Abstract
Complex social structure in eusocial insects can involve worker morphological and behavioural differentiation. Neuroanatomical variation may underscore worker division of labour, but the regulatory mechanisms of size-based task specialization in polymorphic species are unknown. The Australian weaver ant, Oecophylla smaragdina, exhibits worker polyphenism: larger major workers aggressively defend arboreal territories, whereas smaller minors nurse brood. Here, we demonstrate that octopamine (OA) modulates worker size-related aggression in O. smaragdina. We found that the brains of majors had significantly higher titres of OA than those of minors and that OA was positively and specifically correlated with the frequency of aggressive responses to non-nestmates, a key component of territorial defence. Pharmacological manipulations that effectively switched OA action in major and minor worker brains reversed levels of aggression characteristic of each worker size class. Results suggest that altering OA action is sufficient to produce differences in aggression characteristic of size-related social roles. Neuromodulators therefore may generate variation in responsiveness to task-related stimuli associated with worker size differentiation and collateral behavioural specializations, a significant component of division of labour in complex social systems.
Keywords: neuromodulation, octopamine, territorial aggression, polymorphism, division of labour
1. Introduction
Colony organization in complex insect societies is based on division of labour. Differentiation of sterile workers into polymorphic subcastes specialized on tasks according to morphology is widespread and has evolved independently in diverse eusocial insect taxa [1–5]. Some ant species have evolved morphological variation based on growth allometries in association with discretized and potentially size-constrained worker behavioural repertoires [6,7]. Although the number of polymorphic ant species is relatively small, striking physical caste structures characterize the most socially advanced and evolutionarily successful species, including the fungus-growing ants, army ants and members of the hyperdiverse genus Pheidole [1]. Concomitant with increased behavioural specialization, size-variable workers may respond differently to social signals and cues [6,8]; this variation has a significant function in the collective organization and ecology of social insect colonies [9]. Worker polyphenism has been demonstrated to be under hormonal [10,11] and genetic [12,13] control. Neuroanatomical differentiation accompanies polymorphism [14,15], and neuropeptides have been suggested to regulate division of labour [16]. The role of neuromodulators in worker size-based behavioural differentiation, however, remains poorly understood.
Biogenic amines such as dopamine (DA), serotonin (5-HT) and octopamine (OA) are neurotransmitters that affect sensorimotor systems and behaviour in insects [17–21]. Recent investigations in ants suggest linkages between neurotransmitters and colony organization by correlating monoamine brain titres and social behaviour [22,23] or causally linking amines to behaviour through pharmacological manipulations of brain neuromodulator levels [24,25]. Monoamine titres in the brains of worker ants are associated with age-related task performance [26] and possibly genetic variation [22]. Studies indicate that biogenic amines can be correlated with worker body size [19,22,27], but their causal role in the genesis of behavioural diversity among polymorphic workers has not been demonstrated.
The morphological differentiation of worker subcastes in ants often involves variation in worker aggression in the context of defence of nest and territory [6,28]. Worker phenotypes specialized on colony security are larger in body size, physically adapted for fighting [7,29] and quick to respond aggressively to threats from intruders. The Australian weaver ant, Oecophylla smaragdina, considered to be one of the most socially complex invertebrates [30], can serve as a model to advance our understanding of the neuromodulation of worker size-related division of labour. Oecophylla smaragdina, which cooperatively construct arboreal nests from leaves ‘woven’ together by silk produced by larvae, dominate rainforest canopy through their large colony size, social organization and territoriality [30]. Weaver ant task specialization is based on worker size: small minor workers generally care for brood within nests, whereas larger major workers construct nests, forage and aggressively maintain exclusive territories by preventing intrusion from neighbouring conspecific [30–33] and interspecific [34] competitors. As many as approximately 500 000 workers inhabit a single colony, and territories may span 1500 m2 [31]. The iconic aggressive threat posture of majors during territorial defence is marked by flaring the mandibles while raising the gaster (figure 1). We tested the hypothesis that division of labour by morphologically differentiated workers in complex insect societies is regulated by biogenic amines. We examined variation in levels of monoamines between O. smaragdina minor and major workers and determined their role in controlling subcaste-specific differences in aggressive behaviours associated with territorial defence.
Figure 1.

Oecophylla smaragdina major worker. Mandibles are flared and the gaster is raised in a threat posture (photo courtesy of P. Smallhorn-West). (Online version in colour.)
2. Material and methods
(a). Colony collection and maintenance
Oecophylla smaragdina colonies were collected from approximately 1 km2 of savannah woodlands on the James Cook University campus in Townsville, Queensland, Australia. Depending on colony size, multiple 19 × 10 × 13.5 cm Fluon®-lined plastic boxes connected by plastic tubes or a single 52 × 30 × 40 cm box were used to house colonies. Colonies were fed 1 : 3 honey water and flies or crickets every other day ad libitum and were kept on a 12 L : 12 D cycle at 55% humidity and 25.5°C.
(b). Aggression assays
Fully pigmented, mature major (average head width 1.57 mm, range 1.31–1.71 mm) and minor (average head width 1.02 mm, range 0.93–1.16 mm) O. smaragdina workers from two colonies were allowed to habituate in a 5 cm diameter closed Petri dish for 1 min. A non-nestmate conspecific mature major worker collected from a colony not adjacent to the colony of the focal worker was used as an intruder stimulus and was gently placed in the dish after removing a small segment of one pretarsus to distinguish focal ants. Behavioural categories were defined to characterize the full range of O. smaragdina aggressive actions [32,35,36]. Behavioural responses directed towards non-nestmates were quantified for 5 min. The number of times a focal worker performed a particular action was tallied according to the following categorization of aggression using a behavioural scale of 1–6, with 6 being the highest level of agonistic response:
(1) Non-aggressive behaviour such as reversing direction to avoid a non-nestmate or not altering behaviour when located within an antenna-length from a non-nestmate.
(2) Olfactory assessment, marked by antennae waving in the direction of a non-nestmate [1].
(3) Flaring the mandibles in the direction of a non-nestmate.
(4) Adopting a threat posture directed towards a non-nestmate, characterized by an immediate stop, flaring the mandibles and raising the gaster.
(5) Lunging towards a non-nestmate a single time or several times in a rapid burst.
(6) Prolonged biting of a non-nestmate.
To compare response frequencies, we divided behaviours into three categories: non-aggressive behaviours, olfactory assessment and aggressive actions (e.g. flared mandibles, startle posture, lunging and biting), as described in our categorization. Within these categories, frequencies of behaviours performed were recorded and analysed. To account for variation in activity levels and time spent interacting with non-nestmates, each worker was also given an aggression score calculated as the product of the number of times a worker performed an action in a given category and the level of aggression, divided by the total number of responses recorded during the assay [32].
The degree of aggression between O. smaragdina and conspecifics may vary depending on colony proximity [32], genetic relatedness [33] and the intensity of ecological interaction between O. smaragdina and interspecific competitors [34]. Therefore, we performed additional aggression assays with behaviourally mature major and minor workers from three weaver ant colonies and workers from queenright colonies of Atta cephalotes, an ant species that does not overlap geographically but nevertheless elicits a strong aggressive response. Using A. cephalotes workers, which are polymorphic, as a model stimulus also allowed us to match intruder body size to that of O. smaragdina major workers. A single A. cephalotes media worker was grasped with forceps (Dumont No. 5, Inox, standard) at the petiole and inserted through a 2 mm hole in the side of a Petri dish (5 cm diameter). A weaver ant worker was then placed in the closed dish. Ants acclimated for 5 min and were then observed for 10 min. Behaviours were quantified using the scale described above. All behavioural assays were filmed using a Canon FS400 camcorder. Petri dishes were cleaned with 70% ethanol between trials. Each focal ant was tested once and stimulus ants were not used more than three times.
(c). Biogenic amine analysis
Immediately after interspecific assays were completed, worker head width was measured and brains of majors and minors were prepared to quantify monoamine titres using high-performance liquid chromatography with electrochemical detection (HPLC-ED). Workers from two colonies were used in these analyses. Brains were dissected from the head capsule in cold Insect Ringer's solution following established monoamine quantification methods [24]. Cuticle, which contains DA, was meticulously removed to ensure samples were not contaminated and brain concentrations of DA were thus accurately measured. Brains were then homogenized in a microcentrifuge tube containing 55 µl of mobile phase solution (50 mM citrate/acetate buffer, 1.5 mM sodium dodecyl sulfonate, 0.01% triethylamine and 22% acetronitrile in MilliQ water; modified from [24] to optimize OA quantification) and kept on ice to prevent amine degradation. Solutions were centrifuged at 1500 r.p.m. for 10 min at 0°C and injected into the HPLC-ED system (model 584 pump, model 5020 guard cell, model MD-150 reversed-phase analytical column, model 5011A dual-channel coulometric analytical cell, and a Coulochem III electrochemical detector; ESA Inc., Chelmsford, MA, USA). Electrode potentials were 375 mV for the first channel, which measured 5-HT and DA, and 600 mV for the second channel, which measured OA. External standards of 5-HT, DA and OA were run daily.
Workers from two colonies of O. smaragdina encompassing the full body size spectrum were analysed separately to determine the relationship between head width and brain mass. Brains were dried for 48 h and weighed on a microbalance (Sartorius CP 2P). The data displayed a linear trend (brain mass = [31.3 × head width] + 13.45; F1,39 = 12.64, R2 = 0.24; p < 0.01; n = 41; electronic supplementary material, figure S1). Environmental humidity and the limit of accuracy and resolution of the microbalance likely account in part for variability in brain measurements. Because brain sizes varied between and within subcastes, titres of biogenic amines were scaled to both head width and estimated brain mass.
(d). Pharmacological manipulations of octopamine
Epinastine (EPN), a specific OA receptor antagonist [37], was used to decrease OA action in mature majors. OA-specific synthesis inhibitors have not been developed and thus could not be employed to lower OA titre. To orally administer EPN, 30–50 workers were placed in a Fluon®-lined box and provided 10 mM EPN in a 3 : 1 honey water solution or 3 : 1 honey water as a control. Both solutions were dyed with red food colouring to confirm that ants had ingested drug-treated and control solutions. Only ants with red-coloured fluid visible in the crop were tested. Dosage was determined in a series of trials that began with a concentration of 20 mM EPN in 1 : 3 honey water [38]. However, this solution produced a strongly aversive response and the dose was lowered to increase its palatability to majors. Intraspecific aggression assays were conducted using workers from two colonies 1–3 h after solution ingestion as described above, except observation time was increased to 10 min for more robust behavioural quantification. Intraspecific assays were used because of their ecological relevance; they also produced similar results to those of interspecific assays. Additionally, worker activity was assayed to determine whether gross locomotion was systemically affected by treatments. Workers were placed in a closed Petri dish (8.5 cm diameter) with lines dividing the bottom into four equal quadrants; ants were allowed 1 min to habituate. Worker movement among quadrants made during a 5 min period was recorded to quantify activity.
To increase brain levels of OA, 0.5 µl of 10.5 mM OA in dimethylformamide (DMF) was topically applied to the thoraces of mature minors from two colonies following methods established in honeybees [39]. The amount of solution applied was reduced owing to the smaller body size of minor workers. OA application was used rather than a receptor agonist to allow quantification of brain OA titres, ensuring titres were elevated after treatment. Controls received topical applications of 0.5 µl DMF. The timings of behavioural assays and dissections were based on sampling times described in [39]. Minors were tested 15–30 min after treatment. Intraspecific aggression assays and activity assays were conducted as described in the EPN experiment. Immediately after testing, ants were placed on ice. Treated workers were repeatedly washed in MilliQ water to rinse any OA potentially remaining on the cuticle and minimize contamination. Brains were then dissected and prepared for HPLC-ED analysis 45–60 min after treatment. Biogenic amines were measured as described above, except the electrode potential for the first channel was 425 mV and 600 mV for the second channel. Changes in sensitivity associated with routine equipment maintenance prevented the accurate comparison of absolute amine titres in interspecific and OA manipulation assays.
(e). Statistical analysis
Aggression scores and the frequency of behaviours in response categories (non-aggressive behaviours, olfactory assessment and aggressive actions) of subcaste or treatment groups were compared using non-parametric Mann–Whitney U-tests. Simple linear regression analyses were used to determine the relationship between amine titre and the natural log of the frequency of behaviours in each category. We added one to all frequencies to correct for frequencies of zero within behavioural categories [40]. We used Bonferroni correction [41] for multiple comparisons of behaviour frequencies and simple linear regression analyses: p < 0.017 and p < 0.0056, respectively, were considered statistically significant. Biogenic amine titres and movement assays were compared with Student's t-tests. Summary statistics are presented as mean ± 95% confidence intervals and all statistical analyses were performed using JMP Pro 11 statistical software.
3. Results
(a). Worker subcaste-related aggression in response to intraspecific and interspecific ants
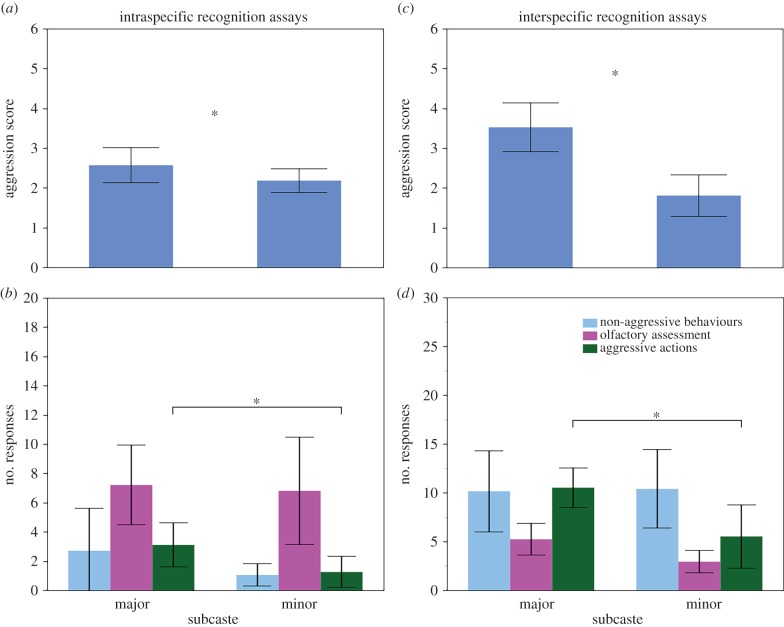
When presented with non-nestmate conspecifics, majors had significantly higher aggression scores than minors (major: 2.57 ± 0.44, minor: 2.18 ± 0.3; Mann–Whitney U, W20,20 = 315.5, p < 0.05; figure 2a). Majors showed a significantly higher frequency of aggressive behaviours (major: 3.10 ± 1.5, minors: 1.25 ± 1.06; W20,20 = 302.5, p < 0.01; figure 2b); there were no subcaste differences in the frequency of non-aggressive behaviours (major: 2.7 ± 2.93, minor: 1.05 ± 0.77; W20,20 = 392, p = 0.56) or olfactory assessment (major: 7.2 ± 2.72, minor: 6.8 ± 3.67; W20,20 = 366.5, p = 0.24).
Figure 2.
Average aggression scores (a,c) and frequency of responses in three behavioural categories (b,d) are presented for intraspecific assays (a,b) and interspecific assays (c,d) for major and minor workers. Error bars indicate 95% CIs and asterisks denote statistical significance: (a) p < 0.05; (b) p < 0.01; (c,d) p < 0.001. Horizontal brackets indicate statistical significance between behavioural groups. (Online version in colour.)
In interspecific assays, major worker aggression scores were significantly greater than those of minors (major: 3.50 ± 0.61, minor: 1.76 ± 0.53; Mann–Whitney U-test, W17,23 = 323, p < 0.001; figure 2c). Majors exhibited aggressive displays more frequently than minors (major: 8.76 ± 2.66, minor: 2.71 ± 1.27; W17,23 = 361, p < 0.001; figure 2d). There were no subcaste differences in the frequency of non-aggressive behaviours (major: 5.24 ± 3.18, minor: 9.0 ± 4.16; W17,23 = 324.5, p = 0.39) or olfactory assessment (major: 4.24 ± 2.12, minor: 3.46 ± 1.45; W17,23 = 484.5, p = 0.60).
(b). Brain biogenic amine titres and behavioural responses to interspecific intruders
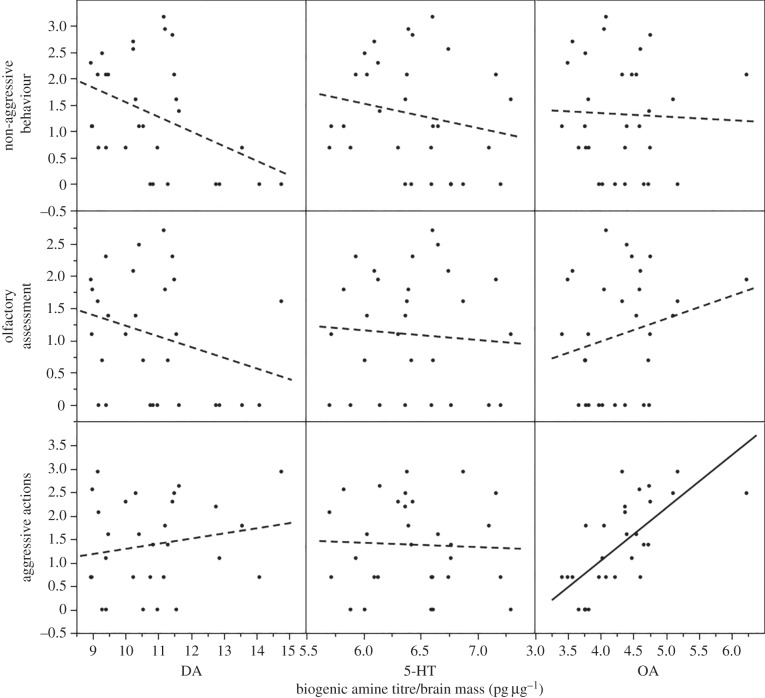
Absolute titres of all biogenic amines were significantly higher in majors than minors (DA: major: 676.72 ± 59.15 pg, minor: 507.55 ± 40.42 pg; 5-HT: major: 398.25 ± 16.92 pg, minor: 304.60 ± 12.56 pg; OA: major: 296.37 ± 14.79 pg, minor: 181.71 ± 14.79 pg; t-test, all p < 0.001; electronic supplementary material, figure S2). OA level, scaled to brain mass, was significantly different between subcastes (major: 4.73 ± 0.28 pg µg−1, minor: 3.89 ± 0.18 pg µg−1; t-test, t28 = 5.39, p < 0.001; figure 3); on average, majors had 19.5% higher OA levels than minors. DA and 5-HT titres of majors and minors were not significantly different when scaled to brain mass (DA: major: 10.79 ± 0.87 pg µg−1, minor: 10.87 ± 0.88 pg µg−1; t28 = 0.13, p = 0.89; 5-HT: major: 6.35 ± 0.22 pg µg−1, minor: 6.52 ± 0.26 pg µg−1; t28 = 1.03, p = 0.31). There were no significant relationships between behaviour and either DA or 5-HT (all p > 0.02). There were no relationships between the frequency of non-aggressive behaviours and olfactory assessment recorded and OA titre (simple linear regression, F1,28 = 0.04, p = 0.84 and F1,28 = 1.53, p = 0.23, respectively). Only the frequency of aggressive behaviours showed a significant positive correlation with OA level (F1,28 = 27.33, R2 = 0.49, p < 0.001; figure 4). When scaled to head width, OA was higher in majors than minors (major: 188.55 ± 11.21, minor: 170.87 ± 7.95; t28 = 2.76, p < 0.05; electronic supplementary material, figure S2). DA level was similar between subcastes (major: 430.37 ± 34.82, minor: 477.82 ± 39.25; t28 = 1.94, p = 0.063), whereas 5-HT was significantly higher in minors than in majors (major: 253.54 ± 9.39, minor: 286.66 ± 11.95; t28 = 4.68, p < 0.001). Only the positive correlation of OA titre scaled for head width and frequency of aggressive behaviour was significant (F1,28 = 11.49, R2 = 0.29, p < 0.005; all other p > 0.01).
Figure 3.
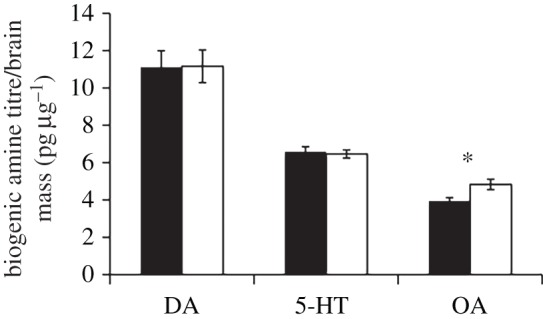
Mean ± 95% CIs (error bars) of dopamine (DA), serotonin (5-HT) and octopamine (OA) brain titres corrected for estimated brain mass are presented for minors (black) and majors (white). Asterisk denotes statistical significance at p < 0.001.
Figure 4.
Best-fit regression lines of dopamine (DA), serotonin (5-HT) and octopamine (OA) titres in both majors and minors and the number of times a worker performed non-aggressive behaviours, olfactory assessment and aggressive action (ln). Solid line indicates regression of statistical significance.
(c). Effects of epinastine treatment on behavioural responses of major workers
EPN-treated majors had significantly decreased aggression scores compared with those of control majors (control: 3.08 ± 0.34, EPN: 2.64 ± 0.38; Mann–Whitney U-test, W17,20 = 258.5, p < 0.05; figure 5a). With respect to the frequency of behaviours, both groups showed similar levels of non-aggressive acts (control: 0.70 ± 0.57, EPN: 0.82 ± 0.78; W17,20 = 376, p = 0.89; figure 5b), olfactory assessment (control: 6.30 ± 1.33, EPN: 7.24 ± 2.34; W17,20 = 373, p = 0.83) and aggressive behaviours (control: 12.70 ± 8.95, EPN: 6.35 ± 4.2; W17,20 = 274, p = 0.13). Workers in both groups showed similar activity levels (control: 16.10 ± 9.44, EPN: 23.18 ± 7.96; t-test, t35 = 1.18, p = 0.25).
Figure 5.
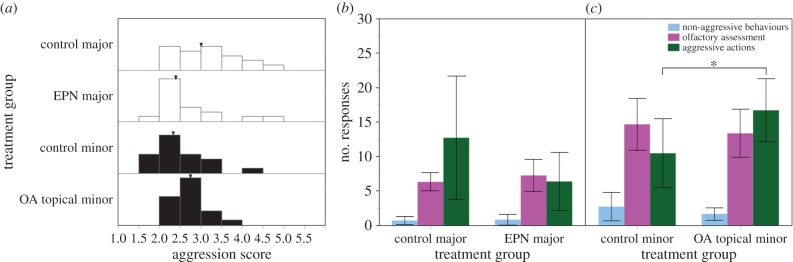
Distribution of aggression scores for control and pharmacologically treated majors (white) and minors (black) are presented with arrowheads indicating mean (a). The mean frequencies of behaviours are shown in control and epinastine (EPN)-treated majors (b) and control and OA topically treated minors (c). Error bars indicate 95% CIs and asterisk denotes statistical significance at p < 0.05. Horizontal bracket indicates statistical significance between behavioural groups. (Online version in colour.)
(d). Effects of octopamine treatment on biogenic amine titres and behavioural responses of minor workers
OA-treated minors had significantly increased titres of brain OA compared with control minors when correcting for brain mass (control: 5.11 ± 1.14 pg µg−1, OA-treated: 8.00 ± 1.76 pg µg−1; t-test, t31 = 2.96, p < 0.01); the average increase in brain OA level was 44%. DA and 5-HT levels were similar between groups (DA: control: 10.47 ± 0.87 pg µg−1, OA-treated: 10.22 ± 0.82 pg µg−1, t37 = 0.45, p = 0.65; 5-HT: control: 6.06 ± 1.07 pg µg−1, OA-treated: 5.56 ± 0.47 pg µg−1, t37 = 0.92, p = 0.36). Similar relationships were found when comparing absolute biogenic amine titres and titres scaled to head width (electronic supplementary material, table S1 and figure S3). OA-treated minors were significantly more aggressive than controls, with higher aggression scores (control: 2.49 ± 0.28, OA-treated: 2.74 ± 0.19; Mann–Whitney U-test, W20,20 = 335, p < 0.05; figure 5a) and an increased frequency of aggressive acts (control: 10.45 ± 5.02, OA-treated: 16.70 ± 4.59; W20,20 = 318, p < 0.05; figure 5c). Frequencies of non-aggressive behaviours (control: 2.70 ± 2.05, OA-treated: 1.65 ± 0.89; W20,20 = 396.5, p = 0.70) and olfactory assessment (control: 14.65 ± 3.79, OA-treated: 13.35 ± 3.5; W20,20 = 396.5, p = 0.71) were similar between groups. Workers in both groups showed similar activity levels (control: 15.59 ± 8.83, OA-treated: 8.95 ± 4.88; t-test, t36 = 1.44, p = 0.16).
4. Discussion
Morphological subcastes and behavioural differentiation have coevolved in social insects [42]. To understand the control mechanisms associated with division of labour by physical caste, we assayed major and minor worker aggression in the context of intruder recognition and territorial defence, quantified monoamine neurotransmitter levels in individual worker brains and pharmacologically altered monoamine action. Using a socially complex ant species as a model, we determined that OA can act as a modulator of task specializations characteristic of worker physical castes. Major workers of O. smaragdina, renowned for their stereotypical territorial aggression, had significantly higher titres of OA than minors, whose labour appears to be primarily focused on within-nest tasks such as nursing. 5-HT was significantly higher in minors when scaled to head width. However, only OA titre scaled to estimated brain mass or head width was positively and specifically correlated with frequencies of aggressive responses triggered by nestmate recognition stimuli that allow territorial intruders to be detected. DA and 5-HT did not appear to regulate aggression, olfactory assessment, or non-aggressive responses towards intruders. These results could be explained by a direct effect of OA on aggression, an effect of OA on nestmate recognition [43], and/or a positive effect of aggression on OA expression [38,44], all supporting a function of OA in the task specializations of polymorphic weaver ant workers. Effectively switching OA action in major and minor worker brains by pharmacological treatments reversed the aggression level characteristic of each worker subcaste. Although we cannot rule out the possibility that aggressive encounters caused an increase in OA titres [38], we nonetheless provide pharmacological evidence that OA contributes to the behavioural differentiation of major and minor workers with respect to aggressive behaviour. Our study is the first to demonstrate that neuromodulators can underscore size-related variation in worker responsiveness to task-related stimuli—a core element of division of labour for socially complex polymorphic insects.
The results of our pharmacological manipulations of OA in both major and minor workers suggest that OA specifically impacts aggressive behaviour without affecting general activity. We are confident that using interspecific assays for correlative analyses and intraspecific assays involving drug manipulations did not bias our results or interpretation because significant subcaste differences were seen in both assays. The use of an OA antagonist to block monoamine action and OA topical application to increase monoamine titre produced results consistent with those of studies in other insects: our treatments therefore effectively altered OA action in the manner intended [37,39]. Majors treated with EPN had lower aggression scores than controls, suggesting that inhibiting OA action decreased aggressive behaviour. There was no difference in the frequency of aggressive actions between treatment groups, which may be owing to a change in the ratio of aggressive to non-aggressive acts rather than a change in the absolute frequency of aggressive behaviours. Inter-individual variation in both groups may also lead the discrepancy. Variation in the experimental group could have been a result of majors ingesting different amounts of EPN, which we could not quantify. Minors with increased OA titres exhibited higher aggression scores than controls and had a greater frequency of aggressive acts. Non-aggressive actions, olfactory assessment and rates of movement were similar across treatment groups, indicating that increasing OA specifically increased aggressive actions and did not affect overall activity or behavioural responsiveness. Contrasting with our results, OA has been shown to affect general activity in other insects [45,46]. However, worker activity during the aggression assay, which was not measured, may be affected by OA.
The causal relationship between OA and aggression in O. smaragdina could be owing to an enhanced ability to recognize non-nestmate cuticular hydrocarbon profiles, an effect on motor outputs generating aggression [45], or both. These behaviours are closely linked in ants: nestmate recognition enables workers to distinguish colony-specific chemical signatures and is therefore crucial for defending nest and territory from intruders [43]. OA has previously been implicated in both nestmate recognition and aggression in insects and other invertebrates [19,45,47–51]. The causal coupling of OA and aggression towards non-nestmates in O. smaragdina worker subcastes is consistent with the conserved neuromodulatory function of OA. However, the role of biogenic amines in modulating aggression is complex and varies among invertebrates [52–54]. For example, the role of 5-HT in decreasing aggression in some species [53,54] is supported by our data, which show significantly higher 5-HT titres in minors than majors when scaled to head width. 5-HT may be modulating behaviours associated with decreased aggression such as retreat or reduced impulsivity [47]. Allometric relationships of brain mass and head width may account for the observed inconsistencies in amine titre comparisons between subcastes. The role of monoamines in the control of social behaviour in ants and other eusocial insects is unclear, largely owing to the sampling of only approximately 10 of the more than 14 000 described species of ants and the lack of broader phylogenetic analysis of the evolution of aminergic systems in the Hymenoptera.
Aminergic systems affected by gene expression [55] could regulate the behavioural differentiation that accompanied the evolution of physical castes and task specialization in eusocial insects [22,56]. The mechanistic relationships between genes, neuromodulators and behavioural specializations associated with worker morphology, however, are poorly understood. Genetic techniques developed in Drosophila have targeted specific aminergic circuitry, enabling detailed analyses that may provide insight for ants [54,57]. Direct relationships between genes and phenotypic differentiation are nevertheless not well defined in ants. Gene expression has been suggested to control subcaste differences in task performance and plasticity [12,58,59]. In the completely dimorphic ant Pheidole pallidula, changes in foraging or defence in major workers are correlated with the expression of the PPFOR protein in a small number of cells; this does not occur in minors [60]. Downstream neurobiological effects contributing to division of labour between subcastes and the higher defensive aggression shown by major workers have not been determined in P. pallidula or other ants; however, studies on honeybees suggest the nature of the mechanisms involved [61].
Morphology, neuroanatomy and behaviour are interrelated in brain evolution [62–64]. Subcaste differences in macroscopic and cellular neuroanatomy may interact with neuromodulators to generate specialized behaviour in social insects. In O. smaragdina, the relative sizes of brain compartments that function in the primary processing of olfactory signals and cues and visual information are larger in majors, reflecting their subcaste-specific sensory ecologies (J.F.K & J.F.A.T. 2015, unpublished data). OA receptor expression is likely widespread throughout the neuropil [65], and receptor expression density, which varies by brain region, could correlate with social role [66]. Differences in relative investment in brain regions may therefore affect OA receptor profiles and consequently OA-regulated tasks. The association of serotonergic circuitry with subcaste behavioural differentiation in the dimorphic ant Pheidole dentata supports this model. In comparison to minor workers, defensive majors have more 5-HT-immunoreactive cells in primary visual regions, more extensive serotonergic varicosities in sensory and integrative brain regions [67], and more elaborate axonal arborization in a serotonergic neuron integral to sensory integration [27]. Although relationships between neuromodulators and circuit development have been described in other taxa [68,69], the influence of neuroanatomical differences in 5-HT circuitry on 5-HT signalling and behaviour remains to be studied in ants. In O. smaragdina, major and minor worker differences in endogenous OA titres may alter circuitry to generate subcaste-related aggression. Alternatively, workers may retain behavioural flexibility through similar distributions of OA receptors [56] that enable responses to changing titres of OA acting on neural circuits underscoring aggression, despite differences in endogenous OA titres and brain compartment sizes. Behavioural differentiation of polymorphic workers may be based on variation in neuromodulator levels that produce worker size-related task responsiveness and thus distributed intelligence at the colony level.
Supplementary Material
Acknowledgements
We thank Patrick Smallhorn-West for the generous use of his photograph and four anonymous reviewers for their critical reading of the manuscript. Andrew Hoadley and Darcy G. Gordon provided thoughtful suggestions and discussions.
Ethics
Animals were collected in Australia with Permit numbers WITK09412611 and WITK14544014 and exported with Wildlife Trade Permit numbers WT2012-4106, PWS2013-AU-000415 and PWS2014-AU-001493. Animals were imported into the US with USDA Permit number P526P-12-04067 and cultured under containment conditions simulating a tropical environment. Experiments minimized pain and used universally accepted methods of euthanizing ants.
Data accessibility
The datasets supporting this article have been uploaded to Dryad (doi:10.5061/dryad.36s2m).
Authors' contributions
J.F.K. and K.N. collected laboratory data, J.F.K. and S.K.A.R. analysed results, J.F.K. and J.F.A.T. drafted the manuscript, and all authors contributed to the design of the study and gave final approval for publication.
Competing interests
There are no competing interests.
Funding
This work was supported by a National Science Foundation (NSF) East Asia and Pacific Summer Institute grant 1209967 to J.F.K., NSF grants IOB-0725013 and IOS-1354291 to J.F.A.T., and Australian Research Council Discovery grant 1093553 to S.K.A.R.
References
- 1.Hölldobler B, Wilson EO. 1990. The ants. Cambridge, MA: Harvard University Press. [Google Scholar]
- 2.Grüter C, Menezes C, Imperatriz-Fonseca VL, Ratnieks FL. 2012. A morphologically specialized soldier caste improves colony defense in a neotropical eusocial bee. Proc. Natl Acad. Sci. USA 109, 1182–1186. ( 10.1073/pnas.1113398109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eggleton P. 2011. An introduction to termites: biology, taxonomy and functional morphology. In Biology of termites: a modern synthesis (eds. Bignell DE, Roisin Y, Lo N.), pp. 1–26, 2nd edn New York, NY: Springer Science and Business Media. [Google Scholar]
- 4.Aoki S. 1987. Evolution of sterile soldiers in aphids. In Animal societies: theories and facts (eds Itô Y, Brown JL, Kikkawa J.), pp. 53–65. Tokyo, Japan: Scientific Societies Press. [Google Scholar]
- 5.Crespi BJ. 1992. Eusociality in Australian gall thrips. Nature 359, 724–726. ( 10.1038/359724a0) [DOI] [Google Scholar]
- 6.Mertl AL, Traniello JFA. 2009. Behavioral evolution in the major worker subcaste of twig-nesting Pheidole (Hymenoptera: Formicidae): does morphological specialization influence task plasticity? Behav. Ecol. Sociobiol. 63, 1411–1426. ( 10.1007/S00265-009-0797-3) [DOI] [Google Scholar]
- 7.Wilson EO. 1980. Caste and division of labor in leaf-cutter ants (Hymenoptera, Formicidae, Atta). 1. The overall pattern in A. sexdens. Behav. Ecol. Sociobiol. 7, 143–156. ( 10.1007/BF00299520) [DOI] [Google Scholar]
- 8.Wilson EO. 1985. Between-caste aversion as a basis for division of labor in the ant Pheidole pubiventris (Hymenoptera, Formicidae). Behav. Ecol. Sociobiol. 17, 35–37. ( 10.1007/Bf00299425) [DOI] [Google Scholar]
- 9.Robinson GE. 1992. Regulation of division of labor in insect societies. Annu. Rev. Entomol. 37, 637–665. ( 10.1146/Annurev.Ento.37.1.637) [DOI] [PubMed] [Google Scholar]
- 10.Nijhout HF, Wheeler DE. 1982. Juvenile hormone and the physiological basis of insect polymorphisms. Q. Rev. Biol. 57, 109–133. ( 10.1086/412671) [DOI] [Google Scholar]
- 11.Robinson GE, Strambi C, Strambi A, Huang ZY. 1992. Reproduction in worker honey bees is associated with low juvenile hormone titers and rates of biosynthesis. Gen. Comp. Endocrinol. 87, 471–480. ( 10.1016/0016-6480(92)90055-O) [DOI] [PubMed] [Google Scholar]
- 12.Hughes WOH, Sumner S, Van Borm S, Boomsma JJ. 2003. Worker caste polymorphism has a genetic basis in Acromyrmex leaf-cutting ants. Proc. Natl Acad. Sci. USA 100, 9394–9397. ( 10.1073/pnas.1633701100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schluns EA, Wegener BJ, Robson SKA. 2011. Genetic polyethism and nest building in the weaver ant Oecophylla smaragdina (FABRICIUS, 1775) (Hymenoptera: Formicidae). Myrm. News 15, 7–11. [Google Scholar]
- 14.Muscedere ML, Traniello JFA. 2012. Division of labor in the hyperdiverse ant genus Pheidole is associated with distinct subcaste- and age-related patterns of worker brain organization. PLoS ONE 7, e31618 ( 10.1371/journal.pone.0031618) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gronenberg W, Heeren S, Hölldobler B. 1996. Age-dependent and task-related morphological changes in the brain and the mushroom bodies of the ant Camponotus floridanus. J. Exp. Biol. 199, 2011–2019. ( 10.1007/BF00225821) [DOI] [PubMed] [Google Scholar]
- 16.Castillo P, Pietrantonio PV. 2013. Differences in sNPF receptor-expressing neurons in brains of fire ant (Solenopsis invicta Buren) worker subcastes: indicators for division of labor and nutritional status? PLoS ONE 8, e83966 ( 10.1371/journal.pone.0083966) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Libersat F, Pflueger HJ. 2004. Monoamines and the orchestration of behavior. Bioscience 54, 17–25. ( 10.1641/0006-3568(2004)054[0017:MATOOB]2.0.CO;2) [DOI] [Google Scholar]
- 18.Barron AB, Robinson GE. 2008. The utility of behavioral models and modules in molecular analyses of social behavior. Genes Brain Behav. 7, 257–265. ( 10.1111/j.1601-183X.2007.00344.x) [DOI] [PubMed] [Google Scholar]
- 19.Kamhi JF, Traniello JFA. 2013. Biogenic amines and collective organization in a superorganism: neuromodulation of social behavior in ants. Brain Behav. Evol. 82, 220–236. ( 10.1159/000356091) [DOI] [PubMed] [Google Scholar]
- 20.Anstey ML, Rogers SM, Ott SR, Burrows M, Simpson SJ. 2009. Serotonin mediates behavioral gregarization underlying swarm formation in desert locusts. Science 323, 627–630. ( 10.1126/Science.1165939) [DOI] [PubMed] [Google Scholar]
- 21.Schulz DJ, Barron AB, Robinson GE. 2002. A role for octopamine in honey bee division of labor. Brain Behav. Evol. 60, 350–359. ( 10.1159/000067788) [DOI] [PubMed] [Google Scholar]
- 22.Smith AR, Muscedere ML, Seid MA, Traniello JF, Hughes WO. 2013. Biogenic amines are associated with worker task but not patriline in the leaf-cutting ant Acromyrmex echinatior. J. Comp. Physiol. A 199, 1117–1127. ( 10.1007/s00359-013-0854-2) [DOI] [PubMed] [Google Scholar]
- 23.Cuvillier-Hot V, Lenoir A. 2006. Biogenic amine levels, reproduction and social dominance in the queenless ant Streblognathus peetersi. Naturwissenschaften 93, 149–153. ( 10.1007/S00114-006-0086-1) [DOI] [PubMed] [Google Scholar]
- 24.Muscedere ML, Johnson N, Gillis BC, Kamhi JF, Traniello JFA. 2012. Serotonin modulates worker responsiveness to trail pheromone in the ant Pheidole dentata. J. Comp. Physiol. A 198, 219–227. ( 10.1007/S00359-011-0701-2) [DOI] [PubMed] [Google Scholar]
- 25.Falibene A, Rössler W, Josens R. 2012. Serotonin depresses feeding behaviour in ants. J. Insect Physiol. 58, 7–17. ( 10.1016/J.Jinsphys.2011.08.015) [DOI] [PubMed] [Google Scholar]
- 26.Seid MA, Traniello JFA. 2005. Age-related changes in biogenic amines in individual brains of the ant Pheidole dentata. Naturwissenschaften 92, 198–201. ( 10.1007/S00114-005-0610-8) [DOI] [PubMed] [Google Scholar]
- 27.Giraldo YM, Patel E, Gronenberg W, Traniello JFA. 2013. Division of labor and structural plasticity in an extrinsic serotonergic mushroom body neuron in the ant Pheidole dentata. Neurosci. Lett. 534, 107–111. ( 10.1016/j.neulet.2012.11.057) [DOI] [PubMed] [Google Scholar]
- 28.Powell S, Clark E. 2004. Combat between large derived societies: a subterranean army ant established as a predator of mature leaf-cutting ant colonies. Insectes Soc. 51, 342–351. ( 10.1007/S00040-004-0752-2) [DOI] [Google Scholar]
- 29.Mertl AL, Sorenson MD, Traniello JFA. 2010. Community-level interactions and functional ecology of major workers in the hyperdiverse ground-foraging Pheidole (Hymenoptera, Formicidae) of Amazonian Ecuador. Insectes Soc. 57, 441–452. ( 10.1007/S00040-010-0102-5) [DOI] [Google Scholar]
- 30.Crozier RH, Newey PS, Schluns EA, Robson SKA. 2010. A masterpiece of evolution—Oecophylla weaver ants (Hymenoptera: Formicidae). Myrm. News 13, 57–71. [Google Scholar]
- 31.Hölldobler B. 1983. Territorial behavior in the green tree ant (Oecophylla smaragdina). Biotropica 15, 241–250. ( 10.2307/2387648) [DOI] [Google Scholar]
- 32.Newey PS, Robson SKA, Crozier RH. 2010. Weaver ants Oecophylla smaragdina encounter nasty neighbors rather than dear enemies. Ecology 91, 2366–2372. ( 10.1890/09-0561.1) [DOI] [PubMed] [Google Scholar]
- 33.Newey PS, Robson SKA, Crozier RH. 2010. Know thine enemy: why some weaver ants do but others do not. Behav. Ecol. 21, 381–386. ( 10.1093/Beheco/Arp201) [DOI] [Google Scholar]
- 34.Majer JD. 1976. Maintenance of ant mosaic in Ghana cocoa farms. J. Appl. Ecol. 13, 123–144. ( 10.2307/2401933) [DOI] [Google Scholar]
- 35.Carlin NF, Hölldobler B. 1986. The kin recognition system of carpenter ants (Camponotus spp.). Behav. Ecol. Sociobiol. 19, 123–134. ( 10.1007/BF00299947) [DOI] [Google Scholar]
- 36.van der Burg NMD, Lavidis N, Claudianos C, Reinhard J. 2014. A novel assay to evaluate olfactory modulation of honeybee aggression. Apidologie 45, 478–490. ( 10.1007/s13592-013-0263-0) [DOI] [Google Scholar]
- 37.Roeder T, Degen J, Gewecke M. 1998. Epinastine, a highly specific antagonist of insect neuronal octopamine receptors. Eur. J. Pharmacol. 349, 171–177. ( 10.1016/S0014-2999(98)00192-7) [DOI] [PubMed] [Google Scholar]
- 38.Rillich J, Stevenson PA. 2011. Winning fights induces hyperaggression via the action of the biogenic amine octopamine in crickets. PLoS ONE 6, e28891 ( 10.1371/journal.pone.0028891) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barron AB, Maleszka J, Vander Meer RK, Robinson GE, Maleszka R. 2007. Comparing injection, feeding and topical application methods for treatment of honeybees with octopamine. J. Insect Physiol. 53, 187–194. ( 10.1016/J.Jinsphys.2006.11.009) [DOI] [PubMed] [Google Scholar]
- 40.Box GEP, Cox DR. 1964. An analysis of transformations. J. R. Stat. Soc. B 26, 211–252. [Google Scholar]
- 41.Rice WR. 1989. Analyzing tables of statistical tests. Evolution 43, 223–225. ( 10.2307/2409177) [DOI] [PubMed] [Google Scholar]
- 42.Wilson EO. 1953. The origin and evolution of polymorphism in ants. Q. Rev. Biol. 28, 136–156. ( 10.1086/399512) [DOI] [PubMed] [Google Scholar]
- 43.Vander Meer RK, Morel L. 1998. Nestmate recognition in ants. In Pheromone communication in social insects (eds Vander Meer RK, Breed M, Winston M, Espelie KE.), pp. 79–103. Boulder, CO: Westview Press. [Google Scholar]
- 44.Adamo SA, Baker JL. 2011. Conserved features of chronic stress across phyla: the effects of long-term stress on behavior and the concentration of the neurohormone octopamine in the cricket, Gryllus texensis. Horm. Behav. 60, 478–483. ( 10.1016/J.Yhbeh.2011.07.015) [DOI] [PubMed] [Google Scholar]
- 45.Stevenson PA, Dyakonova V, Rillich J, Schildberger K. 2005. Octopamine and experience-dependent modulation of aggression in crickets. J. Neurosci. 25, 1431–1441. ( 10.1523/Jneurosci.4258-04.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fussnecker BL, Smith BH, Mustard JA. 2006. Octopamine and tyramine influence the behavioral profile of locomotor activity in the honey bee (Apis mellifera). J. Insect Physiol. 52, 1083–1092. ( 10.1016/j.jinsphys.2006.07.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simpson SJ, Stevenson PA. 2015. Neuromodulation of social behavior. In The Oxford handbook of molecular psychology (ed. Canli T.), pp. 27–51. New York, NY: Oxford University Press. [Google Scholar]
- 48.Vander Meer RK, Preston CA, Hefetz A. 2008. Queen regulates biogenic amine level and nestmate recognition in workers of the fire ant, Solenopsis invicta. Naturwissenschaften 95, 1155–1158. ( 10.1007/s00114-008-0432-6) [DOI] [PubMed] [Google Scholar]
- 49.Zhou C, Rao Y, Rao Y. 2008. A subset of octopaminergic neurons are important for Drosophila aggression. Nat. Neurosci. 11, 1059–1067. ( 10.1038/Nn.2164) [DOI] [PubMed] [Google Scholar]
- 50.Hoyer SC, Eckart A, Herrel A, Zars T, Fischer SA, Hardie SL, Heisenberg M. 2008. Octopamine in male aggression of Drosophila. Curr. Biol. 18, 159–167. ( 10.1016/J.Cub.2007.12.052) [DOI] [PubMed] [Google Scholar]
- 51.Jones TC, Akoury TS, Hauser CK, Neblett MF, Linville BJ, Edge AA, Weber NO. 2011. Octopamine and serotonin have opposite effects on antipredator behavior in the orb-weaving spider, Larinioides cornutus. J. Comp. Physiol. A 197, 819–825. ( 10.1007/S00359-011-0644-7) [DOI] [PubMed] [Google Scholar]
- 52.Okada Y, Sasaki K, Miyazaki S, Shimoji H, Tsuji K, Miura T. 2015. Social dominance and reproductive differentiation mediated by dopaminergic signaling in a queenless ant. J. Exp. Biol. 218, 1091–1098. ( 10.1242/jeb.118414) [DOI] [PubMed] [Google Scholar]
- 53.Kravitz EA. 2000. Serotonin and aggression: insights gained from a lobster model system and speculations on the role of amine neurons in a complex behavior. J. Comp. Physiol. A 186, 221–238. ( 10.1007/s003590050423) [DOI] [PubMed] [Google Scholar]
- 54.Alekseyenko OV, Lee C, Kravitz EA. 2010. Targeted manipulation of serotonergic neurotransmission affects the escalation of aggression in adult male Drosophila melanogaster. PLoS ONE 5, e10806 ( 10.1371/journal.pone.0010806) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsalik EL, Niacaris T, Wenick AS, Pau K, Avery L, Hobert O. 2003. LIM homeobox gene-dependent expression of biogenic amine receptors in restricted regions of the C. elegans nervous system. Dev. Biol. 263, 81–102. ( 10.1016/S0012-1606(03)00447-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Young LJ, Nilsen R, Waymire KG, MacGregor GR, Insel TR. 1999. Increased affiliative response to vasopressin in mice expressing the V-1a receptor from a monogamous vole. Nature 400, 766–768. ( 10.1038/23475) [DOI] [PubMed] [Google Scholar]
- 57.Andrews JC, Fernández MP, Yu Q, Leary GP, Leung AKW, Kavanaugh MP, Kravitz EA, Certel SJ. 2014. Octopamine neuromodulation regulates Gr32a-linked aggression and courtship pathways in Drosophila males. PloS Genet. 10, e1004356 ( 10.1371/journal.pgen.1004356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Libbrecht R, Oxley PR, Kronauer DJC, Keller L. 2013. Ant genomics sheds light on the molecular regulation of social organization. Genome Biol. 14, 212 ( 10.1186/gb-2013-14-7-212) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Corona M, Libbrecht R, Wurm Y, Riba-Grognuz O, Studer RA, Keller L. 2013. Vitellogenin underwent subfunctionalization to acquire caste and behavioral specific expression in the harvester ant Pogonomyrmex barbatus. PLoS Genet. 9, e1003730 ( 10.1371/journal.pgen.1003730) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lucas C, Sokolowski MB. 2009. Molecular basis for changes in behavioral state in ant social behaviors. Proc. Natl Acad. Sci. USA 106, 6351–6356. ( 10.1073/Pnas.0809463106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alaux C, et al. 2009. Honey bee aggression supports a link between gene regulation and behavioral evolution. Proc. Natl Acad. Sci. USA 106, 15 400–15 405. ( 10.1073/pnas.0907043106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Farris SM, Roberts NS. 2005. Coevolution of generalist feeding ecologies and gyrencephalic mushroom bodies in insects. Proc. Natl Acad. Sci. USA 102, 17 394–17 399. ( 10.1073/pnas.0508430102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Striedter GF. 2005. Principles of brain evolution. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 64.Ilies I, Muscedere ML, Traniello JF. 2015. Neuroanatomical and morphological trait clusters in the ant genus Pheidole: evidence for modularity and integration in brain structure. Brain Behav. Evol. 85, 63–76. ( 10.1159/000370100) [DOI] [PubMed] [Google Scholar]
- 65.Sinakevitch I, Mustard JA, Smith BH. 2011. Distribution of the octopamine receptor AmOA1 in the honey bee brain. PLoS ONE 6, e14536 ( 10.1371/journal.pone.0014536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reim T, Scheiner R. 2014. Division of labour in honey bees: age- and task-related changes in the expression of octopamine receptor genes. Insect Mol. Biol. 23, 833–841. ( 10.1111/Imb.12130) [DOI] [PubMed] [Google Scholar]
- 67.Seid MA, Goode K, Li C, Traniello JFA. 2008. Age- and subcaste-related patterns of serotonergic immunoreactivity in the optic lobes of the ant Pheidole dentata. Dev. Neurobiol. 68, 1325–1333. ( 10.1002/Dneu.20663) [DOI] [PubMed] [Google Scholar]
- 68.Shakiryanova D, Zettel GM, Gu T, Hewes RS, Levitan ES. 2011. Synaptic neuropeptide release induced by octopamine without Ca2+ entry into the nerve terminal. Proc. Natl Acad. Sci. USA 108, 4477–4481. ( 10.1073/pnas.1017837108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Neckameyer WS, Bhatt P. 2012. Neurotrophic actions of dopamine on the development of a serotonergic feeding circuit in Drosophila melanogaster. BMC Neurosci. 13, 26 ( 10.1186/1471-2202-13-26) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded to Dryad (doi:10.5061/dryad.36s2m).