Abstract
Conservation biology is increasingly concerned with preserving interactions among species such as mutualisms in landscapes facing anthropogenic change. We investigated how one kind of mutualism, mixed-species bird flocks, influences the way in which birds respond to different habitat types of varying land-use intensity. We use data from a well-replicated, large-scale study in Sri Lanka and the Western Ghats of India, in which flocks were observed inside forest reserves, in ‘buffer zones' of degraded forest or timber plantations, and in areas of intensive agriculture. We find flocks affected the responses of birds in three ways: (i) species with high propensity to flock were more sensitive to land use; (ii) different flock types, dominated by different flock leaders, varied in their sensitivity to land use and because following species have distinct preferences for leaders, this can have a cascading effect on followers' habitat selection; and (iii) those forest-interior species that remain outside of forests were found more inside flocks than would be expected by chance, as they may use flocks more in suboptimal habitat. We conclude that designing policies to protect flocks and their leading species may be an effective way to conserve multiple bird species in mixed forest and agricultural landscapes.
Keywords: anthropogenic disturbance, biodiversity loss, human-modified ecosystems, mixed-species bird flocks, mutualisms, species interaction networks
1. Introduction
Conservation biologists are increasingly calling for strategies that preserve species interaction networks, such as mutualisms, as they may be even more sensitive to anthropogenic change than the species themselves [1–3]. Here, we explore the conservation implications of one such mutualism, mixed-species animal groups, by asking whether the dynamics of these groups influence how animals respond to human disturbance. Mixed groups are commonly found in fish [4] and mammals [5], although they have been most studied in birds, in which ‘mixed-species flocks' are a prominent form of social organization in temperate climates during the non-breeding season and year-round in the tropics [6]. Animals in mixed groups gain advantages in foraging and anti-predatory vigilance (reviewed in [7]), and this may translate into superior fitness [8]. Given that mixed-species groups may be an important part of anti-predatory and foraging behaviour, integrating an understanding of such groups into conservation plans could thus be an important topic in the emerging field of conservation behaviour [9].
An especially interesting question related to the conservation of species interaction networks is whether there are some species that are particularly important to such networks, which could be targeted in conservation plans in order to protect multiple species simultaneously [10–12]. A repeated observation in the mixed-species flock literature is that some ‘nuclear’ or leading species are especially important for the formation or maintenance of flocks ([13] and references therein). Observational and experimental evidence points to the presence of nuclear species being important to the fitness of following species [14,15]. Hence, there is a potential for a ‘non-trophic cascade’ where factors that influence the persistence of a nuclear species in disturbed areas have consequences for following ‘attendant’ species, analogous to a trophic cascade, in which changes in the abundances of species at one trophic level reverberate at lower trophic levels [16].
Early reports on mixed-species flocks suggested that flocking species were particularly vulnerable to anthropogenic disturbance [17,18], and a number of studies have now investigated how flocks respond to fragmentation [19–24] and to different land uses or successional stages [25–30], with the near-universal finding of fewer and smaller flocks in more disturbed conditions. But the larger question of how and why the flocking phenomenon might influence how birds respond to land-use intensity is still unclear. We see at least three ways in which flocking could aggravate or mitigate the effect of human disturbance on forest birds. First, a potentially negative, aggravating effect: mixed-species flock participants may have larger territories than solitary species, and hence may be more sensitive to habitat fragmentation because their space requirements are greater [21]. Indeed, mixed-species flocks do especially poorly in the smallest forest fragments [18,19,22,23]. Second, flocking species may be dependent on other species in flocks, especially on nuclear species [13]. If nuclear species respond poorly to disturbance, this could lead to attendant species also having low resilience [18,20,22,24,27]. However, if nuclear species do well in disturbed areas, attendants could have high resilience to disturbance; hence, this effect could either aggravate or mitigate the effect of land use, depending on the nuclear species involved. Third, a potentially positive, mitigating effect: forest-interior species might be more likely to travel into, and forage in, disturbed areas when they are in flocks. Dolby & Grubb [31] and Sieving et al. [32] have shown that flock attendant species may travel more into open areas when nuclear species of flocks were present. Tubelis et al. [29] and Péron & Crochet [26] found that some species characterized as forest-interior species also visited forest edge areas when in flocks. As a consequence, flocks outside of protected areas may include more forest-preferring species than the overall bird community does in these areas, and flocks could be targeted in conservation plans as an effective way of protecting multiple species at once in production landscapes.
Here, we examine how mixed-species flocking influences the response of birds to varying intensities of human land use. We use data from a large-scale study of flocks in Sri Lanka and the Western Ghats of India [28], a biodiversity hotspot in which flocks have been well studied [33], though previously mostly inside protected areas (but see [22]). We sampled flocks in three land-use types: inside forest reserves, in buffer areas containing degraded forest or tree plantations, and in agricultural areas. We investigated three hypotheses as to how flocking is important to understanding how birds respond to this land-use intensity gradient: (i) if flocking makes species more vulnerable to anthropogenic disturbance, then we should find that species' propensity to flock in forests (that is, the percentage of individuals in forest that are in flocks) predicts their response to non-forest land-use types; (ii) if leading species' habitat preferences influence the habitat selection of other species, then we should find that different leading species vary in their sensitivity to land use, and also show that attendant species' flocking behaviour depends on what leading species are present; (iii) if flocking allows forest-interior species to enter non-forest areas, then we should find that observed flocks have more individuals of forest-interior species, and fewer open-landscape species, than simulated null flocks, whose composition is based purely on species' abundances.
2. Material and methods
(a). Data collection
We worked in areas of moist evergreen forest in Sri Lanka and southern India. From November 2006 to December 2007, we sampled three sites of varying elevation in Sri Lanka: Sinharaja World Heritage Reserve (SWHR), western sector, 300–500 m.a.s.l.; SWHR, eastern sector, 900–1100 m; and Nuwara Eliya region, 1800–2000 m. At each site, we laid down eight 2 km transects: three transects were placed in relatively undisturbed forest inside protected reserves, three transects in ‘buffer zones' of degraded forest and non-native timber plantations near the borders of protected reserves, and two transects in areas of intensive agriculture. In April 2007, and then from January 2008 to May 2008, we expanded the same sampling design to two Indian sites: Thattekad reserve in Kerala, 40–80 m; and Anamalai Hills in Tamil Nadu, 850–1000 m. From January 2008 to January 2009, we increased the sampling in Sri Lanka, adding 17 more 1 km transects (six forest, seven buffer and four agriculture) over the altitudinal gradient from the Gillimalle Forest Reserve (90 m) to the Horton Plains Reserve (2180 m). For a map and more information, see Goodale et al. [28].
When walking a transect, we noted all birds seen or heard, their group size (number of individuals within 10 m distance of each other), their distance from the transect, as estimated visually, the specific land use of the habitat they were seen in, and whether they were in a mixed-species flock, defined as two or more species definitively moving in the same direction [33]. When a flock was encountered, we noted the distance from the transect to the middle of the flock, and observed it for a minimum of 5 min to a maximum of 15 min, before continuing the transect. Most flocks were considered complete, in that we believed 80–100% of the individuals were identified. We also noted incomplete flocks and flocks encountered while returning after finishing a transect, provided that a flock had not been seen previously that day in that 500 m section of the transect. Observers, who all had experience with bird watching before the project and were further trained for at least one month, usually worked in a group of two; in total, eight observers collected data for the project. Transects were generally walked in the morning (8.00–10.00) and the afternoon (15.00–17.00).
The average transect was walked 7.2 times over a year (s.d. = 4.0), with visits staggered over the full annual cycle [28]. Over the 2 years and 2 months of sampling, we collected 34 867 observations of birds inside and outside of flocks, including 204 species, following the taxonomy of Gill & Donsker [34]. The flock data included 329 complete and 159 incomplete observations while walking transects, and 69 additional complete observations that were made on transects, but not while walking a transect. The majority of the data were from Sri Lanka (27 234 observations, 327 complete flocks).
(b). Density estimation
As our statistical analyses (in particular the simulations) required species abundance data, we estimated species' densities, taking into account differences in how easily detected they are. For any one species, we first needed to estimate the number of individuals outside of flocks, which have a species-specific detectability, and add them to the number of individuals inside of flocks, assuming that all the individuals of all species in flocks were detected together as a flock. To make these estimations, we used the software DISTANCE [35]; for more specifics on this analysis, see electronic supplementary material, methods I.
(c). Species classification
As most species that participate in mixed-species flocks in this region are insectivores [36], for some analyses we used only insectivorous species. Diets were classified according to Rasmussen & Anderton [37] and BirdForum (http://www.birdforum.net), into five diet classes: insectivores, frugivores, carnivores, nectarivores and granivores (eating a majority of arthropods, fruit, vertebrates, nectar and seeds, respectively; electronic supplementary material, appendix S1).
We classified ‘forest-interior’ and ‘open-landscape’ species independently from the literature, using Ali & Ripley [38] and Grimmett et al. [39] to make these classifications. Using this method, we classified 57 species as forest-interior species, and 52 species as open-landscape species (electronic supplementary material, appendix S1); the rest were classified as ‘unclear’.
For analyses about leading species, we only used data from Sri Lankan transects, because there is already published quantitative (front-to-back organizational) data identifying the leading species: the orange-billed babbler leads lowland flocks [36], and the Sri Lanka white-eye leads montane flocks [40] (see the electronic supplementary material, methods II for further discussion of these species’ leading roles in flocks).
(d). Analysis
(i) To determine whether flocking propensity predicts species' response to land use, we measured species' response to land-use by calculating the proportion of the total density of a species that was observed on forest transects. A species's propensity to flock was defined as the proportion of the total density of a species that was observed in flocks, following Thiollay [41], and was calculated only for forest transects (as flocks were less frequent outside of forests). Both proportional variables were arcsine transformed to minimize departures from normality and homoschedasticity. We tested whether propensity to flock in forests predicted response to land use, using a general linear model (generalized linear models with binomial distributions were not applicable to the data because the densities were not integers). To make sure this result was not simply due to flocking species being insectivores, and insectivores being more sensitive to land-use, we repeated the analysis using insectivores only. All statistical analyses were performed using R software (R Development Core Team, 2014).
(ii) To determine whether the habitat preferences of nuclear species have cascading effects on the habitat selection of other flocking species, we first investigated whether the two leading species in Sri Lanka differed in their habitat sensitivities at the middle elevations, where they both were relatively common. We compared the presence or absence of these species in the 11 transects outside of forests at this elevation with a Fisher's exact test. In these 11 transects, we also compared the presence or absence of ‘babbler-dominated’ flocks (flocks in which babblers were the most gregarious species and white-eyes were absent) with ‘white-eye-dominated’ flocks (flocks in which white-eyes were the most gregarious species and babblers were absent). Statistical analyses of how the densities of these species, or these flock types, responded to land use were qualitatively similar to the results of the Fisher's exact tests, but had low power because of the small number of forest transects at this elevation (4).
These two leading species are of different sizes, with the babbler being relatively large (66 g) and the white-eye being quite small (13 g) [42] (E.G. 2002–2003, unpublished data). Recent work suggests that birds will associate in flocks with other species of similar body sizes [43]; therefore, we hypothesized that larger birds would prefer joining babbler flocks. To test this hypothesis, we used the phi coefficient as an association measure, calculated by modifying the sp.pair function in the library spaa in R. Other association measures, including the path length (geodesic distance) between nodes in network analyses [44] and simulations of null flocks [45], gave qualitatively similar results (see the electronic supplementary material, methods III). To avoid associations that are based on similar habitat preferences rather than leader preference [46], we only used flock observations that were made in the same specific habitat type of the same transect. To avoid problems with small sample sizes, we only used a transect/habitat combination if more than four flocks were observed there (n = 30), only analysed attendant species that were found in at least five flocks in the whole study (n = 40 such species) and only used a species at a transect if it was found at a density more than 0.1 bird per hectare. We then calculated the phi coefficient for each attendant species's relationship with orange-billed babbler and with Sri Lanka white-eye, for each transect/habitat combination, and averaged these measures for all the Sri Lankan transects.
The relationship between association strength with the leading species and the body weight of the attendant species was determined for the two leading species separately by simple linear regression. For body mass, we used the text of Dunning [42]; for species without data, we either used some of our own unpublished data (8 species) or used the weight of a similar-sized congeneric (17 species; electronic supplementary material, appendix S1). Assumptions of regression were met once body mass was log transformed. To see whether species with extreme body sizes differed in their preferences for flock leaders, we divided the attendant species into three size classes with equal sample sizes (small, 6–15 g; medium, 16–43 g; large, 56–196 g), and for each species we calculated a measure of its preference for orange-billed babbler over Sri Lanka white-eye as the difference between its association values with each species. We then performed a Welch's t-test to investigate whether these preferences differed between species of small and large body masses. The size of the effect was measured using the Cohen's d index [47].
(iii) As background information to understanding how forest-interior species use flocks outside of forests, we first investigated how propensity to flock changed inside and outside of forests for these and other kinds of species (see the electronic supplementary material, methods IV).
We then simulated ‘null’ flocks and compared them with observed flocks, following the general approach of Srinivasan et al. [45], and analysed only insectivorous species because they make up the great majority of flock participants. The pool of birds at a transect was first estimated by summing together the estimates of bird density per hectare inside and outside of flocks, and then calculating the number of birds in a 10 ha area (as shown by Mokross et al. [23], areas smaller than this size do not retain flocks well in the Neotropics, although comparable data are not available for Asia). We conducted 500 simulations of the same number of flocks as observed (n = 394 flocks in which there were insectivores), based purely on the abundances of the different species at each transect, to produce an array of null flocks. When a species was selected for inclusion in the flock, we added the mean group size of that species found outside of flocks, subsequently subtracting those individuals from the pool of birds at the site. Flocks were filled up to the number of individuals in the actual observations (for the last species added, the group size was truncated to fit the total number of individuals in a flock).
The total number of forest-interior individuals or open-landscape individuals in the simulated null flocks was then compared with the number of such individuals in the observed flocks, using a paired Student's t-test (n = 52 transects with complete flocks). We further determined whether the factors of land-use type, country and elevation (low, 0–500 m; medium, 800–1300 m; high, 1500–2180 m) affected the difference between observed and simulated flocks, using multi-factor ANOVA. All models were progressively simplified, dropping interaction terms and factors that did not significantly (α > 0.05) explain the variance. We report means and standard deviations of the results of the 500 simulations compared with the observed data.
In order to see how stable the analyses (i)–(iii) were, when replicated in different areas, we also conducted mixed-effects models in which each transect was assigned to one of five main regions (Sri Lanka low, middle and high elevation, India low and middle). In these models, variables were measured for each region separately and region was considered a random factor (see the electronic supplementary material, methods V). These models were in general confirmatory of simpler models, but because such analyses break the data into smaller pieces, and because proportional data in particular become more variable as sample size decreases, they had less power, and we include them only in the electronic supplementary material.
3. Results
(a). Did flocking propensity predict species' response to the gradient of land-use intensity?
Species that had high propensities to flock in forest were more likely to be confined to forest (LM: F1,164 = 18.53, p < 0.0001), although the effect size was relatively small (R2 = 0.10; electronic supplementary material figure S1). This result was also found for insectivores only (LM: F1,92 = 5.17, p = 0.025, R2 = 0.05).
(b). Can habitat preferences of nuclear species have cascading effects on the habitat selection of other flocking species?
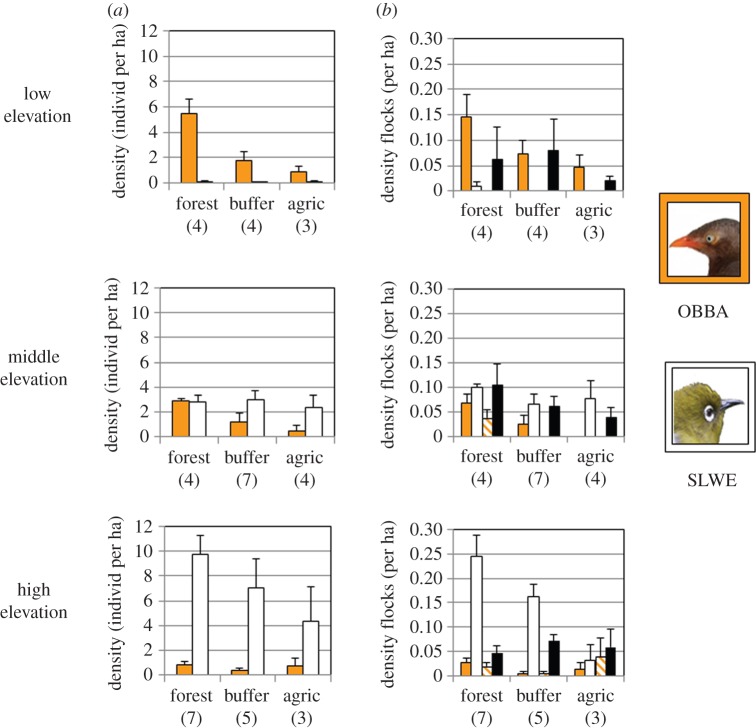
We found the nuclear species to be as expected in Sri Lanka, with the orange-billed babbler and the Sri Lanka white-eye being the most gregarious species in 76% (250 of 326) of Sri Lankan flocks; no other species was observed to be the most gregarious species in more than 10 flocks. Orange-billed babblers were most common in the lowlands (0 to 500 m), while Sri Lanka white-eyes were most common in the montane regions (more than 1500 m), and they each dominated flocks where they were most common (figure 1). Both species led flocks at intermediate elevations. There was some indication that these two species avoided each other in flocks, as they had a strongly negative phi coefficient (mean: −0.46; negative in 8 of 10 transects where it could be tested, sign test: p = 0.11).
Figure 1.
Two nuclear species in Sri Lanka, the orange-billed babbler (OBBA, Turdoides rufescens; orange bars) and the Sri Lanka white-eye (SLWE, Zosterops ceylonensis; white bars), are most common in the lowlands and montane regions, respectively (as shown in (a)), and lead the majority of flocks where they are most common (b). At middle elevations, OBBA are more confined to forest than SLWE, and this leads to babbler-dominated flocks being scarce outside of forests, relative to white-eye-dominated flocks. Cross-hatched bars represent flocks in which both species were present, and black bars represent flocks in which neither was found. Numbers in parentheses represent the numbers of transects in each elevation/land use combination. Error bars are standard errors. Photograph of OBBA copyright Eben Goodale; photograph of SLWE modified from freely accessible image at Wikimedia Commons (https://commons.wikimedia.org), originally created by Lip KeeYap. (Online version in colour.)
Although both these nuclear species had highest densities in forests overall, at middle elevations, where they were both relatively common, they showed different sensitivities to land-use intensity (figure 1). Sri Lankan white-eyes were present on all 11 transects outside of forests there, whereas orange-billed babblers were absent on six of these 11 transects (Fisher's two-tailed exact test for comparison between species' presence/absence: p = 0.013). These differences translated into differences in the distribution of flocks. Babbler-dominated flocks were found only on 2 of 11 transects outside of forests, whereas white-eye-dominated flocks were found on eight such transects (Fisher's two-tailed exact test: p = 0.030).
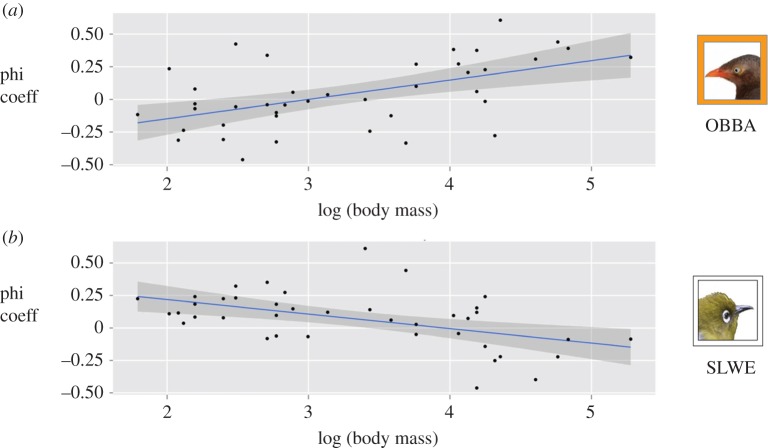
Could differences in the leading species' habitat preferences affect how attendant species select habitat? Such an effect could only occur if species show different flock-joining tendencies based on which species is the leader. We found that attendant species' association strengths with the leader species are significantly influenced by body mass. The larger the species, the more positive were the associations with orange-billed babbler (figure 2a; LM: p = 0.0004, R2 = 0.26), and the more negative were the associations with Sri Lanka white-eye (figure 2b; LM: p = 0.001, R2 = 0.22). Dividing the associate species into three size classes, the extreme classes (big versus small) differed substantially in their preference for leading species (Welch's T-test: t20.83 = 4.70, p < 0.0001, Cohen's d index of effect size = 1.84).
Figure 2.
The association strength between leading species in Sri Lanka and attendant species was influenced by the attendant species' body size. (a) Species with larger body mass associated more strongly with the orange-billed babbler (66 g), while (b) species with smaller body mass associated more strongly with the Sri Lanka white-eye (13 g). Each point is the association strength, measured by the phi coefficient, between an attendant species and the particular leading species. Only attendant species in more than five flocks in Sri Lanka are shown. Deeply shaded areas represent 95% CIs for the regression line. (Online version in colour.)
Attendant species that prefer following a nuclear species that is sensitive to land-use changes (like the orange-billed babbler) may be quite confined in their own habitat selection. For example, take the red-faced malkoha Phaenicophaeus pyrrhocephalus, a species listed as vulnerable in the IUCN Red List [48]. Malkohas are almost always seen inside babbler-dominated flocks (table 1; the 14% of flocks with malkohas that were not babbler-dominated had white-eyes present, but babblers were still numerically the most gregarious species), and this may be one factor underlying why the species is almost always found inside forest, as the only malkohas seen outside forest were in babbler-dominated flocks in the buffer transects. A similar, but less extreme trend of being in a high percentage of flocks and preferring babbler-dominated ones was also seen for two other relatively large, endemic and threatened species (table 1).
Table 1.
Relatively large-sized and threatened species that are often observed in flocks and prefer to follow flocks dominated by orange-billed babblers, as opposed to those dominated by Sri Lanka white-eyes. For definitions of flock types, see text; see the electronic supplementary material, appendix S1 for bird weights and their sources. All three species are endemic to Sri Lanka and listed as vulnerable by the IUCN [48].
| percentage of individuals |
percentage of flocks |
||||||
|---|---|---|---|---|---|---|---|
| species | weight (g) | in forest | in flocks | n | OBBA-dominated | SLWE- dominated | n |
| red-faced malkoha Phaenicophaeus pyrrhocephalus | 119 | 85 | 88 | 26 | 86 | 0 | 21 |
| ashy-headed laughingthrush Garrulax cinereifrons | 70 | 83 | 85 | 497 | 64 | 4 | 45 |
| white-faced starling Sturnus albofrontatus | 56 | 81 | 63 | 32 | 61 | 0 | 18 |
(c). Were forest-interior species observed more often in flocks when outside of forests?
The flocking propensity of forest-interior species declined only slightly outside of forests, but other types of species showed a more precipitous drop, as flocks themselves were substantially less frequent outside of forests (electronic supplementary material, figure S2).
Forest-interior species were more frequent in actual flocks that expected by the null simulations. On average, 14.87 forest-interior species (s.d. = 1.48) had more individuals than expected in observed flocks, whereas 10.97 (s.d. = 0.86) such species had fewer individuals than expected (Student's t-test: average t51 = 7.33, average p < 0.0001). The difference calculated for each transect between the number of species with more individuals in actual flocks than expected from the null models and those with fewer individuals than expected (hereafter referred to as the ‘plus–minus' difference) was higher for Sri Lanka than India (LM: average F1,50 = 9.36, average p = 0.006) but was not influenced by elevation or land use.
By contrast, open-landscape species were less frequent in flocks than expected by the null simulations. On average, 4.82 open-landscape species (s.d. = 0.98) had more individuals than expected in observed flocks, whereas almost twice as many species, 9.53 (s.d. = 1.48), had fewer individuals than expected (Student's t-test: average t51 = 2.75, average p = 0.014). The plus–minus difference was not influenced by country or elevation, but varied by land use (LM: average F2,49 = 4.55, average p = 0.043), being especially prominent in agricultural lands compared to forests (Tukey HSD multiple comparisons: average p = 0.036).
4. Discussion
We conclude that mixed-species flocks can serve as targets of conservation policies in tropical countryside landscapes, perhaps not surprisingly given the dominance of flocking as a form of bird behaviour in the tropics. In most tropical forests, a significant percentage of the birds are found participating in flocks at any one time [41], with an average of 48% of individuals found in flocks in our region [33]. Mixed-species flocking can increase foraging efficiency (reviewed by Sridhar et al. [6]), through social copying [49,50] and the use of other species as beaters by some fly-catching species [51]. Such flocking can also decrease predation risk through a variety of mechanisms, including risk dilution and alarm calling [7,41,52]. Such factors have been shown to increase bird species fitness in the only study to investigate this important subject [8]. Hence, it is essential to understand the implications of such a mutualistic social system on how birds respond to the continuing threat of land-use intensification.
We conducted three separate tests relating flocking to birds' response to land-use intensity in the Sri Lankan and Western Ghats biological diversity hotspot. We found that the flock system may influence species' sensitivities to land use in one of three ways. First, flocking species were more sensitive to human disturbance. Second, attendant species in flocks may prefer associating with particular nuclear species, and nuclear species may themselves vary in their habitat preferences, potentially leading to cascading effects on the attendant species' habitat selection. Third, forest-interior species, when found outside of forests, had higher propensities to flock relative to other species than when inside forests. Observed flocks had more individuals of forest-interior species, and fewer open-landscape species, than did simulated null flocks.
Before continuing, we want to highlight some features of our dataset that make it particularly powerful to answer questions about the importance of flocking. First, the unit of replication is the transect, and all values (e.g. flock density, percentage of flocks in which a species was present) were calculated at the transect level. Multiple site studies are historically rare in studies of mixed-species flocks, especially in South Asia [33], but are preferable because flocks seen at one site are not necessarily independent of each other. Second, for our association calculations, we only use sections of homogeneous habitat on a transect, and thus avoid having similarities in species' habitat preferences drive their associations [46]. Third, we have data from both inside flocks and outside them, allowing us to compare flocks to the general pool of birds at any location. Fourth, while some of our analyses centred on particular parts of the dataset, most analyses used all the transects, which were placed at a wide range of elevations in the two countries and hence give more generality to the results.
In the first analysis, we found that species' flocking propensity influences their exclusivity to forests, with higher-propensity species being more confined to forests. The idea that mixed-species flocking species may be especially vulnerable was first raised by two classic studies of birds' responses to human disturbance [17,18] and was confirmed by Van Houtan et al. [21]. All three of these studies were in Amazonia, with a unique system in which flocks defend interspecific territories [53]. Since such interspecific territories tend to be large, Van Houtan et al. made the plausible argument that such flocks would not be able to live in small fragments, which makes all participating species susceptible to fragmentation (and hence land-use changes, which correlate with fragmentation). But such an explanation would not seem to be applicable to flocks in South and Southeast Asia, because flocks there do not appear to hold such interspecific territories but rather appear to be similar to ‘waves' [54] that birds join when the flock moves through their species-specific territory, and then leave when the flock moves on. How exactly mixed-species flocking makes species more sensitive to land-use change—acknowledging that the effect size is low (R2 = 0.10)—in our region is thus unclear. One possibility has been mentioned by Sridhar & Shankar [22], who actually worked at our Indian middle elevation site: mixed-species flocks might be especially susceptible to human disturbance because attendant species might be adversely affected by the disappearance of a nuclear species, a hypothesis similar to the one we tested in our second analysis.
The second analysis is the most complex, because to demonstrate that flock leadership could be important in influencing how attending species respond to land-use intensity, we need to demonstrate two results: first, that leaders themselves vary in their response to different land-use types; and second, that attendant species prefer some leading species to others. We found evidence for leading species having different sensitivities to land uses in middle-elevation transects in Sri Lanka, where both the lowland flock leader (orange-billed babbler) and montane flock leader (Sri Lanka white-eye) were relatively common. At this elevation, white-eyes and white-eye-dominated flocks persisted outside of forests more than babblers and babbler-dominated flocks.
Can these differences in habitat preferences affect how other species use habitat? Our association analysis, conducted across Sri Lanka, indicates that bird species tend to associate in flocks based on the leader's body size, a result that has also been suggested by a global meta-analysis [43], as well as some site-specific studies [55]. While here we have concentrated on the phi association index, we found the same results with other techniques, including network approaches (which have only recently been applied to flock studies [23,50]) and flock simulations [45] (which use data from both inside and outside of flocks; see the electronic supplementary material, results). While it is possible that species of the same size may associate together because of similar habitat or resource requirements, adherent species in this system have a great diversity of foraging techniques and heights [36], so we believe it is leadership, not similar habitat requirements, that drives this result. Bird species of small or large sizes were very selective about which flock system they associate with, and this effect may contribute to the habitat specificity of some endangered species. Our study demonstrates that such potential costs of mixed-species flocking may be specific to certain sites (in this case, middle elevations in Sri Lanka) and certain species (in this case, large species that prefer to follow babbler-dominated flocks).
Several other studies have suggested that dependencies on nuclear species in flocks may lead to flock breakdown in areas if nuclear species are absent [20,22,24,27], but this is the first study to statistically compare species' associations with different leaders and demonstrate that those leaders prefer different land uses. Our study measures associations observed in nature, and we do not know to what extent such associations represent dependencies. In future work, it would be important to measure the fitness of attendant species in areas with and without specific leaders; because of the difficulties in controlling other factors, such work may best be done experimentally, by removing leading species from isolated patches of forest, as has been done in the USA by Dolby and Grubb, in a study that showed that attendants had poorer body condition when leaders were absent [15].
The data from the third analysis support the idea that flocks can make habitats outside of forests more accessible to forest-preferring species. This hypothesis was supported by the observational work of Tubelis et al. [29] and Péron & Crochet [26], as well as a few experimental studies [31,32]. We found that forest-interior species' propensity to flock is higher relative to other species when they are found outside of forests and that such species are found more inside flocks than would be predicted based on their abundances alone. Flocks are not welcoming to open-landscape species, with almost twice as many species having fewer individuals in flocks than expected from their abundances, and this effect is particularly acute in agricultural areas. In such highly modified areas, the birds in flocks may not be classified as forest-interior species, but they prefer forest more than the average birds do of those areas, and hence can be targets of conservation in working forests and agricultural areas [56].
Our data suggest that conservation plans should target flock systems and their leading species. Note that the density of flocks is often much lower outside of forests than in them, so that in some cases conserving flocks is not possible: for example, flocks were completely absent from agricultural transects in India [28]. Nevertheless, flocks did persist in agricultural transects in Sri Lanka at a low level and were nearly as dense in buffer transects in both countries as they were in forests [28]. A clear priority for further research is to understand what vegetation structural and floristic characteristics are correlated with flock persistence [57] or with the presence of particularly important nuclear species in highly modified landscapes.
In summary, we are able to use a unique, large-scale multi-site study, with data on birds inside and outside of flocks, to ask novel questions about how the flock system influences the participating species' response to a gradient of land-use intensity, adding to a quickly accumulating literature on how flocks respond to anthropogenic disturbance [23,24,27,28,30]. Flocking species are particularly sensitive to disturbance, being more exclusive to forest, and flocks outside of forests are particularly important to the forest-preferring birds that remain in these areas. Further, we show that different attendant species prefer different leading species, and that the ways these leading species respond to land-use intensity themselves can vary; these effects of leadership could potentially aggravate how land-use intensity affects birds in some areas and for some species. This is another example of how ‘strong interactors' may be important to communities [11], and hence targets for community conservation [10].
Supplementary Material
Acknowledgements
We thank A. Jayarathna, R. M. Pathiraja, G. Ramachandran, W. Ranjith, M. V. I. Sanjeewanie and H. Sathischandra for collecting the Sri Lanka data, T. R. Shankar Raman for supervising the Indian data, and R. T. Corlett, R. D. Harrison, D. I. King, R. Sreekar, H. Sridhar and two anonymous reviewers for improving the manuscript.
Ethics
This observational study followed the laws of Sri Lanka and India. We thank the Sri Lanka Forest Department and Wildlife Conservation Department, the Tamil Nadu and Kerala Forest Departments and Tata Coffee Ltd., for their permission and assistance.
Data accessibility
Data are accessible at http://dx.doi.org/10.5061/dryad.vk070.
Authors' contributions
E.G. and S.W.K. found funding for the project. E.G., U.M.G., S.W.K. and S.S. planned and oversaw the field data collection. C.M. led the overall data analysis, in collaboration with E.G. All authors were involved in writing the manuscript.
Competing interests
The authors declare that they have no competing interests.
Funding
This study was supported by the Conservation, Food and Health Foundation and the American Institute for Indian Studies. E.G. is grateful to the National Science Foundation (International Research Fellowship Program grant no. 0601909), and the 1000 Plan Recruitment Program of Global Experts of the People's Republic of China. C.M. appreciates the support of a postdoctoral grant from the Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences.
References
- 1.Tylianakis JM, Laliberté E, Nielsen A, Bascompte J. 2009. Conservation of species interaction networks. Biol. Conserv. 143, 2270–2279. ( 10.1016/j.biocon.2009.12.004) [DOI] [Google Scholar]
- 2.Valiente-Banuet A, et al. 2015. Beyond species loss: the extinction of ecological interactions in a changing world. Funct. Ecol. 29, 299–307. ( 10.1111/1365-2435.12356) [DOI] [Google Scholar]
- 3.Zarnetske P, Skelly DK, Urban MC. 2012. Biotic multipliers of climate change. Science 336, 1516–1518. ( 10.1126/science.1222732) [DOI] [PubMed] [Google Scholar]
- 4.Lukoschek V, McCormick MI. 2000. A review of multi-species foraging associations in fishes and their ecological significance. In Proc. 9th International Coral Reef Symposium, Bali, Indonesia, 23–27 October 2000, pp. 467–474. Bali: Ministry of Environment, Indonesia; Indonesian Institute of Sciences; International Society for Reef Studies. [Google Scholar]
- 5.Stensland E, Angerbjörn A, Berggren P. 2003. Mixed species groups in mammals. Mamm. Rev. 33, 205–223. ( 10.1046/j.1365-2907.2003.00022.x) [DOI] [Google Scholar]
- 6.Sridhar H, Beauchamp G, Shanker K. 2009. Why do birds participate in mixed-species foraging flocks? A large-scale synthesis. Anim. Behav. 78, 337–347. ( 10.1016/j.anbehav.2009.05.008) [DOI] [Google Scholar]
- 7.Greenberg R. 2000. Birds of many feathers: the formation and structure of mixed-species flocks of forest birds. In On the move: how and why animals travel in groups (eds Boinski S, Garber PA.), pp. 521–558. Chicago, IL: University of Chicago Press. [Google Scholar]
- 8.Jullien M, Clobert J. 2000. The survival value of flocking in neotropical birds: reality or fiction? Ecology 81, 3416–3430. ( 10.1890/0012-9658(2000)081[3416:TSVOFI]2.0.CO;2) [DOI] [Google Scholar]
- 9.Blumstein DT, Fernández-Juricic E. 2010. A primer of conservation behavior. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 10.Simberloff D. 1998. Flagships, umbrella, and keystones: is single-species management passé in the landscape era? Biol. Conserv. 83, 247–257. ( 10.1016/S0006-3207(97)00081-5) [DOI] [Google Scholar]
- 11.Soulé ME, Estes JA, Berger J, Del Rio CM. 2003. Ecological effectiveness: conservation goals for interactive species. Conserv. Biol. 17, 1238–1250. ( 10.1046/j.1523-1739.2003.01599.x) [DOI] [Google Scholar]
- 12.Caro TM, Girling S. 2010. Conservation by proxy: indicator, umbrella, keystone, flagship, and other surrogate species. Washington, DC: Island Press. [Google Scholar]
- 13.Goodale E, Beauchamp G. 2010. The relationship between leadership and gregariousness in mixed-species bird flocks. J. Avian Biol. 41, 99–103. ( 10.1111/j.1600-048X.2009.04828.x) [DOI] [Google Scholar]
- 14.Hino T. 1998. Mutualistic and commensal organization of avian mixed-species foraging flocks in a forest of western Madagascar. J. Avian Biol. 29, 17–24. ( 10.2307/3677336) [DOI] [Google Scholar]
- 15.Dolby AS, Grubb TC., Jr 1998. Benefits to satellite members in mixed-species foraging groups: an experimental analysis. Anim. Behav. 56, 501–509. ( 10.1006/anbe.1998.0808) [DOI] [PubMed] [Google Scholar]
- 16.Terborgh J, et al. 2001. Ecological meltdown in predator-free forest fragments. Science 294, 1923–1926. ( 10.1126/science.1064397) [DOI] [PubMed] [Google Scholar]
- 17.Thiollay J-M. 1992. Influence of selective logging on bird species diversity in a Guianan rain forest. Conserv. Biol. 6, 47–60. ( 10.1046/j.1523-1739.1992.610047.x) [DOI] [Google Scholar]
- 18.Stouffer PC, Bierregaard RO., Jr 1995. Use of Amazonian forest fragments by understory insectivorous birds. Ecology 76, 2429–2445. ( 10.2307/2265818) [DOI] [Google Scholar]
- 19.Maldonado-Coelho M, Marini MA. 2000. Effects of forest fragment size and successional stage on mixed-species bird flocks in southeastern Brazil. Condor 102, 585–594. ( 10.1650/0010-5422(2000)102[0585:EOFFSA]2.0.CO;2) [DOI] [Google Scholar]
- 20.Maldonado-Coelho M, Marini MA. 2004. Mixed-species bird flocks from Brazilian Atlantic forest: the effects of forest fragmentation and seasonality on their size, richness and stability. Biol. Conserv. 116, 19–26. ( 10.1016/S0006-3207(03)00169-1) [DOI] [Google Scholar]
- 21.Van Houtan KS, Pimm SL, Bierregaard RO, Jr, Lovejoy TE, Stouffer PC. 2006. Local extinctions in flocking birds in Amazonian forest fragments. Evol. Ecol. Res. 8, 129–148. [Google Scholar]
- 22.Sridhar H, Sankar K. 2008. Effects of habitat degradation on mixed-species bird flocks in Indian rain forests. J. Trop. Ecol. 24, 135–147. ( 10.1017/S0266467408004823) [DOI] [Google Scholar]
- 23.Mokross K, Ryder TB, Côrtes MC, Wolfe JD, Stouffer PC. 2014. Decay of interspecific avian flock networks along a disturbance gradient in Amazonia. Proc. R. Soc. B 281, 20132599 ( 10.1098/rspb.2013.2599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cordeiro NJ, Borghesio L, Joho M, Monoski T, Mkongewa V. 2014. Forest fragmentation in an African biodiversity hotspot impacts mixed species foraging bird flocks. Biol. Conserv. 188, 61–71. ( 10.1016/j.biocon.2014.09.050) [DOI] [Google Scholar]
- 25.Lee TM, Soh MCK, Sodhi N, Koh LP, Lim SL-H. 2005. Effects of habitat disturbance on mixed species bird flocks in a tropical sub-montane rainforest. Biol. Conserv. 122, 193–204. ( 10.1016/j.biocon.2004.07.005) [DOI] [Google Scholar]
- 26.Péron G, Crochet P-A. 2009. Edge effect and structure of mixed-species bird flocks in an Afrotropical lowland forest. J. Ornithol. 150, 585–599. ( 10.1007/s10336-009-0376-4) [DOI] [Google Scholar]
- 27.Zhang Q, Han RC, Zhang M, Huang Z, Zou F. 2013. Linking vegetation structure and bird organization: response of mixed-species bird flocks to forest succession in subtropical China. Biodiv. Conserv. 22, 1965–1989. ( 10.1007/s10531-013-0521-5) [DOI] [Google Scholar]
- 28.Goodale E, Kotagama SW, Raman TRS, Sidhu S, Goodale UM, Parker S, Chen J. 2014. The response of birds and mixed-species bird flocks to human-modified landscapes in Sri Lanka and southern India. Forest Ecol. Manag. 329, 384–392. ( 10.1016/j.foreco.2013.08.022) [DOI] [Google Scholar]
- 29.Tubelis DP, Cowling A, Donnelley C. 2006. Role of mixed-species flocks in the use of adjacent savannas by forest birds in the central Cerrado, Brazil. Austral. Ecol. 31, 38–45. ( 10.1111/j.1442-9993.2006.01541.x) [DOI] [Google Scholar]
- 30.Colorado GJ, Rodewald AD. 2015 Response of mixed-species flocks to habitat alteration and deforestation in the Andes. Biol. Conserv. 188, 72–81. ( 10.1016/j.biocon.2015.02.008) [DOI] [Google Scholar]
- 31.Dolby AS, Grubb TC., Jr 2000. Social context affects risk taking by a satellite species in a mixed-species foraging group. Behav. Ecol. 11, 110–114. ( 10.1093/beheco/11.1.110) [DOI] [Google Scholar]
- 32.Sieving KE, Contreras TA, Maute KL. 2004. Heterospecific facilitation of forest boundary crossing by mobbing understory birds in north-central Florida. Auk 121, 738–751. ( 10.1642/0004-8038(2004)121[0738:HFOFCB]2.0.CO;2) [DOI] [Google Scholar]
- 33.Goodale E, et al. 2009. Regional variation in the composition and structure of mixed-species bird flocks in the Western Ghats and Sri Lanka. Curr. Sci. India 97, 648–663. [Google Scholar]
- 34.Gill F, Donsker D. 2014. International Ornithologists’ Union world bird list (v. 3.3). See http://www.worldbirdnames.org/ (accessed December 2014). [Google Scholar]
- 35.Thomas L, Buckland ST, Rexstad EA, Laake JL, Strindberg S, Hedley SL, Bishop JRB, Marques TA, Burnham KP. 2010. Distance software: design and analysis of distance sampling surveys for estimating population size. J. Appl. Ecol. 47, 5–14. ( 10.1111/j.1365-2664.2009.01737.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kotagama SW, Goodale E. 2004. The composition and spatial organization of mixed-species flocks in a Sri Lankan rainforest. Forktail 20, 63–70. [Google Scholar]
- 37.Rasmussen PC, Anderton JC. 2012. Birds of South Asia: the Ripley guide, 2nd edn Barcelona, Spain: Lynx Editions. [Google Scholar]
- 38.Ali S, Ripley SD. 1987. Compact handbook of the birds of India and Pakistan, together with those of Bangladesh, Nepal, Bhutan and Sri Lanka, 2nd edn New Delhi, India: Oxford University Press. [Google Scholar]
- 39.Grimmett R, Inskipp C, Inskipp T. 1999. A guide to the birds of India, Pakistan, Bangladesh, Bhutan, Sri Lanka and the Maldives. Princeton, NJ: Princeton University Press. [Google Scholar]
- 40.Partridge L, Ashcroft R. 1976. Mixed-species flocks of birds in hill forest in Ceylon. Condor 78, 449–453. ( 10.2307/1367093) [DOI] [Google Scholar]
- 41.Thiollay J-M. 1999. Frequency of mixed-species flocking in tropical forest birds and correlates of predation risk: an intertropical comparison. J. Avian Biol. 30, 282–294. ( 10.2307/3677354) [DOI] [Google Scholar]
- 42.Dunning JB., Jr 2008. CRC handbook of avian body masses, 2nd edn Boca Raton, FL: CRC Press. [Google Scholar]
- 43.Sridhar H, et al. 2012. Positive relationships between association strength and phenotypic similarity characterize the assembly of mixed-species bird flocks worldwide. Am. Nat. 180, 777–790. ( 10.1086/668012) [DOI] [PubMed] [Google Scholar]
- 44.Whitehead H. 2008. Analyzing animal societies. Chicago, IL: University of Chicago Press. [Google Scholar]
- 45.Srinivasan U, Raza RH, Quader S. 2010. The nuclear question: rethinking species importance in multi-species animal groups. J. Anim. Ecol. 79, 948–954. ( 10.1111/j.1365-2656.2010.01707.x) [DOI] [PubMed] [Google Scholar]
- 46.Hutto RL. 1994. The composition and social organization of mixed-species flocks in a tropical deciduous forest in western Mexico. Condor 96, 105–118. ( 10.2307/1369068) [DOI] [Google Scholar]
- 47.Cohen J. 1988. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Lawrence Earlbaum Associates. [Google Scholar]
- 48.International Union for the Conservation of Nature (IUCN). 2014. IUCN Red List of threatened species. See www.iucnredlist.org (accessed September 2014). [DOI] [PubMed] [Google Scholar]
- 49.Hsieh H, Chen C-C. 2011. Does niche-overlap facilitate mixed-species flocking in birds? J. Ornithol. 152, 955–963. ( 10.1007/s10336-011-0678-1) [DOI] [Google Scholar]
- 50.Farine DR, Milburn PJ. 2013. Social organisation of thornbill-dominated mixed-species flocks using social network analysis. Behav. Ecol. Sociobiol. 67, 321–330. ( 10.1007/s00265-012-1452-y) [DOI] [Google Scholar]
- 51.Sridhar H, Shanker K. 2014. Using intra-flock association patterns to understand why birds participate in mixed-species foraging flocks in terrestrial habitats. Behav. Ecol. Sociobiol. 68, 185–196. ( 10.1007/s00265-013-1633-3) [DOI] [Google Scholar]
- 52.Goodale E, Kotagama SW. 2005. Alarm calling in Sri Lankan mixed-species bird flocks. Auk 122, 108–120. ( 10.1642/0004-8038(2005)122[0108:ACISLM]2.0.CO;2) [DOI] [Google Scholar]
- 53.Munn CA, Terborgh JW. 1979. Multi-species territoriality in Neotropical foraging flocks. Condor 81, 338–347. ( 10.2307/1366956) [DOI] [Google Scholar]
- 54.McClure HE. 1967. The composition of mixed-species flocks in lowland and sub-montane forests of Malaya. Wilson Bull. 79, 131–154. [Google Scholar]
- 55.Srinivasan U, Raza RH, Quader S. 2012. Patterns of species participation across multiple mixed-species flock types in a tropical forest in northeastern India. J. Nat. Hist. 46, 2749–2762. ( 10.1080/00222933.2012.717644) [DOI] [Google Scholar]
- 56.Gardner TA, Barlow J, Chazdon R, Ewers RM, Harvey CA, Peres CA, Sodhi NS. 2009. Prospects for tropical forest biodiversity in a human-modified world. Ecol. Lett. 12, 561–582. ( 10.1111/j.1461-0248.2009.01294.x) [DOI] [PubMed] [Google Scholar]
- 57.Raman TRS. 2006. Effects of habitat structure and adjacent habitats on birds in tropical rainforest fragments and shaded plantations in the Western Ghats, India. Biodiv. Conserv. 15, 1577–1607. ( 10.1007/s10531-005-2352-5) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are accessible at http://dx.doi.org/10.5061/dryad.vk070.