marsh sites had no effect on the total yield of this measure at the landscape scale according to our calculations.
From these results, we draw three main conclusions. The first is that mussels significantly enhance the richness, diversity and abundance of resident invertebrates and several important ecosystem functions at the patch scale. Second, the enhancement of patch-level multifunctionality by mussels results from both direct effects through their own engineering activities and indirect effects through their local enhancement of resident fauna. Finally, the local stimulation of multifunctionality by this secondary foundation species is probably translating to marked increases in multifunctionality at the landscape scale in United States salt marshes, despite the fact that mussels only cover a minor percentage of the total marsh surface (0.2–1.1%, figure 3).
Combined, our field experiment and surveys take several decades of research that has demonstrated that biodiversity is a major driver of ecosystem multifunctionality at the patch scale (e.g. [41,47,48]) to the next level. In southeastern United States salt marshes, diverse communities are indeed needed to maintain elevated levels of multiple ecosystem functions. However, our results reveal that these communities arise only where mussels provide additional, complex niche space, habitat that facilitates the local coexistence of high densities of multiple functional groups. Furthermore, we show that any single function cannot be considered a representative indictor of the whole ecosystem response to secondary foundation species, as different ecosystem functions are enhanced through distinct mechanisms and functional relations (figure 2). Thus, where ecosystem managers and policy makers are concerned with maximizing key, individual functions, they must focus on the relationship between the foundation species and their specific, target function(s) [5,8]. Given that secondary foundation species commonly increase habitat complexity and exhibit rather patchy distributions in many other terrestrial, freshwater and marine ecosystems, we predict that our finding that layering of foundation species creates spatial patterns in biodiversity and multifunctionality, is broadly applicable [15,19]. Moving forward, the hierarchical linkages among foundation species, biodiversity and multifunctionality need to be tested across diverse ecosystems, as such studies are critical for evaluating whether or not our conceptual understanding of forces regulating biodiversity–ecosystem functioning relationships should be expanded to integrate these potentially widespread and powerful mechanisms.
Perhaps even more importantly, our initial findings indicate that overlapping foundation species collectively enhance biodiversity and specific ecosystem functions not only on patch, but also on larger, landscape spatial scales (i.e. the scales at which ecosystem services are generated and ecosystem management strategies are often applied). As a result, we anticipate that harnessing the benefits of multiple foundation species holds promise as a new management approach. Specifically, conserving or enhancing the cover of secondary foundation species may offer a practical, effective means of enhancing biodiversity and ecosystem functioning in natural systems. Likewise, integrating multiple foundation species into the design of systems managed for agriculture, timber, aquaculture, livestock and fisheries has the potential to strongly enhance the ability of these ecosystems to sustain not one primary function, but multiple target functions.
3. Material and methods
(a). Study system
We performed our field experiment within the Sapelo Island National Estuarine Research Reserve on Sapelo Island, GA, USA (31°24′26″ N, 81°17′24″ W), a barrier island embedded in an expansive network of salt marshes that spans the mouth of the Altamaha River estuary. We conducted the field experiment and subsequent mussel distribution surveys in marsh platforms, relatively flat expanses of salt marsh that are dominated by ‘short-form’ cordgrass monocultures whose canopy reaches approximately 50 cm in height by the end of the growing season in early autumn. In these platforms, mussels exhibit a widespread distribution and occur in aggregations that vary in density, from 1 to approximately 100 individuals. Our mussel distribution survey was conducted in 12 salt marshes that were accessible by foot and spanned more than 850 km of coastline.
(b). Density-dependent effects of mussels on biodiversity, ecosystem functions and multifunctionality: an experiment
Prior to setting up our experiment to test the effects of mussel aggregate density on invertebrate diversity and ecosystem functioning, we first characterized the range of densities with which mussels naturally aggregate in marsh platforms. To do so, we haphazardly identified 17 mussel aggregations that captured the range of sizes observed within a 50 × 50 m area at a representative of marsh platform site on Sapelo Island (electronic supplementary material, figure S2), extracted each aggregation, and then counted and measured every mussel retained in a 0.5 cm sieve over which aggregations were washed. Natural aggregations contained between 1 and 82 individual mussels, which were 7.2 ± 2.9 cm (mean ± s.d.) in length respectively, and were at the most 0.20 m2 in area.
Within a high elevation marsh platform, we then marked 24 plots, positioned more than 1.5 m apart, in a monoculture of cordgrass. Plots were cleared of resident mussels in April 2012 and randomly assigned one mussel density treatment (0, 1, 3, 5, 10, 20, 40 or 80 mussels, with density levels based on our natural range of mussels per aggregation, n = 3 replicates per treatment). We then transplanted the appropriate number of mussels (length: 50–80 mm) collected from a nearby marsh platform in a cluster to mimic natural aggregations in each plot. Three dead mussels were replaced on day 7 of the experiment, after which aggregations were left undisturbed until the following summer. In June 2012, August 2012, June 2012 and August 2013, we used the following methods to quantify the effect of mussel density on invertebrate functional groups; biodiversity metrics, ecosystem functions (infauna, benthic algae biomass and infiltration were measured in August 2013 only), and two measures of multifunctionality—the average multifunctionality index and threshold index [5]—in 0.5 × 0.5 m patch-scale plots centred on each experimental treatment. The plot area encompassed all 80 mussels transplanted in our largest aggregations. We just present results from August 2013 because this is the only date for which all response variables were measured and because our analyses indicate that the magnitude and pattern in these response variables varied by only 10–25% among sampling dates and, thus, these data are representative of the patterns elucidated by the experiment.
(i). Invertebrate functional group abundance, richness and diversity
To assess the effect of mussel density on resident fauna, we counted every macro-invertebrate in each plot. We classified invertebrates in five functional groups (mud crabs, marsh crabs, adult fiddler crabs, juvenile fiddler crabs and snails) rather than taxonomic species to account both for functional redundancy between species (i.e. Eurythium limosum and Panopeus obesus are generalist predators that excavate similarly sized burrows, thus they are both counted as mud crabs) and functional disparity between life stages of the same species (i.e. as adult fiddler crabs excavate burrows that are 5–10× wider and deeper than those excavated by juvenile fiddler crabs, we counted them separately). As excavations of 20 burrows per functional group revealed that burrow densities correspond closely to crab densities at this field site (0.95 mud crabs per burrow, 1.2 marsh crabs (Sesarma reticulatum) per burrow, 0.85 adult and 0.94 juvenile mud fiddler crabs (Uca pugnax) per burrow), we counted burrows as a non-destructive measure of each crab functional group. Snails (Littoraria irrorata) were counted on the marsh surface and cordgrass canopy. From invertebrate counts, we calculated functional group richness and the inverse Simpson's diversity index, 1/λ in which larger values correspond to higher levels of diversity, as complementary measures of biodiversity. We also collected, sieved and counted the number of each species of infauna within 5, 4 × 10 cm (diameter × depth) soil cores from each plot at the experiment's conclusion. However, because we only found three polychaetes in these 120 cores, we did not include infauna in our analyses.
(ii). Invertebrate biomass
We assessed invertebrate biomass, a proxy of secondary marsh productivity, in each plot by collecting a random sample of 20 mud crabs, 20 marsh crabs, 20 adult and 20 juvenile fiddler crabs, and 20 snails at the site of our experiment. Each invertebrate was dried in a 60°C oven for 48 h and weighed. We then calculated invertebrate biomass by multiplying the density of each functional group observed in August 2013 by the respective average biomass per individual, and summing these values across all functional groups in each plot.
(iii). Soil accretion
We measured soil accretion, the vertical accumulation of settled, but not yet root-bound, soil, by inserting a 3 mm diameter rod perpendicularly into the marsh until it made contact with the rigid root mat [45]. We recorded the unbound soil depth at five haphazardly chosen locations per plot in October 2013 and averaged these values to generate an integrative measure of the extent to which soil accreted on the marsh surface over the duration of the experiment (additional data on short-term soil deposition rates are provided in the electronic supplementary material, figure S6).
(iv). Decomposition
We quantified decomposition, a key process in nutrient cycling, using bait lamina tests (Terra Protecta, Berlin, Germany). In August 2013, we haphazardly inserted three bait strips to a depth of 12 cm in each plot, collected them after 48 h, and counted the number of baits that were decomposed out of the 16 baits per strip [49]. Further methods used to measure decomposition and their results are provided in provided in the electronic supplementary material, Methods S5 and figure S7.
(v). Infiltration rate
We measured infiltration, the rate with which water percolates through marsh soils, by securing a 12 cm diameter double-ring infiltrometer to the marsh surface, filling it with 2 l of creek water 4 h after high tide, and recording the time required for the water to drain [7]. Infiltration is a critical function that prevents the development of water-logged, anoxic marsh soils which limit primary and secondary production and promotes the uptake and filtration of nutrients from terrestrial and estuarine water sources [50].
(vi). Benthic algae biomass
We quantified the density of benthic algae, a major component of salt marsh primary production and a dominant resource consumed by fiddler crabs and snails [36], on the surface of the marsh using a hand-held fluorometer (Bentho-torch, bbe Moldaenke GmbH, Germany). On each of three consecutive sunny days in August 2013, we recorded three readings (µg diatoms + µg cyanobacteria per cm2) per plot, which we then averaged to derive one integrated measure of benthic algae biomass.
(vii). Aboveground cordgrass biomass
We harvested, rinsed, dried in a 60°C oven for 72 h, and weighed all live stems located within plot boundaries in October 2013 to quantify aboveground cordgrass biomass. Standing plant biomass is commonly used as a proxy for primary production and is a function that mediates carbon sequestration and wave attenuation, two key services provided by marsh ecosystems [27].
(viii). Multifunctionality
To distinguish whether the number of mussels in an aggregation broadly enhances multiple ecosystem functions to increase multifunctionality, we calculated the average multifunctionality and multiple threshold indices which are described in detail in Byrnes et al. [5]. To calculate the former, we standardized each function to the same scale by dividing the value of each function measured in each plot by the average of the four highest values measured for that function across all plots and then averaged together all seven standardized function values to derive an ‘average % of maximum functioning’ value for each plot [48]. We assume that the high values of each of our functions indicate a high level of functioning; for example, high soil accretion denotes a high level of performance for this function. The average multifunctionality index can be interpreted as the average level of all seven functions. However, using this index one cannot interpret whether all functions are being performed simultaneously at a high level, as functions that are performed at low levels can be ‘averaged out’ by those performed at high levels. Thus, we also tallied the number of functions, at their standardized function value, in each plot that surpassed each of seven threshold levels: 10, 20, 30, 40, 50, 60, 70, 80 and 90% of maximum functioning. Threshold index scores, which range from 0 to all six functions, can be interpreted simply as the number of functions performed above a given threshold level in a plot [3,5].
(ix). Analyses
Using generalized linear models and nonlinear least-squares models, we fit null (Yi = a), linear (Yi = a + bM), log [Yi = a + b*log(M + 1)], power (Yi = a + cMz) and hyperbolic [Yi = aM/(b + M)] relationships between the number of mussels added (M) and each functional group and ecosystem function response variable (Yi) and selected the best-fitting model using Akaike's information criterion corrected for low sample size, AICc [51].
Model fits, AICc values and AICc weights for invertebrate, ecosystem function and multifunctionality response variables are reported in the electronic supplementary material, tables S1–S3. For response variables in which the best-fit model was linear or log, we report the significance of mussel treatment as the probability (P) of obtaining the slope value, b, given that the null hypothesis (that b equals zero) is true. For response variables in which the best-fit model is a power or hyperbolic function, we report the significance of mussel treatment as the probability (P) of obtaining the slope (c) and exponent (z), or asymptote (d) and half-maximum value (k), value given that the null hypothesis (that each parameter equals zero) is true. Analyses were conducted in R v. 3.0.2 [52] and model comparisons were conducted using the AICcmodavg package [53].
(c). Effects of mussels on multiple ecosystem functions and biodiversity at marsh platform scales
To gauge whether mussels enhance the number of resident invertebrates and level of ecosystem functioning at larger spatial scales, we used our experimental results to scale from the patch to marsh landscape. Specifically, we mapped and measured the area of every mussel aggregation found within 5 × 20 m belt transects (n = 5 per site, total area surveyed per site = 500 m2) run across non-overlapping, haphazard locations within marsh platform areas at each of the 12 salt marsh sites (see ‘Study system’). We then estimated the number of mussels (M) in each aggregation using the following equation derived from extracting and counting mussels from 17 real aggregations: M = 70.34 × A0.67, where M is the number of mussels and A is the aggregation area in m2 (see the electronic supplementary material, figure S2). Next, we divided each 500 m2 surveyed area into 2000, 0.5 × 0.5 m cells, the area of our experimental plots, which we either ‘occupied’ with a mussel aggregation recorded in our survey or left ‘unoccupied’ with 0 mussels. We then used the best-fit model equations from our experiment (figure 1) to estimate the yield of each ecosystem function in each cell and density of each invertebrate functional group and summed each function and invertebrate metric across all mussel-occupied cells (Xi,Mussels) and unoccupied (Xi,No Mussels) cell values. As a reference, we also calculated the yield of each ecosystem function and density of each invertebrate functional group in a salt marsh with no mussel aggregations (i.e. all cells were unoccupied).
To validate that mussels stimulate resident invertebrate densities and marsh ecosystem functions within salt marsh platforms across this geographical region and, thus, justify using the functions derived from our experiment to estimate their effects to marsh platform scales, we counted the number of mud crab, marsh crab, adult and juvenile fiddler crab burrows and snails, as above, within 0.25 m2 sampling frames that were positioned either on intermediate-sized mussel aggregations and no-mussel control areas located 2 m away (n = 8 replicate frames for each condition) at each of our 12 surveyed salt marsh sites. We then harvested all aboveground cordgrass stems in the sampling frame, dried them in a 60°C oven until a constant weight was reached, and weighed them. We analysed the effect size and significance of site and mussel presence within site using a nested ANOVA. For brevity, we have included these results in the electronic supplementary material as they suggest that mussels have similar effects on resident invertebrate richness and abundance and aboveground biomass across sites (electronic supplementary material, figures S4 and S5).
Supplementary Material
Acknowledgement
We appreciate the comments of S. L. Flory, D. Levey, C. Osenberg, Q. He and T. M. Palmer on this manuscript as well as the time and effort of R. Atkins, H. de Paoli, J. van de Koppel, S. M. Buhler, S. J. Sharp and J. O'Donnell.
Data accessibility
All data are archived and freely available through the Georgia Coastal Ecosystems LTER online data portal (doi:10.6073/pasta/ab7dfc03bc228a0962f58993d6d30527).
Authors' contributions
C.A., J.N.G. and B.R.S. designed the experiment and survey; C.A., T.v.d.H., J.N.G., M.D.H., A.J.S., L.P.M.L. conducted the field experiment; J.M.P. and C.A. conducted the survey; C.A. analysed the data; all authors wrote and revised the manuscript and gave final approval for publication.
Competing interests
None of the authors have any competing financial interests to claim.
Funding
Funds supporting this research were provided by NSF GRFP (DGE-0802270) and University of Florida Graduate Alumni Fellowship awards to C.A.; NSF Career award (no. 1056980) to B.R.S.; Climate Change Consortium of Wales (C3W) and Marie Curie Career Integration (no. 618935) awards to J.N.G.; and NWO-VENI (no. 863.12.003) award to T.v.d.H.
References
- 1.Daily G, et al. 1997. Ecosystem services: benefits supplied to human societies by natural ecosystems. Issues Ecol. 2, 1–21. (doi:10.1.1.498.2963) [Google Scholar]
- 2.Kareiva P, Watts S, McDonald R, Boucher T. 2007. Domesticated nature: shaping landscapes and ecosystems for human welfare. Science 316, 1866–1869. ( 10.1126/science.1140170) [DOI] [PubMed] [Google Scholar]
- 3.Zavaleta E, Pasari J, Hulvey K, Tilman D. 2010. Sustaining multiple ecosystem functions in grassland communities requires higher biodiversity. Proc. Natl Acad. Sci. USA 107, 1443–1446. ( 10.1073/pnas.0906829107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gamfeldt L, Hillebrand H, Jonsson P. 2008. Multiple functions increase the importance of biodiversity for overall ecosystem functioning. Ecology 89, 1223–1231. ( 10.1890/06-2091.1) [DOI] [PubMed] [Google Scholar]
- 5.Byrnes J, et al. 2014. Investigating the relationship between biodiversity and ecosystem multifunctionality: challenges and solutions. Methods Ecol. Evol. 5, 111–124. ( 10.1111/2041-210X.12143) [DOI] [Google Scholar]
- 6.Pasari J, Levi T, Zavaleta E, Tilman D. 2013. Several scales of biodiversity affect ecosystem multifunctionality. Proc. Natl Acad. Sci. USA 110, 10 219–10 222. ( 10.1073/pnas.1220333110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hensel M, Silliman B. 2013. Consumer diversity across kingdoms supports multiple functions in a coastal ecosystem. Proc. Natl Acad. Sci. USA 110, 20 621–20 626. ( 10.1073/pnas.1312317110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradford MA, et al. 2014. Discontinuity in the responses of ecosystem processes and multifunctionality to altered soil community composition. Proc. Natl Acad. Sci. USA 111, 14 478–14 483. ( 10.1073/pnas.1413707111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hector A, Bagchi R. 2007. Biodiversity and ecosystem multifunctionality. Nature 448, 05947 ( 10.1038/nature05947) [DOI] [PubMed] [Google Scholar]
- 10.Reich PB, Tilman D, Isbell F, Mueller K, Hobbie SE, Flynn DFB, Eisenhauer N. 2012. Impacts of biodiversity loss escalate through time as redundancy fades. Science 336, 589–592. ( 10.1126/science.1217909) [DOI] [PubMed] [Google Scholar]
- 11.Duffy JE. 2003. Grazer diversity effects on ecosystem functioning in seagrass beds. Ecol. Lett. 6, 637–645. ( 10.1046/j.1461-0248.2003.00474.x) [DOI] [Google Scholar]
- 12.Elmqvist T, Folke C, Nyström M, Peterson G, Bengtsson J, Walker B, Norberg J. 2003. Response diversity, ecosystem change, and resilience. Front. Ecol. Environ. 1, 488–494. ( 10.1890/1540-9295(2003)001[0488:RDECAR]2.0.CO;2) [DOI] [Google Scholar]
- 13.Cardinale B. 2011. Biodiversity improves water quality through niche partitioning. Nature 472, 86–90. ( 10.1038/nature09904) [DOI] [PubMed] [Google Scholar]
- 14.Ludwig J. 1999. Disturbances and landscapes: the little things count. In Issues and perspectives in landscape ecology (eds Weins J, Moss M.), pp. 59–63. Guelph, Canada: International Association for Landscape Ecology; ( 10.1017/CBO9780511614415.007) [DOI] [Google Scholar]
- 15.Angelini C, Altieri AH, Silliman BR, Bertness MD. 2011. Interactions among foundation species and their consequences for community organization, biodiversity, and conservation. Bioscience 61, 782–789. ( 10.1525/bio.2011.61.10.8) [DOI] [Google Scholar]
- 16.Dayton PK. 1972. Toward an understanding of community resilience and the potential effects of enrichments to the benthos at McMurdo Sound, Antarctica. In Proc. Colloquium on Conservation Problems in Antarctica, Lawrence, Kansas: Allen Press. [Google Scholar]
- 17.Ellison A, et al. 2005. Loss of foundation species: consequences for the structure and dynamics of forested ecosystems. Front. Ecol. 3, 479–486. ( 10.1890/1540-9295(2005)003[0479:LOFSCF]2.0.CO;2) [DOI] [Google Scholar]
- 18.Bruno JF, Stachowicz JJ, Bertness MD. 2003. Inclusion of facilitation into ecological theory. Trends Ecol. Evol. 18, 119–125. ( 10.1016/S0169-5347(02)00045-9) [DOI] [Google Scholar]
- 19.Thomsen MS, Wernberg T, Altieri A, Tuya F, Gulbransen D, McGlathery KJ, Holmer M, Silliman BR. 2010. Habitat cascades: the conceptual context and global relevance of facilitation cascades via habitat formation and modification. Integr. Comp. Biol. 50, 158–175. ( 10.1093/icb/icq042) [DOI] [PubMed] [Google Scholar]
- 20.Altieri A, Silliman B, Bertness M. 2007. Hierarchical organization via a facilitation cascade in intertidal cordgrass bed communities. Am. Nat. 169, 195–206. ( 10.1086/510603) [DOI] [PubMed] [Google Scholar]
- 21.Angelini C, Silliman B. 2014. Secondary foundation species as drivers of trophic and functional diversity: evidence from a tree-epiphyte system. Ecology 95, 185–196. ( 10.1890/13-0496.1) [DOI] [PubMed] [Google Scholar]
- 22.Bishop M, Byers J, Marcek B, Gribben P. 2012. Density-dependent facilitation cascades determine epifaunal community structure in temperate Australian mangroves. Ecology 93, 1388–1401. ( 10.1890/10-2296.1) [DOI] [PubMed] [Google Scholar]
- 23.Thomsen MS, McGlathery KJ, Schwarzschild A, Silliman B. 2009. Distribution and ecological role of the non-native macroalga Gracilaria vermiculophylla in Virginia salt marshes. Biol. Invasions 11, 2303–2316. ( 10.1007/s10530-008-9417-9) [DOI] [Google Scholar]
- 24.Byers J, Gribben P, Yeager C, Sotka E. 2012. Impacts of an abundant introduced ecosystem engineer within mudflats of the southeastern US coast. Biol. Invasions 14, 2587–2600. ( 10.1007/s10530-012-0254-5) [DOI] [Google Scholar]
- 25.Ellwood MDF, Foster WA. 2004. Doubling the estimate of invertebrate biomass in a rainforest canopy. Nature 429, 549–551. ( 10.1038/nature02560) [DOI] [PubMed] [Google Scholar]
- 26.Watson DM, Herring M. 2012. Mistletoe as a keystone resource: an experimental test. Proc. R. Soc. B 279, 3853–3860. ( 10.1098/rspb.2012.0856) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barbier E, Hacker S, Kennedy C, Stier A, Silliman B. 2011. The value of estuarine and coastal ecosystem services. Ecol. Monogr. 81, 169–193. ( 10.1890/10-1510.1) [DOI] [Google Scholar]
- 28.Gedan KB, Silliman BR, Bertness MD. 2009. Centuries of human-driven change in salt marsh ecosystems. Ann. Rev. Mar. Sci. 1, 117–141. ( 10.1146/annurev.marine.010908.163930) [DOI] [PubMed] [Google Scholar]
- 29.Craft C, Clough J, Ehman J. 2008. Forecasting the effects of accelerated sea-level rise on tidal marsh ecosystem services. Front. Ecol. Environ. 7, 73–78. ( 10.890/070219) [DOI] [Google Scholar]
- 30.Dijkstra JA, Boudreau J, Dionne M. 2012. Species-specific mediation of temperature and community interactions by multiple foundation species. Oikos 121, 646–654. ( 10.1111/j.1600-0706.2011.19712.x) [DOI] [Google Scholar]
- 31.Bertness M, Grosholz E. 1985. Population dynamics of the ribbed mussel, Geukensia demissa: the costs and benefits of an aggregated distribution. Oecologia 67, 192–204. ( 10.1007/BF00384283) [DOI] [PubMed] [Google Scholar]
- 32.Stiven A, Gardner S. 1992. Population processes in the ribbed mussel Geukensia demissa (Dillwyn) in a North Carolina salt marsh tidal gradient: spatial pattern, predation, growth and mortality. J. Exp. Mar. Bio. Ecol. 160, 81–102. ( 10.1016/0022-0981(92)90112-N) [DOI] [Google Scholar]
- 33.Nielsen KJ, Franz DR. 1995. The influence of adult conspecifics and shore level on recruitment of the ribbed mussel Geukensia demissa (Dillwyn). J. Exp. Mar. Bio. Ecol. 188, 89–98. ( 10.1016/0022-0981(94)00190-O) [DOI] [Google Scholar]
- 34.Jost J, Helmuth B. 2007. Morphological and ecological determinants of body temperature of Geukensia demissa, the Atlantic ribbed mussel, and their effects on mussel mortality. Biol. Bull. 213, 141–151. ( 10.2307/25066630) [DOI] [PubMed] [Google Scholar]
- 35.Silliman B, Layman C, Geyer K, Zieman J. 2004. Predation by the black-clawed mud crab, Panopeus herbstii, in mid-Atlantic salt marshes: further evidence for top-down control of marsh grass production. Estuaries 27, 188–196. ( 10.1007/BF02803375) [DOI] [Google Scholar]
- 36.Teal J. 1958. Distribution of fiddler crabs in Georgia salt marshes. Ecology 39, 186–193. ( 10.2307/1931862) [DOI] [Google Scholar]
- 37.Silliman BR, Newell S. 2003. Fungal-farming in a snail. Proc. Natl Acad. Sci. USA 100, 15 643–15 648. ( 10.1073/pnas.2535227100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kneib RT. 1984. Patterns of invertebrate distribution and abundance in the intertidal salt marsh: causes and questions. Estuaries 7, 392–412. ( 10.2307/1351621) [DOI] [Google Scholar]
- 39.Schalles J, Hladik C, Lynes A, Pennings SC. 2013. Landscape estimates of habitat types, plant biomass, and invertebrate densities in a Georgia salt marsh. Oceanography 26, 88–98. ( 10.5670/oceanog.2013.50) [DOI] [Google Scholar]
- 40.Jost L. 2006. Entropy and diversity. Oikos 113, 363–375. ( 10.1111/j.2006.0030-1299.14714.x) [DOI] [Google Scholar]
- 41.Cardinale B, Matulich K, Hooper DU, Duffy JE, Gamfeldt L, Balvanera P, O'Connor MI, Gonzalez A. 2011. The functional role of producer diversity in ecosystems. Am. J. Bot. 98, 572–592. ( 10.3732/ajb.1000364) [DOI] [PubMed] [Google Scholar]
- 42.Zedler JB. 1980. Algal mat productivity: comparisons in a salt marsh. Estuaries 3, 122–131. ( 10.2307/1351556) [DOI] [Google Scholar]
- 43.Bertness M. 1985. Fiddler crab regulation of Spartina alterniflora production on a New England salt marsh. Ecology 66, 1042–1055. ( 10.2307/1940564) [DOI] [Google Scholar]
- 44.Griffin J, Butler J, Soomdat NN, Brun KE, Chejanovski ZA, Silliman BR. 2011. Top predators suppress rather than facilitate plants in a trait-mediated tri-trophic cascade. Biol. Lett. 7, 710–713. ( 10.1098/rsbl.2011.0166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith J, Frey R. 1985. Biodeposition by the ribbed mussel Geukensia demissa in a salt marsh, Sapelo Island, Georgia. J. Sediment. Res. 55, 817–828. [Google Scholar]
- 46.Jordan T, Valiela I. 1982. A nitrogen budget of the ribbed mussel, Geukensia demissa, and its significance in nitrogen flow in a New England salt marsh. Limnol. Oceanogr. 27, 75–90. ( 10.4319/lo.1982.27.1.0075) [DOI] [Google Scholar]
- 47.Tilman D. 1999. The ecological consequences of changes in biodiversity: a search for general principles. Ecology 80, 1455–1474. ( 10.2307/176540) [DOI] [Google Scholar]
- 48.Hooper DU, Vitousek PM. 1998. Effects of plant composition and diversity on nutrient cycling. Ecol. Monogr. 68, 121–149. ( 10.1890/0012-9615(1998)068[0121:EOPCAD]2.0.CO;2) [DOI] [Google Scholar]
- 49.Simpson J, Slade E, Riutta T, Taylor M. 2012. Factors affecting soil fauna feeding activity in a fragmented lowland temperate deciduous woodland. PLoS ONE 7, e29616 ( 10.1371/journal.pone.0029616) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hemond H, Nuttle W. 1984. Surface infiltration in salt marshes: theory, measurement, and biogeochemical implications. Water Resour. Res. 20, 591–600. ( 10.1029/WR020i005p00591) [DOI] [Google Scholar]
- 51.Burnham KP, Anderson DR. 2002. Model selection and inference: a practical information- theoretical approach, 2nd edn New York, NY: Springer. [Google Scholar]
- 52.R Core Development Team. 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation of Statistical Computing. [Google Scholar]
- 53.Mazerolle MJ. 2013. Model selection and multimodel inference based on (Q)AIC(c).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are archived and freely available through the Georgia Coastal Ecosystems LTER online data portal (doi:10.6073/pasta/ab7dfc03bc228a0962f58993d6d30527).