Abstract
The development of biomaterials for cardiac tissue engineering (CTE) is challenging, primarily owing to the requirement of achieving a surface with favourable characteristics that enhances cell attachment and maturation. The biomaterial surface plays a crucial role as it forms the interface between the scaffold (or cardiac patch) and the cells. In the field of CTE, synthetic polymers (polyglycerol sebacate, polyethylene glycol, polyglycolic acid, poly-l-lactide, polyvinyl alcohol, polycaprolactone, polyurethanes and poly(N-isopropylacrylamide)) have been proven to exhibit suitable biodegradable and mechanical properties. Despite the fact that they show the required biocompatible behaviour, most synthetic polymers exhibit poor cell attachment capability. These synthetic polymers are mostly hydrophobic and lack cell recognition sites, limiting their application. Therefore, biofunctionalization of these biomaterials to enhance cell attachment and cell material interaction is being widely investigated. There are numerous approaches for functionalizing a material, which can be classified as mechanical, physical, chemical and biological. In this review, recent studies reported in the literature to functionalize scaffolds in the context of CTE, are discussed. Surface, morphological, chemical and biological modifications are introduced and the results of novel promising strategies and techniques are discussed.
Keywords: biofunctionalization, cardiac tissue engineering, biomimetic scaffolds, chemical modification, biological modification
1. Introduction
Myocardial infarction (MI) or heart attack occurs as a consequence of abrupt blocking of one or more of the blood vessels supplying the heart (coronary arteries). In an adult heart, the damaged tissue does not repair spontaneously, and scar tissue is formed instead, as the mature contracting cardiac cells (cardiomyocytes) have limited capacity to proliferate [1]. When occlusions in the coronary arteries occur, a sudden decrease in nutrient and oxygen supply to the portion of the heart muscle supplied by the occluded arteries is observed. Therefore, if the flow of blood is not rapidly restored within the affected area of the heart muscle, permanent cell death occurs. The scar tissue replacing the heart muscle cannot conduct electrical or mechanical stimuli thus leading to a reduction in the pumping efficiency of the heart's main pumping chambers: the ventricles. Owing to the reduced cardiac output, several compensatory mechanisms are initiated which at first stabilize the damaged heart and maintain the cardiac output, such as: vasoconstriction, leading to an increase in blood pressure in order to return more blood to the heart; increase in heart rate; hypertrophy or enlargement of the heart muscles for greater pumping force and left ventricles (LVs) enlargement or dilation, so that more blood can fill into the heart. However, extra complications may affect the weakened heart leading to further deterioration of the cardiac function, ultimately reaching heart failure. Thus, the heart fails to pump enough blood around the body at the right pressure and as a result body functions fail. For example, kidneys functions fail causing retention of fluids and salts, therefore, fluid builds up in the arms, legs, ankles, feet, lungs and/or other organs and the body becomes congested [2]. The only therapeutic option available for patients with end-stage heart failure is to undergo heart transplantation or the use of mechanical ventricular assist devices (VADs) [3]. Unfortunately, many patients die while being on a waiting list, owing to the limited availability of organ donors and to the limitations of VADs, such as peri-operative and post-operative bleeding complications, thrombotic events and infection, occasional thrombus in the circuit, failure of the electrical motor and haemolysis owing to non-laminar blood flow. In addition, it has to be considered that not all patients have a body size that allows the device to be implanted into the chest cavity. Moreover, the majority of available devices are somewhat bulky, making normal patient movement difficult. Finally, the high costs must be taken into account.
Lately, replacement of the defective myocardial tissue with fetal or neonatal cardiomyocytes, skeletal myoblasts, embryonic stem cells, bone marrow-derived mesenchymal and haematopoietic stem cells and adipose-derived stem cells [4–7] has been proposed. Currently, the injection of cells in suspension, either into the circulating blood or directly into the myocardium, is the preferred method. However, the efficiency of these methods for cell delivery may be low owing to a significant loss of cells [8]. Therefore, alternative methods for cell delivery are being investigated to open new approaches for cardiac tissue engineering (CTE) [9]. The goal of CTE is thus to repair or replace damaged and injured heart tissue.
In general terms, a tissue engineering/regenerative medicine (TERM) approach consists of cell seeding on a scaffold, followed by in vitro tissue maturation and construct implantation in the host environment. However, alternative TERM approaches exist, lacking some elements or steps of the basic TERM paradigm. Among them, the most commonly implemented approaches in cardiac TERM are (i) cell-seeded' (in vitro maturation); (ii) cell injection (no scaffold and no in vitro maturation); and (iii) scaffolds that attract endogenous cells (no cells and in vitro maturation) [10]. All these approaches involve the design of a pre-formed or injectable scaffold, made using a biomaterial, able to properly interact with seeded or endogenous recruited cells. Therefore, surface functionalization can be exploited both in seeded and unseeded scaffolds.
The development of suitable biodegradable biomaterials as candidates for CTE is an active field of research [7,11]. Different fabrication methods are being continuously studied to develop three-dimensional scaffolds with a specific shape, thickness, mechanical strength and porosity to promote cell growth [7,12–14]. The specific physical properties of CTE constructs that are crucial for the success of this approach are biocompatibility, ability to foster cells, tailored degradation rate, permeability (for biomolecule diffusion), suitable mechanical properties, contractility and electrophysiological stability [15,16]. Both natural (gelatin [17], alginate [18], collagen type I [19–21] and fibrin glue [22,23]) and synthetic polymers (polyglycerol sebacate (PGS), polyethylene glycol (PEG) [24,25], polyglycolic acid (PGA), poly-l-lactide (PLA), poly(lactide-co-glycolide) (PLGA), polyvinyl alcohol (PVA), polycaprolactone, polyurethanes and poly(N-isopropylacrylamide) are being considered to develop cardiac patches. For both classes, pros and cons are summarized in table 1.
Table 1.
Summary of pros and cons of both natural and synthetic materials.
| advantages | disadvantages | |
|---|---|---|
| natural | —biological origin —biocompatibility —presence of adhesive sequences that facilitate cell adhesion and cell differentiation |
—inadequate mechanical properties —rapid degradation —variable properties depending on extraction procedure —risk of contamination —high production costs |
| synthetic | —suitable mechanical properties —highly reproducible chemical and mechanical properties —low production costs —low immune response |
—low biocompatibility —risk of biodegradation side effects |
Despite various advancements made, incomplete understanding of the interactions between biomaterials and biological systems still limits the advancement of CTE in clinical settings. Indeed, specific and complex mechanisms govern the reactions that occur at the interface between the biomaterial and the cellular environment. Schematically, figure 1 describes the initial interactions between biomaterials and cells. These interactions are governed by surface energy, chemical composition, stiffness, as well as roughness and topography of the biomaterial surface in contact with the biological environment [26].
Figure 1.
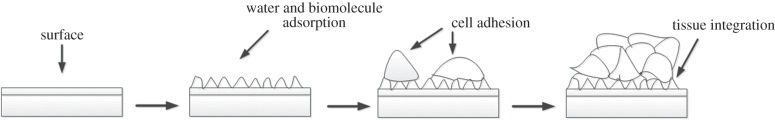
The interaction of cells with biomaterials is governed by the surface properties of the biomaterial.
Over the years, surface modification techniques have been adopted to enhance biocompatibility, haemocompatibility [27,28] and to promote vascularization [29] of scaffolds. The most promising synthetic materials investigated for CTE are polyurethanes [30,31] and polyesters [32,33]. However, these polymers lack cell recognition sites. Therefore, it is crucial to introduce functional groups on the surface of the scaffold that will function as cell recognition sites or may act as focal points for additional modification with bioactive molecules [34,35]. Moreover, surface modification can be useful to prevent thrombotic deposition and occlusion triggered by the activation of the coagulation cascade and platelets. Biomolecular modifications should lead to promising bioactive materials with the ability to control interactions with cell receptors (e.g. integrins) thus enhancing cell proliferation, differentiation, production and organization of the extracellular matrix (ECM).
There are basically two strategies for the biofunctionalization of polymers. The first one is pre-polymerization functionalization via polymerization of functional monomers [36] (e.g. alcohols, carboxylic acids, amines, acrylates). This procedure provides, for example, functional polyesters or polyurethanes with a defined chemical structure that allow for further modification following polymerization [37]. The second strategy is post-polymerization functionalization, which is the modification of the polymer after the polymerization process [35]. Post-polymerization techniques might be specific, targeting functional groups present in the polymer via carbodiimide or UV-initiated radical coupling, or non-specific, using azide- or glutaraldehyde-based couplings. A disadvantage of the non-specific covalent functionalization method is that it may result in the destruction of biomolecule bioactivity and/or can involve side reactions such as hydrolysis, chain-degradation or cross-linking [38].
This review focuses on the post-polymerization functionalization of scaffolds, covering different methods and techniques being investigated for application in CTE. In particular, chemical and biological modifications are reviewed in detail, discussing relevant examples. Table 2 presents a summary of techniques for the post-polymerization functionalization proposed or being investigated to modify biomaterials to induce a desired cell response.
Table 2.
Techniques used to modify biomaterials (post-polymerization) to obtain better interaction with the cells.
| modification methods | modified biomaterial properties |
|---|---|
| morphological modifications | coatings containing pores to enhance tissue ingrowth |
| topography induced (groves, morphology, roughness) | |
| fibrous materials | |
| porous materials | |
| chemical modifications | glow discharge to increase surface energy and tissue adhesion |
| cross-linked polymeric surfaces to decrease surface permeability and increase surface hardness | |
| plasma treatment with reactive gases to create new functional groups on polymer surface | |
| grafting macromolecules such as polyethylene glycol to reduce protein adsorption and cell adhesion | |
| functional groups used to produce positively or negatively charged surface | |
| biological modifications | immobilization of biomolecules to promote cell adhesion and growth |
| heparin, heparin sulfate binding peptides | |
| natural ECM proteins (fibronectin, laminin, collagen) | |
| peptide sequences (RGD) | |
| growth factors |
2. Bioactive molecules
Bioactive molecules can promote angiogenesis and engrafting by improving viability and survival of the grafted engineered tissue [39,40]. Scaffolds can be incorporated with bioactive molecules, such as chemicals in the form of ionic dissolution products, drugs, peptide sequences and/or growth factors (GFs). Bioactive molecules, which can be discharged from the scaffold by controlled release, diffusion or network breakdown, are capable of interacting with cells when they are released. The cells produce additional GFs that, in turn, stimulate multiple generations of growing cells to self-assemble into tissues in situ [41].
The bioactive molecules can be whole protein molecules such as ECM proteins or short peptide sequences (cell binding domains) isolated from ECM proteins. Short peptides are generally preferable to whole proteins, owing to the tendency of whole proteins to randomly fold, so that the receptor binding domains are not always available. Moreover, during the modification process, short peptides tend to be more stable. Short peptides can be mass-produced in laboratories. Arg–Gly–Asp (RGD), which is derived from fibronectin [42,43], is the most common short peptide sequence used.
In the next paragraphs, the attention will be focused on biomolecules already tested for CTE or that can reasonably find application in this field.
2.1. Extracellular matrix proteins and short peptide sequences
2.1.1. Collagen
Collagen is one of the most popular bioactive coatings used [44,45]. Collagen provides a biomimetic environment for cell growth [46–48], as collagen has a fibrous structure and appropriate mechanical properties [49]. In addition, it is highly biocompatible and biodegradable [47,49]. Collagen type I provides structural support (stiffness and resistance to deformation). Collagen type III plays an important role by linking contractile elements of adjacent myocytes together [50].
Collagen is usually incorporated onto polymer surfaces by immersion of the scaffold into a solution of the protein, to obtain a layer of collagen physically attached to the polymer surface. Park et al. [51] seeded neonatal rat heart cells suspended in Matrigel (a protein mixture of laminin, entactin, collagen and heparan sulfate (HS) proteoglycans) into a composite scaffold made of poly(dl-lactide-co-caprolactone), poly(dl-lactide-co-glycolide) (PLGA) and type I collagen at a density of 1.35 × 108 cells cm−3 and cultivated them in cartridges perfused with culture medium for 8 days; a collagen sponge (Ultrafoam) and a PLGA sponge served as controls. Results showed improved presence of cardiac markers and contractile properties in the composite scaffolds in comparison with both controls [51]. Collagen sponge exhibited a fast degradation rate and poor mechanical properties, even over short culture periods, whereas the PLGA sponge was hydrophobic and lacked cell attachment. The composite scaffold had several advantages, including a surface that supported cell attachment, a structure that allowed seeding and nourishment of cells at higher densities, mechanical properties that supported construct contractions and tunable degradation rate.
In a related study, Bai et al. [52] fabricated novel alginate/collagen composite microbeads encapsulating neonatal rat cardiomyocytes cells. The composite microbeads showed the proliferation of cardiac cells, the formation of interconnected multilayer heart-like tissues, the presence of well-organized and dense cell structures, and the spontaneous synchronized contractility of the grafts after two weeks in culture (at a rate of 20–30 beats min−1).
2.1.2. Fibronectin
Fibronectin is a high molecular weight glycoprotein of the ECM crucial for cell adhesion, spreading and migration. Previous studies have shown that ECM proteins influence cardiac cell morphology and physiology [53]. Hidalgo-Bastida et al. showed that fibronectin was the optimal ECM protein to enhance cell adhesion on poly(1,8-octanediol-co-citric acid) (POC) film. This could possibly be related to the fact that a higher level of fibronectin expression is associated with cell proliferation and migration at the embryonic stage of heart development, whereas during heart growth, fibronectin levels decrease, and laminin and collagen levels increase [53,54]. In another study, fibronectin was shown to be the most appropriate component, in comparison with laminin, gelatin and collagen I, to maintain the highest cell adhesion and proliferation rate [55].
2.1.3. Laminin
Laminin is a glycoprotein of the basal lamina and it has been demonstrated to play an important role in supporting and stimulating migration, adhesion, growth and differentiation of various cell types [56–60]. Because cardiac muscle is made of highly aligned fibres, lanes of laminin have been microcontact-printed onto non-adhesive surfaces to direct the organization of cultured cardiomyocytes to more closely resemble that found in vivo [61]. Adherent cardiomyocytes responded to the spatial constraints by forming elongated, rod-shaped cells whose myofibrils aligned parallel to the laminin lanes [61]. With the final aim of better matching the mechanical properties of the native tissue, a similar study but using a different substrate was carried out. Similar cardiomyocyte patterns were achieved on biodegradable polyurethane (PU). PU scaffolds are known for their elastomeric behaviour, which is a desired property for the regeneration of myocardial tissue [62]. Thin films based on PU synthesized from poly(ε-caprolactone) diol, 1,4-butandiisocyanate and l-lysine ethyl ester dihydrochloride chain extender were patterned by microcontact printing of laminin moieties and seeded with neonatal rat cardiomyocytes. Seeded cells produced a multi-layered, dense and highly aligned tissue, with the ability to contract within the thin films [63]. Alperin et al. [64] seeded embryonic stem cell derived cardiomyocytes on PU films coated with laminin or collagen IV and the coated surfaces showed higher numbers of contracting cells compared with unfunctionalized PU controls, thus showing the potential of PU elastomers for the repair of damaged heart. POC scaffolds coated with fibronectin, collagen and laminin showed better proliferation of the mouse cardiac muscle cell line HL-1 than untreated scaffolds. In particular, fibronectin-coated films showed the highest proliferation rate, compared with collagen and laminin coating [53]. In a related study, LaNasa et al. [65] seeded neonatal rat cardiomyocytes on flexible two-dimensional hydrogels consisting of covalently bounded collagen I, laminin and cell adhesive oligopeptides (RGD). Cells were sensitive to the different modified substrates: whole proteins, collagen and laminin, were effective in promoting cardiomyocyte interaction with hydrogels and cardiomyocyte maturation, whereas RGD did not provide adequate ECM cues for cardiomyocytes.
2.1.4. Nephronectin
Nephronectin (Npnt) is an ECM protein investigated for use as a natural adhesive material for CTE. It contains an N-terminal signal peptide followed by EGF-like repeats, an RGD sequence and a C-terminal MAM domain [66]. Nephronectin is expressed in cardiomyocytes throughout the heart and it is secreted into the cardiac jelly, which is in direct contact with cardiomyocytes and endocardial cells [67]. Neonatal rat cardiomyocytes were seeded on nephronectin-coated glass coverslips, demonstrating the excellent properties of nephronectin for cardiomyocyte adhesion and function [68]. Nephronectin enhanced cell-to-cell communication, sarcomere maturation and alignment and synchronized contractions.
Other studies have shown that all major cellular components of the heart, such as cardiomyocytes, cardiac fibroblasts, vascular smooth muscle cells and endothelial cells, can attach and spread on Npnt. Cardiomyocytes have been shown to attach and spread markedly faster on Npnt than on gelatin [67]. This was in part due to the presence of the integrin-binding RGD sequence in Npnt [69]. In addition, cardiomyocytes grown on Npnt exhibited a matured contractile apparatus with well-aligned sarcomeres. Connexin 43 expression data suggest, moreover, that the cells were electrically coupled. Consequently, cardiomyocytes on Npnt contracted synchronously and exhibited a higher beating frequency than cardiomyocytes on gelatin or fibronectin. Npnt was shown to maintain differentiation, promote sarcomere maturation as well as electrical signal propagation and consequently cardiomyocyte contractility [70–73].
2.1.5. Gelatin
Gelatin is derived from collagen by denaturation of the triple-helix structure. Gelatin is highly biodegradable and biocompatiable, in addition it exhibits excellent non-antigenicity, antithrombogenicity and it is cost effective [74–76].
Miskon et al. [77] reported that a gelatin-coated surface has a high ability not only to maintain the cardiac phenotype, but also to enhance cardiac differentiation. In another study, gelatin was demonstrated to maintain most of the cellular characteristics of cardiomyocytes in comparison with collagen I, laminin and fibronectin [55].
2.1.6. Short peptide sequences
RGD peptide is one of the most physiologically ubiquitous binding motifs commonly used, which is found in many natural adhesive proteins such as fibronectin, vitronectin, laminin and collagen type I [78]. The intracellular cytoskeleton is linked by cellular integrins to the ECM via RGD. Many studies with different cell types cultured on synthetic scaffolds immobilized with RGD demonstrated good adhesion and often comparable results to those obtained using entire proteins [79].
Silicone surfaces modified with both RGD and YIGSR have shown the same degree of adhesion obtained with native proteins (fibronectin and laminin, respectively) [80]. Furthermore, RGD-modified chitosan hydrogels were developed and C2C12 myoblasts proliferated and differentiated on these hydrogels and not on the unmodified ones [81]. In another study, three-dimensional alginate scaffolds modified with RGD peptide promoted neonatal rat cardiac fibroblasts viability in vitro [82]. Moreover, it was observed that tissue culture surfaces coated with RGD enhanced cardiomyocyte contractility and doubled cell viability compared with fibronectin-coated wells [83]. In a comparative study, collagen I, laminin and RGD were covalently bound to two-dimensional flexible hydrogels: RGD-modified hydrogels demonstrated good bioactivity when tested with skeletal myoblasts; however, when neonatal rat myocytes were cultured on RGD-modified hydrogels, the myocytes were self-associated and formed aggregates that exhibited a disorganized cytoarchitecture, suggesting that RGD does not provide adequate ECM cues for cardiomyocytes [65]. In another study, alginate was immobilized with cell adhesion peptides, containing the sequences RGD and YIGSR, or with a non-specific peptide (RGE) and implanted 7 days after infarction [84]. After 60 days, peptide-modified alginate reduced the therapeutic effects when compared with unmodified alginate, as revealed by the extent of scar thickness and by LV dilatation and function [84]. These findings were explained by structural changes occurring in alginate following covalent attachment of the peptides. The apparent viscosity of the cross-linked alginate, when modified with RGD/YIGSR or RGE peptides, increased by four- to sevenfold. The distribution of the biomaterial in the infarct region depends on solution viscosity, thus the peptide-modified biomaterial covered a smaller scar area in comparison with unmodified alginate. The influence of surface modification on myocardial microenvironment and the effect on myocardial function was also investigated in vitro and in vivo (in a rat model of ischaemic cardiomyopathy) [85]. In vitro, RGD-modified alginate improved the proliferation and adhesion of human umbilical vein endothelial cells (HUVECs) when compared with non-modified alginate [85]. Results also demonstrated that both modified and unmodified alginate improved heart function when injected into the infarct area of rats five weeks post-MI. RGD-modified alginate and unmodified alginate increased the arteriole density; however, the greatest angiogenic response was shown by the RGD-modified alginate [85].
The RGD motif has been also shown to enhance cell adhesion and contractility. For example, RGD immobilized on collagen scaffolds has been shown to improve cardiomyocyte viability, differentiation and contractile performance [86]. In vitro studies of encapsulated human mesenchymal stem cells (hMSCs) in RGD-modified alginate microspheres demonstrated that RGD-modified alginate improved cell attachment, growth and increased angiogenic growth factor expression [87]. Surface modification combined with microencapsulation technique was also able to maintain the LV geometry, preserve LV function, increase angiogenesis and improve cell survival. Moreover, immobilization of macroporous alginate scaffolds with RGD peptide has been proven to be a key parameter in promoting the formation of functional cardiac muscle tissue and in enhancing the preservation of the regenerated tissue in culture [88]. Neonatal rat cardiac cells were cultured onto unmodified, RGD-modified and heparin-binding peptide-RGD-modified (HBP/RGD) alginate porous scaffolds. The HBP/RGD-modified scaffolds revealed the best features of a functional cardiac muscle tissue, demonstrating isotropic myofibre arrangement [89]. Cells spread extensively on such RGD-modified scaffolds, with the F-actin fibres well stretched and showing many focal adhesion points. The strong adhesion and spreading inhibited the cell organization process into the myofibril structure. Thus, the introduction of HBP was shown to alter the effect of RGD, reducing cell spreading and promoting proper cell organization [89]. In addition to RGD tripeptide, a great number of longer peptides containing the RGD sequence have been investigated. In particular, GRGDS and GRGDSP peptides were shown to promote the adhesion of several cell types, including myoblasts, and they were found to stimulate integrins that are relevant in early cardiac development [90,91].
In related studies, in vitro cell culture tests on three-dimensional matrix metalloproteinase (MMP)-sensitive PEG-based hydrogels, modified with different quantities of RGDSP peptide have been performed using P19 embryonal carcinoma cells, as a model of pluripotent cardioprogenitors [92]. It was demonstrated that indicators of cardiac maturation were promoted by RGDSP-mediated stimulation of integrins that are relevant in early cardiac development, with a sixfold increased amount of myosin heavy chain-positive cells when compared with cells in suspension [92].
2.2. Growth factors
GFs are small, soluble, natural cell-signalling polypeptides, which are secreted by cells and can stimulate specific activities in a biological environment such as cell growth, proliferation and differentiation. Usually, GFs have slow diffusion and short half-life and as a result they act locally. Typical GFs are listed in table 3. GFs promote myocardial healing and repair at the infarct site [103]. Examples of GFs are granulocyte colony-stimulating factor (G-CSF) [104,105], stromal-derived growth factor (SDF-1) [106], leukaemia inhibitory factor [107], insulin-like growth factor (IGF-1) [108], vascular endothelial growth factor (VEGF) and erythropoietin (EPO) [109]. GFs-based regenerative therapy is a promising therapeutic strategy for MI.
Table 3.
Different GFs and their functions in cardiac tissue engineering.
| growth factor | function | critical issues | reference |
|---|---|---|---|
| VEGF | migration, proliferation and survival of endothelial cells; endothelial capillary formation |
rapid degradation due to short half-life; excessive amounts cause vascular leakage | [93–96] |
| bFGF | heparin-binding protein; proliferation of endothelial cells, smooth muscle cells; endothelial capillary formation |
rapid diffusion, requiring controlled release; mitogen for a wide variety of cell types | [95,97,98] |
| HGF | mitogen for hepatocytes and other cell types; growth of endothelial cells | short half-life, rapid diffusion; large amounts of protein required for response | [99] |
| PDGF | mitogen for connective tissue cells, released from platelets; recruitment of smooth muscle cells to endothelial linings; vessel maturation | vessel destabilization for high levels; increased activity linked with several diseases | [100–102] |
2.2.1. Vascular endothelial growth factor
VEGF is a dimeric glycoprotein, secreted and synthesized by many cell types. VEGF binds to the ECM through indirect interaction via linker molecules (heparin sulfate proteoglycans), so that ECM regulates the bioavailability of VEGF. As a result of hypoxic or inflammatory stimuli, VEGF is secreted by cardiomyocytes [110–112], in order to regulate the formation and maturation of blood vessels, acting mainly on vascular endothelial cells [113]. VEGF promotes angiogenesis and coronary collateral formation in infarcted myocardium, inducing the growth of new blood vessels, by promoting the dissolution of existing blood vessels, the migration and proliferation of endothelial cells and the formation of tubes from endothelial cells [114].
Ozawa et al. studied the effect of VEGF dosage on the morphology and function of newly formed blood vessels in adult mice muscles implanted with retro virally transduced myoblasts that constitutively express VEGF [115]. The study showed that high levels of VEGF may lead to the growth of abnormal blood vessels and haemangiomas, whereas when VEGF concentration is maintained between low-to-medium levels, it leads to normal angiogenesis. Thus, this issue should be taken into consideration while designing a cardiac tissue construct incorporating VEGF.
2.2.2. Granulocyte colony-stimulating factor
G-CSF is a hormone crucial in regulating the proliferation, differentiation and survival of myeloid progenitor cells. Moreover, G-CSF showed a significant increase in the release of haematopoietic stem cells into the peripheral blood circulation. It has been recently demonstrated that G-CSF stimulates healing and repairing, improving cardiac function [104], and reduces mortality after acute MI [116,117]. The mechanism by which G-CSF alters cardiac dysfunction, however, is not fully understood. One possible rational is that G-CSF regenerates cardiac myocytes and blood vessels through mobilization of bone marrow stem cells [117].
2.2.3. Erythropoietin
EPO is a hormone of importance for erythrocyte differentiation and survival. EPO has the ability to maintain vascular autoregulation and reduces primary (apoptosis) and secondary (inflammation) causes of cell death. Calvillo et al. [109] showed that EPO decreased cardiac myocyte loss by 50% in a rat model of infarction, which is sufficient to normalize haemodynamic function.
2.2.4. Insulin-like growth factor
IGF-1 is a GF that can delay cardiomyocyte ageing and death [118]. IGF-1 in an injured muscle can enhance cell homing, healing and regeneration [119].
GFs have been supplied to cells via different methods, including addition to the culture medium, loading in controlled release systems and covalent immobilization onto the biomaterial. Immobilization of GFs onto biomaterials protects them against cellular inactivation and digestion, sustaining their activity [120]. Moreover, GFs immobilization overcomes diffusional limitation of soluble GFs [121].
One of the classical means to functionalize biomaterials is to incorporate GFs, such as VEGF, basic fibroblast growth factor (bFGF) and many others, as summarized in table 4 [93,94,101,139,140]. Some GFs act as angiogenic growth factors (AGFs) which provide an efficient means for stimulating localized vessel recruitment to the scaffold. By improving the vascularization of transplanted engineered tissues, AGFs improve cell survival and function. The local delivery of GFs prevents serious unfavourable effects such as hyper permeability, oedema, hypotension and accelerated atherosclerosis [141]. It has been demonstrated that the incorporation of VEGF in CTE scaffolds facilitates blood vessel growth within the scaffold [142].
Table 4.
Summary of cardiac scaffolds with immobilization of GFs developed in the last years.
| incorporation method | polymer | angiogenic factor | method of preparation | model | result | reference |
|---|---|---|---|---|---|---|
| porous composite scaffold loaded with microspheres | PLGA microspheres alginate |
bFGF | myocardial implantation in rat | controlled release of bFGF promoted vascularization | [102] | |
| composite scaffold | poly(ether)urethane–poly-(di-methylsiloxane) fibrin |
VEGF bFGF |
spray phase inversion method | subcutaneous implant and unilateral hindlimb ischaemia model in rat | enhanced angiogenesis | [122] |
| hollow fibre | cellulose acetate | VEGF SIP |
double injection extrusion precipitation method | subcutaneous implant model in mice | [123] | |
| covalently immobilized GF | collagen | VEGF Ang-1 |
commercial scaffold (Ultrafoam collagen sponge) | chicken chorioallantoin membrane angiogenesis assay | enhanced angiogenesis compared with soluble VEGF and Ang-1 | [124] |
| polymeric injectable carrier | poly(trimethlene carbonate) | VEGF | subcutaneous injection in rat | local GF delivery | [125] | |
| dual-layered scaffold | poly(trimethlene carbonate) | VEGF HGF |
cross-linking | in vitro HAECs | release of dual GFs at similar and controlled rates | [126] |
| fibrous membranes | dextran PLGA |
VEGF | coaxial electrospinning | in vitro | promoted cell proliferation | [127] |
| alginate hydrogel | alginate | VEGF | cross-linking | Hindlimb ischaemia in mice | [128] | |
| hydrogel with surface cross-linked heparin | star-PEG | VEGF FGF-2 |
cross-linking | HUVECs in vitro and chicken chorioallantoin membrane angiogenesis assay | enhanced angiogenic activity compared with signal factor immobilization | [129,130] |
| covalently immobilized VEGF | collagen | VEGF | commercial scaffold (Ultrafoam collagen sponge) | replacement after resection of the right ventricle | promoted tissue formation | [131] |
| biometric hydrogel with adhesive peptide sequence RGD | PEG diacrylate | VEGF | photopolymerization | HUVECs and hMECs in vitro | promoted EC proliferation, migration and viability | [132] |
| surface cross-linked heparin | polycarprolactone | VEGF | solvent casting particulate leaching |
subcutaneous implant model in mice | improved VEGF efficacy | [133] |
| temperature-sensitive injectable hydrogels | PVL-b-PEG-b-PVL | VEGF | metal free cationic method | myocardial implantation in rat | stabilization of the infarct | [134] |
| collagen patch | collagen | VEGF | myocardial implantation in rat | improved LV function enhanced vascularization |
[135] | |
| PEG | EPO | rat model | improvement in fractional shortening LV end diastolic diameter LV end systolic diameter |
[136] | ||
| poly(N-isopropylacrylamide-co-propylacrylic acid-co-butyl acrylate) (p(NIPAAm-co-PAA-co-BA)) |
bFGF | rat model | increase in vessel density and fractional shortening | [137] | ||
| fibrous membranes | PLLA | G-CSF | electrospining | C2C12 in vitro | cell elongation and appearance of cellular junctions | [138] |
An overview of the different methods used for GFs inclusion in scaffolds will be provided in §3.2.
2.3. Synthetic chemicals
In addition to protein-based cytokines and GFs, synthetic chemical compounds have also demonstrated ability to promote cardiomyogenic differentiation in vitro. Synthetic chemicals present some advantages with respect to protein-based cytokines and GFs; they have a longer half-life in solution, permitting in vitro cell culture to be prolonged over several days or even weeks, and, being manufactured by chemical reactions, are more structurally and chemically defined. However, it is known that they may negatively affect cell genomic stability, limiting their potential application in clinical practice.
Among the synthetic chemicals that are known to promote differentiation towards the cardiac phenotype there are dimethyl sulfoxide (DMSO), all-trans retinoic acid (RA), dynorphin B, ascorbic acid and 5-aza-2′-deoxycytidine (5-aza-dC). 5-aza-dC is a synthetic nucleoside that is commonly used as an inhibitor of DNA methylation. It has been demonstrated that it is a potent inducer of cardiomyogenic differentiation in both embryonic [143] and adult stem cells, in particular bone marrow-derived mesenchymal stem cells [144–148].
Both ascorbic acid (vitamin C) and retinoic acid (derivative of vitamin A) have been shown to promote cardiomyogenic differentiation of embryonic stem cells [149,150]. DMSO is a commonly used cryoprotectant but it has also been shown to induce cardiomyogenic differentiation in both embryonic stem cells [151] and embryonal carcinoma cells [152,153]. Additionally, the cardiomyogenic differentiation of embryonal carcinoma cells has been shown to be stimulated by dynorphin B, which is a naturally occurring κ-opioid [151].
3. Functionalization strategies
3.1. Surface chemical modification
Cell adhesion is affected by surface hydrophilicity, surface charge density, surface micro-morphology, free energy and specific chemical groups present on the surface of the scaffold. Influencing cell spreading and signalling, material surface properties regulate cell growth, migration, differentiation, synthesis of ECM components and tissue morphogenesis. Given that surface chemistry is crucial for the biocompatibility of the scaffold, specific surface modifications are requested to inhibit unfavourable effects and to enhance specific biological responses. Different surface modification methods can be exploited to change surface property or to add functional groups to be used for biomolecule grafting (figure 2).
Figure 2.
Schematic diagram showing three different surface chemical modification methods that can be exploited to modify the surface properties to add functional groups to the surface.
3.1.1. Chemical methods
Chemical modification plays a key role in the design of functional biosurfaces by chemical grafting of functional groups (acetylation, fluorination, silanization, incorporation of sulfonate groups) or by modifications of existing functional groups (oxidation, reduction) [12–14].
Numerous studies have demonstrated that chemical modifications enhance the cytocompatibility of polymers as presented in this section. In one study, the influence of NaOH treatment on fibroblast compatibility was investigated. Cells cultured on untreated films for 6 and 24 h showed more rounded morphology compared with that observed on treated films, on which cells assumed a more stretched morphology. It has been demonstrated that biodegradable polymer surfaces can be modified for better cell attachment in an alkaline solution to provide hydrophilic, rough surfaces [154,155]. Biodegradable aliphatic polyesters degrade in water, owing to scission of the main ester bonds. Degradation results in the generation of carboxylic acid and hydroxyl groups at the incised chain ends.
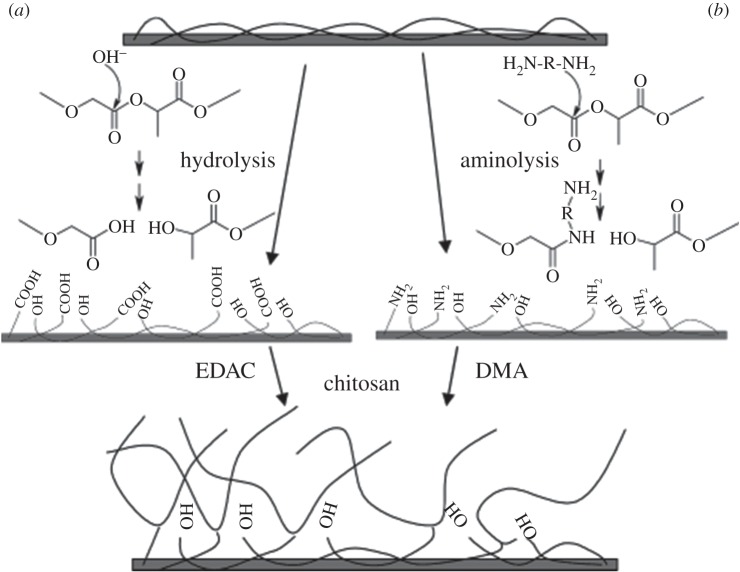
In addition, surface chemical treatments are performed to introduce functional groups on the polymer surface for a subsequent modification of biomaterial surfaces with biomolecules. In one study, two wet techniques were used to introduce carboxylic acid by hydrolysis and primary and secondary amine groups using aminolysis, on PLGA surfaces [156] (figure 3). For the hydrolysis process, films were immersed in a suitable concentration of aqueous sodium hydroxide (NaOH) for a desired period of time [157–159]. For aminolysis, films were immersed in various concentrations of ethylenediamine (ED) or N-aminoethyl-1,3-propanediamine (AEPDA) in either water or isopropyl alcohol for a desired period of time [156,160,161].
Figure 3.
Schematic diagram showing two methods for the functionalization of PLGA surface, first activated (a) by hydrolysis (b) aminolysis and then further functionalized by covalently binding chitosan using EDAC or DMA, respectively. Reproduced with permission from ref. [156].
Hydrolysis is a simple and frequently used method; however, it is pH dependent and might lead to unwanted degradation of the polymer surface. Moreover, carboxylic acid groups first need to be ‘activated’ by turning the acid (anhydride, acid halide) into a more reactive derivative or by using a coupling agent. If a wrong agent is used, it can lead to racemization. Aminolysis is a very simple reaction, which however could produce a salt of the organic acid and base. High temperatures (more than 200°C) could be used to overcome salt formation, but the polymer could decompose at these elevated temperatures.
3.1.2. Photo-induced grafting methods
Photo-induced grafting and photo-induced polymerization are well-established low-cost methods for surface modification [162]. These are modification methods with mild reaction conditions and local surface chemistry modification, without affecting the bulk polymer properties. UV-induced photo polymerization has been used to modify PLLA increasing the hydrophilicity of its surface, by grafting a single monomer or a combination of two hydrophilic monomers (vinyl acetate, acrylic acid and acrylamide) [163]. When copolymerized with vinyl acetate or acrylic acid, acrylamide contributes to the hydrophilicity of the polymer surface. The contact angle of the modified surface varied with the feed composition [163]. Other functional groups such as hydroxyl, carboxyl and amide groups, have also been introduced onto PLLA surfaces, using the photo-induced grafting method, by grafting hydroxyethyl methacrylate, methacrylic acid (MAA) or acrylamide, respectively [164].
3.1.3. Plasma grafting and plasma treatment
Plasma grafting and plasma treatment are effective methods for surface modification.
Plasma strongly interacts with polymer surfaces leading to chemical and physical modifications of the biomaterial surface via electrons, ions, radicals and neutral molecules. Cell culture devices such as Petri dishes, microcarriers and membranes are modified to enhance cell adhesion and growth using plasma treatment. It has been demonstrated that plasma treatment generally acts locally on the surface and it does not alter bulk polymer properties [165].
Moreover, if plasma treatment is applied using non-polymerizing gases, functional groups on the biomaterial surface are generated such as amine or carboxyl groups. For example, a hydrophobic surface was made hydrophilic by oxygen plasma treatment, whereas a hydrophilic surface was modified into a hydrophobic one by using tetrafluormethan (CF4) plasma [166]. In another study, a PLGA surface was modified using oxygen treatment [167]. Analysis showed that contact angles of plasma-treated samples decreased from 78° to 45° after 2 min treatment, meaning that the hydrophilicity of PLGA increased after oxygen plasma modification. The treatment also resulted in increased surface roughness, owing to the formation of peaks and valleys. In addition, cell culture tests performed on the treated PLGA surface showed enhanced attachment of mouse NIH 3T3 fibroblasts [167].
Plasma grafting is a surface graft polymerization, covalently binding functional groups to the polymer surface. It allows the modification of the polymer surface, through a choice of different monomers, to obtain desired properties. Bulk properties of the polymer are not altered through the grafting of high-density chains, in a precise location on the polymer surface. In addition, the chemical bond formed between the polymer surface and the grafted functional group results in a more durable functionalization in comparison with the physical coating [168,169]. Plasma grafting is restricted to localized surface areas and to a depth from several hundred angstroms to 10 nm [154]. In one study, PLLA and PLGA nanofibres were chemically modified using oxygen plasma treatment and by in situ grafting of hydrophilic acrylic acid to add carboxyl groups on the surface. The modified scaffolds showed improved fibroblast attachment and proliferation in vitro [170]. Brown et al. investigated plasma grafting of acrylic acid on PLGA followed by activation of carboxylic groups by 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and N-hydroxysuccinimide (NHS) for the coupling of fibronectin. They demonstrated better spreading of neonatal rat cardiomyocytes and myofibril development on modified PLGA with respect to unmodified PLGA [171]. In another study, derived hMSCs, cultured on PLGA supports, plasma grafted with fibronectin strips, showed highly elongated morphology and markers associated with myogenesis. It was also observed that protein surface micropatterns can highly influence the differentiation process [172].
3.2. Surface biological modification with bioactive molecules
Surface biological modification is achieved by adsorption or chemical bonding of biomolecules to the polymer surface in order to stimulate a specific cell response. Different modifications are discussed in this section.
3.2.1. Chemical incorporation
There are two main groups of methods to chemically incorporate GFs into polymeric scaffolds: (i) non-covalent incorporation by physical adsorption, owing to protein–protein hydrogen bonding or protein–protein hydrophobic interaction with an intermediate molecule and (ii) covalent incorporation of GF.
3.2.1.1. Covalent surface bonding
In covalent surface bonding, biomolecules are chemically bonded to the scaffold surface exposing relevant functional groups. Covalent surface bonding results in a more efficient coating, with the biomolecules being retained over a longer period of time when compared with physical adsorption [173]. To accomplish covalent coating two steps are required: the first is the exposure of functional groups and the second is the covalent binding of the biomolecules to the exposed functional groups.
Carboxyl and amine groups are the most common functional groups exploited in surface functionalization. Carboxyl groups in polymers such as PLLA and PCL can be exposed on the surface by hydrolysis [158,159], amine groups by aminolysis [160,161]. In addition, plasma treatment can be applied to graft functional groups on the surface for further covalent grafting [174], as previously mentioned.
Attachment of biomolecules requires the activation of functional groups on both polymer surface and on biomolecules. Hydrolysed films have been activated by treatment in MES buffer solution containing EDC for an adequate period [174–176]. By adding NHS, EDC efficiency is improved [174,175]. On the other hand, aminolysed films were activated by treatment in triethanolamine buffer containing glutaraldehyde and 1-hydroxbenzol (HOBt), followed by EDC exposure for an adequate period of time [160,176]. After a preliminary chemical treatment, the surface is modified by immobilization of the biomolecules via an amide bond between the amine group/carboxylic group of the biomolecule and the exposed carboxylic group/amine group on the biomaterial surface, respectively [177].
Linker molecules are used when functional molecules cannot be directly grafted onto the polymer surface. A linker molecule is a chemical substance capable of reacting with both the polymer and the functional molecules. In one study, di-amino-poly(ethylene glycol) (di-NH2-PEG) was used as a linker molecule to add functional amine groups to previously exposed carboxyl groups, by soaking PLLA nanofibres in NaOH solution [178]. Using a similar strategy, carboxyl functional groups were grafted on polyethersulfone by using a linker molecule (polyacrylic acid) introduced on the polymer surface by photo polymerization [179]. Moreover, polyacrylic acid was used as linker molecule to covalently bond collagen in the form of lamellae or sheets on PMMA and other polymers [180]. It is very important to consider the properties of the linker molecules before using them, as several studies have demonstrated that linker molecule properties influence the interaction between cells and the immobilized biomolecules, such as cell adhesion ligands [179,181,182].
Covalent bonding is more complex and may limit the type of biomolecule that can be attached owing to the harsh conditions sometimes required to gain a satisfactory functionalization. For instance, biomolecules such as GFs can be deactivated by the organic solvents used during covalent bonding procedures. Alternatively, combined techniques employing both covalent bonding and physical adsorption can be exploited to biofunctionalize polymer surfaces. These techniques will be discussed in detail later.
Covalent immobilization is a promising approach to bind GFs to biomaterials [124,183]. The covalent attachment is achieved by a reaction between functional groups of the polymer and GFs amino acids such as lysine and cysteine [184]. Enhanced cell survival and spreading of MSC have been demonstrated on a PEG scaffold covalently modified with VEGF [185]. GFs can be also covalently immobilized on scaffolds by homo- and heterobifunctional cross-linking agents. A homo-bifunctional cross-linker (disuccinimidyl-disuccinate-polyethyleneglycol (SS-PEG-SS)) was used to immobilize VEGF on collagen matrices. It was demonstrated by in vitro endothelial cell growth and in vivo vessel growth in chorioallantois membrane that VEGF covalently immobilized on collagen matrix produced increased angiogenic effects compared with free VEGF matrices [186].
In one study, VEGF was first modified by introducing a cysteine tag in its structure and then immobilized onto a fibronectin-coated surface using free sulfhydryl group [187]. In a different approach, EDC chemistry was applied to immobilize GFs onto scaffold surfaces, for instance, VEGF and fibronectin were immobilized on PLLA surfaces, demonstrating improved growth of HUVECs. [188]. Collagen scaffolds have also been incorporated with VEGF using EDC chemistry [121]. As a result of VEGF immobilization, survival and proliferation of D4T endothelial cells were enhanced [121]. In a related study, Miyagi et al. demonstrated increased angiogenesis and patch stability in defective rat hearts upon transplantation of scaffolds uniformly immobilized with VEGF, compared with VEGF-free controls [131]. In another study, VEGF and angiopoietin-1 (Ang-1) were co-immobilized onto collagen scaffolds [124]. The co-immobilized scaffolds showed increased cell growth and proliferation in vitro compared with unfunctionalized controls without GF. Furthermore, increased vessel density was found when scaffolds were implanted in chicken chorioallantoic membrane [124]. VEGF and Ang-1 were also covalently immobilized onto porous collagen scaffolds using EDC chemistry. The effect of different reaction buffers (PBS, distilled water and MES) on covalent immobilization was investigated. PBS showed the highest VEGF and Ang-1 immobilization, leading to the highest proliferation rate and lactate metabolism [189].
3.2.2. Physical adsorption
Physical adsorption is one of the simplest methods to biofunctionalize biomaterials, by incubating the scaffold in solutions containing biomolecules. The biomolecules attach to the material surface owing to surface interactions, such as Van der Waal forces, electrostatic forces, hydrophobic interactions and hydrogen bonds. The physical adsorption efficiency can be enhanced by treating the material with air plasma to increase its hydrophilicity. Generally, hydrophilic surfaces tend to improve adhesion strength, biocompatibility and other pertinent properties [190,191]. Surface functionalization via physical adsorption has the advantage of being a simple and gentle procedure accompanied by limited damage to fragile structures and biomolecules; on the contrary, biomolecule binding to scaffold surfaces is relatively weak.
Non-covalent immobilization is based on electrostatic interactions. For example, ionic complex of gelatin and transforming growth factor-1 (TGF-1) can be obtained when gelatin microparticles loaded with TGF-1 are encapsulated in oligo[poly(ethylene glycol) fumarate] hydrogels at pH 7.4 [192]. The interactions between gelatin and TGF-1 occur because of negatively charged chemical groups on the gelatin surface and TGF-1 positive charge [192]. Typical adsorption of a GF onto a polymer is either a charge to charge or a secondary interaction between the polymer surface and the GF. This result can be also achieved through an indirect interaction using an intermediate biomolecule [193–195].
3.2.3. Combined techniques
Chemical reagents and solvents used for chemical conjugation can damage biomolecules (proteins and peptides) such as GFs that are susceptible to inactivation or denaturation. An alternative approach for biomolecule binding is the use of molecules that bind to both the biomolecule and the biomaterial surface. Therefore, intermediate molecules such as glycosaminoglycans, ECM proteins, small oligopeptides mimicking ECM proteins and avidin–biotin molecules can be chemically or physically deposited onto the scaffolds to offer biological sites for GF incorporation. It has been proven that GF binding via ECM linkers regulates GF activity [196]. For example, several groups of GFs interact with ECM proteins, heparin or HS, including IGF, fibroblast growth factor (FGF), TGF-1,2,3, endothelial growth factor (EGF), VEGF, PDGF and HGF [95,196]. As a result, stable and strong covalent bonds are formed between the biomaterial surface and the linker molecules, avoiding the exposure of biomolecules to harsh solvents that could damage their bioactivity. In addition, thanks to the presence of the linker molecules the surface of the material exhibits a strong charge or hydrophobicity favouring the attraction of biomolecules [197]. Combinational techniques are also fast, and do not require substantial incubation time for the biomolecule to attach to the functionalized surface [198]. Moreover, they can be applied using a wider choice of buffer systems in comparison with chemical bonding, where some amine-based buffers (Tris, glycine) cannot be used [198]. The only drawback is that combinational techniques involve many steps.
3.2.3.1. Interaction with glycosaminoglycans
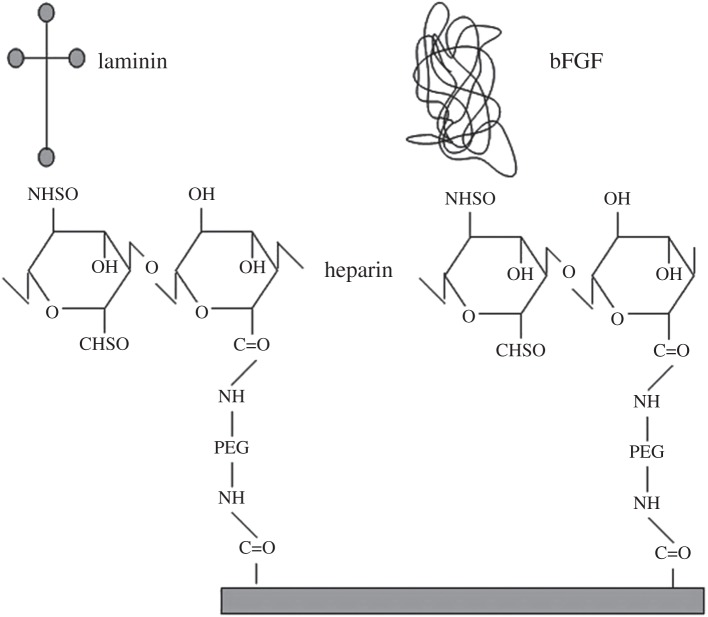
Heparin and HS are linear sulfated polysaccharides with high negative charge, which bind to different proteins, such as FGF and their tyrosine kinases receptor, TGF, bone morphogenetic proteins (BMPs), chemokines, interleukins, enzymes, enzyme inhibitors, lipases, apolipoproteins, ECM and plasma proteins [199]. The specific sulfation motifs function as molecular recognition elements for GFs. The existence of a ‘sulfation code’ has been proposed, whereby glycosaminoglycans encode functional information in a sequence-specific manner analogous to that of DNA, RNA and proteins [200]. GFs become more resistant to proteolysis and thermal denaturation when bound to HS or heparin [201]. GAGs can be therefore used in binding, modulation and sustained release of GFs. GAGs have been incorporated in different polymers. For example, heparin-conjugated PLGA nanospheres (HCPNs), trapped in cross-linked fibrin gel, were developed for long-term and zero-order delivery of bFGF. Heparin was immobilized on PLGA nanospheres via a coupling reaction in the presence of EDC and the bFGF was physically bonded to heparin [202]. The release kinetics of bFGF from HCPNs suspended in fibrin gel was of zero order, sustained for three weeks with no initial burst effect. The release rate was inversely proportional to the fibrinogen concentration in the fibrin gel. In vitro cell culture demonstrated that bFGF released from HCPNs supported HUVEC growth for 15 days. In vivo tests (implantation in a mouse limb ischaemia model) also offered successful results [202]. The use of maleimide reactive groups (figure 4) was demonstrated to be a possible strategy to attach heparin to PEG hydrogels [203]. PEG was immobilized with heparin by the reaction between PEG terminal thiol and maleimide-functionalized low molecular weight heparin. bFGF incorporated in such system exhibited controlled release. The release kinetics of bFGF were the same as the matrix erosion kinetics and the delivery lasted several days [203].
Figure 4.
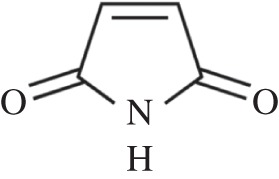
Chemical formula of maleimide investigated to attach heparin to PEG hydrogels [203].
In order to prolong and slow down the release of VEGF, which stimulates both angiogenesis and vasculogenesis, a novel self-assembling system was developed, where the heparin-binding domain sequence was attached to a self-assembling peptide [204]. Results of in vivo tests in infarcted rat model demonstrated myocardial protection, sufficient angiogenesis and improvement in cardiac function [204]. In one study, heparin was covalently bonded to the biomaterial, and then the biomolecule was attached to heparin by physical adsorption, as shown in figure 5 [178]. A different approach for covalent attachment of heparin was also investigated, where heparin was attached by modifying its carboxyl groups to obtain thiol groups [205]. Thiol groups reacted with PEG diacrylate, undergoing Michael addition to form thioether linkages. In vivo studies demonstrated that fibroblast cells encapsulated in the bFGF–heparin-modified PEG remained viable after gelation, showing enhanced proliferation [205].
Figure 5.
Immobilization of bFGF and laminin on PLLA using di-NH2-PEG and heparin as linkers. Reproduced with permission from [178].
3.2.3.2. Interaction with extracellular matrix components
Gelatin is a charged biopolymer which can interact with GFs. Gelatin can be negatively charged (acidic gelatin) or positively charged (basic gelatin), depending on the extraction process. Acidic gelatin can be used to incorporate basic proteins, whereas basic gelatin can be used to incorporate acidic proteins under physiological conditions [206]. A study carried out by Layman et al. [207] has shown the inclusion of bFGF into acidic gelatin hydrogels, comparing the release profile with those from basic gelatin and PAA anionic hydrogels. In this study, the effect of solution temperature and ionic strength on the absorption and desorption was investigated. It was demonstrated that the bFGF absorption increased with increasing the number of carboxylic groups in acidic gelatin hydrogel. This study also demonstrated also the possibility of delivering multiple GFs from gelatin hydrogels. bFGF and G-CSF were immobilized in these hydrogels and a controlled release for several days for both GFs was reported [207].
Collagen is a ubiquitous protein in the human body able to interact with GFs. For example, bFGF binds to collagen type I both in vitro and in vivo, obtaining a protection effect. Incorporation of bFGF in collagen matrices showed a prolonged release of the GF, depending on the degradation rate of the matrix, leading to controlled local delivery with angiogenic activity. Therefore, collagen type I is a suitable candidate material to be used as a reservoir for bFGF delivery [208].
3.2.3.3. Functionalization by the avidin–biotin binding system
Avidin is a tetrameric glycoprotein found in egg white (MW 68 kDa) with a size of 5 nm, which exhibits a specific and extraordinarily high interaction with biotin (also known as vitamin H; MW 244.3 kDa). Avidin contains four biotin binding sites and therefore, the two molecules make a highly specific and stable complex, with an affinity constant of 1015 M−1. Avidin–biotin binding approaches have been extensively applied to biotechnology, such as affinity chromatography, histochemistry, diagnostic, immunoassay and drug delivery [209].
The feasibility of introducing an avidin–biotin binding system in biomedical relevant approaches for increasing cell adhesion has been also demonstrated. The conjugation of biotin on the cell membranes of non-adhesive cells of the Ehrilich ascites carcinoma cell line produced their adhesion to avidin-coated substrata [210]. Similarly, biotinylation of endothelial cells was used to enhance the initial cell attachment to avidin-treated surfaces, in order to improve in vivo patency of vascular grafts [211–215]. The application of this strategy in cell culture and tissue engineering was also explored. It was demonstrated that biotinylated chondrocytes adhered to avidin-coated substrates (tissue culture polystyrene or flat films based on biodegradable polymers), more quickly than untreated chondrocytes to bare substrates [216,217]. The efficiency of the avidin–biotin binding system was also shown for the initial attachment of a biotinylated human hepatoma cell line to avidin adsorbed on the surface of two-dimensional and three-dimensional PLLA scaffolds [218]. Using this approach, avidin-, streptavidin- and neutravidin-modified molecules could be introduced onto scaffold surfaces.
Davis et al. [219] conjugated biotinylated IGF-1 with streptavidin and then bound it to biotinylated self-assembling peptides. The biotin–streptavidin–biotin system enabled IGF-1 attachment to the peptides, without effecting their self-assembly behaviour. After injection into rat myocardium, biotinylated IGF-1 nanofibres provided sustained IGF-1 delivery for 28 days, and delivery of IGF-1 in vivo increased activation of protein kinase B in the myocardium. Moreover, cardiomyocytes added to biotinylated IGF-1 nanofibres showed improved systolic function, a decrease in caspase-3 cleavage by 28% and an increase in the myocyte cross-sectional area by 25%, compared with cells embedded within nanofibres alone or with untethered IGF-1.
3.3. Bulk biological modification with biomolecules
Bulk biomolecule incorporation is another method to introduce biomolecules into polymers during the fabrication process. There are three common techniques for bulk biomolecule incorporation: direct mixing, coaxial electrospinning and self-assembling (amphiphile) peptide nanofibres. When compared with surface functionalization, bulk biomolecule incorporation enables more biomolecules to couple onto the scaffolds. Blending is considered a rapid and simple modification technique in comparison with covalent immobilization and physical adsorption that involve several steps to achieve modification of the scaffold. In addition, the presence of biomolecules both on the surface and inside the material can provide the necessary signals for cell interaction as the synthetic polymer degrades [173]. The biomolecules are embedded in the bulk material and therefore they can be continuously released by diffusion through the polymer or, in the case of biodegradable polymers, by degradation of the matrix.
3.3.1. Direct mixing of biomolecules
Direct mixing or incorporation of biomolecules into the polymer bulk during fabrication is possible if both are soluble in the same solvent. Biomolecules can be dissolved directly into the polymer solution [220], incorporated as a suspension by dissolution in a miscible solvent [221,222], and they can be incorporated as an emulsion by dissolution in an immiscible solvent [223–225]. Alternatively, a multicomponent system can be used when two miscible solvents are not available for a particular polymer–biomolecule system. For example, a three-component system was used for the incorporation of water-soluble heparin into dichloromethane (DCM)-soluble PCL. Heparin was dissolved in water, which is miscible with methanol, which is miscible with DCM [226]. Direct loading is one of the simplest techniques used to incorporate proteins into polymeric matrices. As a drawback, incorporation of proteins using direct loading without further modification usually leads to a fast burst release during the initial swelling phase, as a significant amount of protein originally trapped in the gel network is released [184]. Therefore, controlled release cannot generally be achieved using the direct loading technique, and the release rate is diffusion-controlled [227].
This observation is exemplified in a study reported by Kanematsu et al. [228], in which GFs were added to hydrogels based on collagen type 1. The hydrogels were prepared in aqueous solutions containing bFGF, hepatocyte growth factor (HGF), platelet-derived growth factor-BB (PDGF-BB), VEGF, IGF-1, or heparin-binding epidermal growth factor-like growth factor (HB-EGF). Each GF showed a distinct release profile, as a consequence of the fact that the interaction mechanism between collagen and the different GFs varies. However, in general, the release lasted for some days, and a burst effect was observed [228,229]. Figure 6 schematically shows that VEGF release profile can be modified according to the incorporation method [230].
Figure 6.
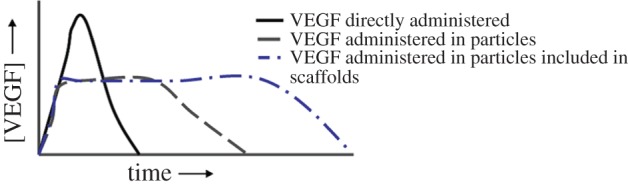
Simplified diagram showing the modulation of VEGF release profile acting on the carrier used to administer it (direct administration, encapsulation in nanoparticles, encapsulation in nanoparticles embedded in a scaffold). Reproduced with permission from [230]. (Online version in colour.)
Structural and surface modifications, such as variations of cross-linking density of the polymer network, change the release profiles [184,230]. For example, degradation time and storage modulus could be varied and controlled for dextran hydrogels (which were formed by mixing aqueous solutions of dextran vinyl sulfone conjugates and tetrafunctional mercapto-poly(ethylene glycol)), varying the composition of vinyl sulfone and dextran molecular weight [231]. bFGF was loaded into dextran hydrogels by mixing the protein with polymer in aqueous solution and it was released with first-order kinetics in 28 days, without showing a burst effect [231].
In recent years, there has been an increasing number of experimental approaches to regenerate the infarcted myocardium by using injectable scaffolds loaded with GFs. Iwakura et al. [232] delivered bFGF via injectable gelatin microspheres, with an average diameter of 10 μm, and reported increased angiogenesis as well as improved cardiac function. An alternative concept was reported by Hsieh et al. [233], who employed self-assembling peptides as a delivery vehicle for PDGF. They reported sustained delivery for 14 days, which decreased cardiomyocytes death and preserved cardiac function, compared with either peptides or GFs alone. Moreover, they demonstrated reduction in infarct size.
In another relevant study, chitosan-gel-containing bFGF was injected into the ischaemic myocardium of rabbits with chronic MI [234], which was found to induce angiogenesis and improve the collateral circulation in the infarcted area. Synthetic hydrogels mimicking the ECM have been also created by cross-linking a thiol-modified analogue of heparin with thiol-modified hyaluronan or chondroitin sulfate with poly(ethylene glycol) diacrylate [235]. The covalently bound heparin provided a cross-linkable analogue of an HS proteoglycan, thus leading to a multivalent biomaterial capable of controlled release of bFGF. This system was found to increase the neovascularization in mice models. The sequential release of VEGF and PDGF from alginate gels was investigated in Fischer rats [236]. The results obtained demonstrated an increased capillary density and blood vessel formation with improved cardiac function in the case of dual delivery compared with the individual delivery of VEGF or PDGF [236]. More recently, the potential application of an affinity binding alginate gel for controlled delivery of HGF was studied in a hindlimb ischaemia model. The HGF-containing alginate gel was demonstrated to induce matured blood vessel network formation [237]. A thermoreversible scaffold, forming a gel at body temperature, has been also developed for VEGF delivery [238]. Such gel exhibited temperature-dependent reversible sol–gel transitions near body temperature and it was found useful for cell/protein delivery in a minimally invasive manner. Not only growth and angiogenic factors, but also other bioactive agents have been loaded into polymeric supports for CTE application. For example, increased neovascularization was reported by delivering a plasmid encoding the angiogeneic growth factor pleiotrophin in fibrin glue [239]. Increased neovasculature formation, reduced infarct size and improved cardiac function have been reported when EPO was delivered intramyocardially through α-cyclodextrin/MPEG–PCL–MPEG hydrogel [137]. In another study, an MMP responsive hydrogel delivering thymosin, a pro-angiogenic and pro-survival factor, was investigated [240]. The gel was able to substitute the degrading ECM in the infarcted myocardium of rats and to promote structural organization of native endothelial cells, whereas some of the delivered hESC-derived vascular endothelial cells formed de novo capillaries in the infarct zone. Moreover, the bioactive hydrogel preserved contractile performance, attenuated LV dilation and decreased infarct size, when compared with infarcted rats treated with PBS injection as a control. In a study published by Ito et al. [241], VEGF was immobilized in photoreactive gelatin. Gelatin and VEGF were mixed in water, cast and photoirradiated. Samples with immobilized VEGF showed growth of HUVECs in comparison with the non-treated surface. Growth of HUVEC increased significantly with an increase in the amount of immobilized VEGF. Micropatterning of HUVEC cultures was also achieved using micropattern-immobilized VEGF using a photomask. In direct incorporation of proteins into hydrogels, the difficult and challenging step of loading the protein has to be considered. If, for example, chemical cross-linking is involved in hydrogel preparation, it is important to evaluate the chemical integrity of the added proteins. Additionally, if loading is performed after cross-linking, the even distribution of the biomolecule within the gel must be verified [184].
Another approach for the direct incorporation of GFs is by electrospinning for the fabrication of fibrous cardiac scaffolds. For example, PLLA electrospun cardiac scaffolds functionalized with G-CSF have been produced. G-CSF was added to a PLLA polymer solution (13 w/w % in DCM) at a concentration of 250 UI g−1. C2C12 murine skeletal myoblasts were seeded on G-CSF modified PLLA electrospun scaffolds. Results demonstrated morphological alteration and induction of Cx43 expression, resembling the usual cardiomyocyte arrangement, characterized by cell elongation and appearance of cellular junctions [138].
In another study, Gao et al. [135] investigated collagen membranes loaded with a fusion protein (consisting of VEGF and a collagen binding domain, CBD-VEGF), natural VEGF and phosphate-buffered saline (PBS), in a rabbit model. The CBD-VEGF/collagen group could effectively induce more cells (endothelial cells and myocardial cells) to penetrate into the collagen membrane after four weeks and promoted vascularization in infarcted myocardium after 12 weeks, compared with control groups. Echocardiography and haemodynamic studies both showed cardiac function improvement in the CBD-VEGF/collagen group. The study showed that CBD-VEGF could be retained longer on the collagen membrane when compared with VEGF, which was rapidly released and diffused by blood flow and body fluid in vivo. It was reported that VEGF concentration plays a crucial role during vascularization [242]. It was shown that the CBD-VEGF/collagen group leads to better results, thanks to a higher content of VEGF than controls.
3.3.2. Carrier systems
When a long-term immobilization of proteins is required, carriers can be added to the scaffold to retain the bioactive molecules for long-term application [243]. This can be achieved by the immobilization of microscopic carriers or drug-releasing implants into the scaffolds [184]. Such delivery systems provide local delivery of biomolecules (local concentration C) in the form of temporal gradients (d[C]/dt) and spatial gradients (d[C]/dx). Moreover, by using carrier systems, proteins can be combined with other biomolecules [244]. Carriers can also provide protection to proteins against inactivation occurring in the biological environment contributing to preserving their bioactivity. Such carriers can be designed in shapes such as micro- or nanoparticles and matrices, to respectively achieve zero- or first-order release kinetics for different biological applications [133]. There are different methods and techniques to prepare biopolymer microparticles, for example the water in oil in water (W/O/W) emulsion. In this method, an aqueous solution of GF is prepared that is emulsified in an organic solution containing the polymer. This primary emulsion is mixed with an external aqueous solution to obtain a double emulsion. Organic solvents are then extracted from the resulting double emulsion to obtain the particles [245]. Both degradable and non-degradable materials can be used in the fabrication of carriers; however, only degradable polymers are being used in CTE.
Biodegradable polymer systems have attracted wide interest as matrices for GFs delivery. In particular, thermoplastic aliphatic polyesters, such as PLA and PLGA, have been investigated largely owing to their demonstrated biocompatibility [34,246]. The degradation of the polymeric matrix determines the release of the bioactive molecule. Indeed, the incorporation of degradable microparticles embedded with GF inside a matrix has been shown to lead to controlled protein release upon microparticle degradation [184]. An example of such a system is a novel scaffold based on degradable PLGA microspheres loaded with bFGF, which were incorporated in a porous alginate matrix [102]. The bFGF led to enhanced proliferation of cardiac fibroblast. The microsphere system was capable of controlling the release of bFGF, thereby promoting vascularization by boosting the formation of large and mature vessels [102]. In another study, heat shock protein 27 (HSP27), fused with transcriptional activator (TAT) derived from human immunodeficiency virus (TAT-HSP27), was loaded in a combination system made of PLGA microspheres and alginate hydrogel. Alginate was cross-linked by calcium ions after the incorporation of TAT-HSP27-loaded PLGA microspheres. It was possible to control the delivery behaviour for a long period of time by changing the microsphere/hydrogel ratio [247]. Apoptotic pathways of cardiomyoblasts cultured under hypoxic conditions were effectively inhibited by the release of TAT-HSP27.
In another relevant development, human embryonic stem cells were encapsulated in dextran-based hydrogel enriched with VEGF-loaded PLGA microparticles [248]. A double emulsion solvent evaporation technique was used to produce PLGA microparticles, and the effect of GF release on vascular differentiation was studied. VEGF-loaded microparticles release kinetics showed a burst effect in the initial phase followed by a lower steady-state release. The release of VEGF from the loaded microparticles was assessed by evaluating the VEGF effect on the survival of an endothelial cell line in vitro [248]. Indeed, biodegradable polymers provide controlled delivery of bioactive molecules enabling specific release concentrations over relatively long periods of time. Furthermore, this strategy gives the opportunity to deliver more than one bioactive agent at different pre-programmed rates, according to the needs of a specific application [246]. Following this approach, a system has been proposed, in which two or more GFs could be delivered with a controlled dose and rate of delivery [249]. VEGF was incorporated by mixing with polymer particles before processing them into a scaffold structure, whereas PDGF was pre-encapsulated into polymeric microspheres. To incorporate the two GFs into the same scaffold, particles encapsulating PDGF and lyophilized VEGF-containing particles were mixed together before scaffold fabrication (figure 7). When implanted in the subcutaneous pockets of the dorsal side of rats, the scaffolds showed enhanced formation of a mature vascular network owing to the dual delivery of VEGF and PDGF [249]. This dual GF system permitted rapid release of VEGF, promoting blood vessel formation and a slower release of PDGF, for blood vessel maturation.
Figure 7.
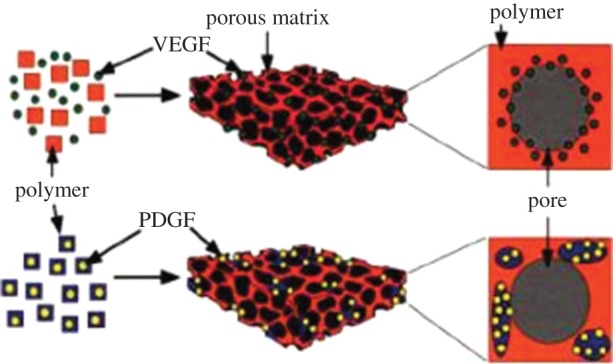
Schematic representation of the scaffold fabrication process with dual GF delivery. Growth factors were incorporated into polymer scaffolds by either mixing with polymer particles before processing into scaffolds (VEGF), or pre-encapsulating the factor (PDGF) into polymer microspheres used to form scaffolds according to Richardson et al. Reproduced with permission from [249]. (Online version in colour.)
Another example of a dual-release system was a scaffold with TGF-1 and IGF-1 immobilized in oligo [poly (ethylene glycol) fumarate] (OPF) hydrogel-containing gelatin microspheres. The GFs were loaded either in the OPF gel or in the gelatin microspheres phase [250,251]. Release profiles were successfully controlled by altering the phase of GF loading and the degree of microparticle cross-linking.
3.3.3. Coaxial incorporation (electrospinning)
In coaxial electrospinning, core shell nanofibres are formed using a special coaxial nozzle, in which one material makes up the core and the other forms the shell. This represents an alternative method when compared with direct mixing of biomolecules in the electrospinning solution [252]. Through a special nozzle, multiple solutions are fed in a concentric extruder, forming a two-phase liquid electrospun jet. As the solvent evaporates, solid core shell nanofibres are formed. Biomolecules can be incorporated inside the nanofibre core, avoiding exposure to the toxic harsh solvents used in the production of the shell [253].
3.3.4. Incorporation of bioactive molecules into self-assembling (amphiphile) peptide nanofibres
Another strategy for bulk incorporation of biomolecules is the incorporation of biofunctional peptide sequences into amphiphile peptides (RAD16-I and RAD16-II), which self-assemble into nanofibres. Several studies have demonstrated that direct injection of biomaterials into the infarcted myocardium prevents deleterious remodelling and reduces cardiac dysfunction [21,254,255]. Using intramyocardial injection of self-assembling peptide nanofibres, a highly biocompatible and biodegradable material was obtained [233,256]. In both small [219,257] and large [258] animals, self-assembling peptide nanofibres have been used as a platform for controlled local delivery of GFs. If GFs or cells are directly injected, the efficacy is reduced by the rapid diffusion from the injection site.
Lin et al. [258] injected mini pigs at the site of infarction with autologous bone marrow mononuclear cells in saline solution or RAD16-II peptide nanofibres or a combination of both. Injection of cells with the nanofibres improved both diastolic and systolic function. More recently, self-assembling RAD16-I peptides were used for dual delivery of PDGF and FGF-2 into a rat infarct, showing a reduction in infarct size, and an increase in capillary and arteriole density, at four and eight weeks post-MI [259]. In another study, PDGF-BB was delivered into rat-infarcted myocardium using injectable self-assembly peptides [233]. PDGF-BB is an endothelium-secreted paracrine factor known to have direct anti-apoptotic effects on cardiomyocytes. The study showed a decrease in cardiomyocyte death and better preservation of systolic function after MI, in comparison with the injection of PDGF-BB or peptide nanofibres by themselves [233].
4. Molecular imprinting technology: an innovative functionalization strategy
Innovative functionalization strategies are gaining increasing interest in the literature. An interesting approach for the creation of advanced synthetic support structures for cell adhesion and proliferation is based on molecular imprinting technology [260–262]. This technology permits the introduction, into a polymeric material, of recognition sites for specific molecular species (templates) through the polymerization of a monomer in the presence of a template [263,264], or through the dissolution of a pre-formed polymer in a solution containing the molecule to be recognized [265]. In both cases, the spatial arrangement is maintained by the polymer, after template extraction, and confers a selective ‘memory’ towards this molecule.
Molecular imprinting technology is a valid alternative to molecular recognition features present in biological systems, such as those activated by antibodies [266]. Macromolecular matrices prepared with this procedure, in fact, can be stable even in critical chemical and physical conditions [267], they can have a life of several years without any apparent reduction in performance and can be used repeatedly without any alteration to the ‘memory’. The most extensively studied applications of molecularly imprinted polymers are mainly in the field of affinity chromatography [268–270], and also in determining the level of drugs in human serum in substitution of natural antibodies. Other applications are in the field of biomimetic sensors [260,261] and intelligent polymers [271]. Interesting developments in the biomaterial field in general [272,273] and in tissue engineering in particular [274,275] have been proposed. Intelligent matrices usable in the field of tissue engineering should be characterized by their particular capacity for molecular recognition of extracellular proteins (collagen, fibronectin, laminin, vitronectin, etc.) that favour cellular processes. These polymeric matrices will be able to improve the adhesion and growth characteristics of cells onto the scaffolds, thanks to the presence of sites that are complementary and selective towards extracellular proteins, recognized by integrins. Molecular imprinting is preferentially performed on biostable polymers in order to guarantee the stability of the recognition sites over time. Considering that biodegradable polymers are a fundamental requisite for tissue-engineered scaffolds, imprinted nanoparticles based on biostable polymers can be used to modify degradable scaffolds. The fate of these particles, upon scaffold degradation, has not been completely ascertained. However, considering the very small amount of particles used, simple elimination by the normal metabolic pathways is likely to occur.
The use of entire proteins as template molecules has often been often unsuccessful because of large molecular dimensions and flexibility of the chains, which limit molecular recognition capacity and selectivity. To overcome these limitations, stable short peptide sequences, representative of accessible fragments of larger proteins of interest are used. These fragments (epitopes) are located in the receptor domains or in other parts directly involved in the molecular recognition process Therefore, if the material can recognize a peptide that represents the exposed part of a protein structure, it will also be able to bind the entire protein [276].
Two different molecular imprinted polymer systems (MIPs), capable of recognizing, respectively, a pentapeptide segment of an exposed part of a fibronectin functional domain and a pentapeptide segment of an exposed part of a laminin functional domain, were synthesized and characterized by some of the authors of this review [274,275]. The pentapeptides H–Gly–Arg–Gly–Asp–Ser–OH (GRGDS) and H–Tyr–Ile–Gly–Ser–Arg–OH (YIGSR) were used as template molecules. GRGDS is a short peptide sequence from fibronectin, the prototype of cell adhesive and bioactive matrix proteins. It has been shown to regulate cell growth, cell shape, cytoskeletal organization, differentiation, migration and apoptosis of almost all cells, including cardiac ones [277–279]. Moreover, GRGDS is able to stimulate integrins (α5β1, αvβ3) that are relevant in early cardiac development [90,91]. YIGSR derives from laminin, the most bioactive component in the basement membrane [280,281]. It is able to increase the ability of stem cells to differentiate into beating cardiomyocytes. The polymers were prepared by polymerization of MAA (in the presence of the template molecule). An appropriate cross-linking agent, trimethylolpropane trimethacrylate or pentaerythritol triacrylate, was added during polymerization to create stable recognition sites in the polymer structure. The obtained materials were characterized for morphological and physico-chemical properties. For both formulations, polymers in the form of densely fused microgels were obtained. The template molecules were removed from the polymers by solvent extraction, and the rebinding ability was verified. An epitope effect was observed, as the extracted polymer was able to recognize both the pentapeptide used as template and larger protein fragments containing the template sequence. Both MIPs showed good performance in terms of recognition capacity and selectivity. Cytotoxicity tests showed viable C2C12 myoblasts cultured in MIPs eluates. MIPs were used for surface modification of tissue engineering supports. The deposition of nanoparticles did not alter their specific recognition and binding behaviour. Functionalized films showed higher quantitative binding than free nanoparticles, suggesting the creation of a preferred microenvironment for the rebinding process. The most remarkable result was obtained in the biological characterization: bioactive scaffolds were able to promote C2C12 myoblast proliferation. These results can be considered very promising and suggested that MIP can be used as an innovative functionalization technique to prepare bioactive scaffolds with an effective capacity of improving tissue regeneration.
5. Conclusions
Infarcted myocardial tissue cannot be treated by conventional methods, thus tissue engineering represents a promising approach. In a common tissue engineering strategy, for the regeneration of cardiac tissue, a scaffold (patch) is required to host the cells and mechanically support the infarcted heart. An ideal CTE scaffold should promote cell attachment and mimic the native tissue, both biologically and mechanically. Many synthetic polymers fit the required mechanical aspect but they lack effective cell–material interaction ability. Therefore, surface and bulk functionalization strategies have been extensively investigated. This review summarizes different functionalization techniques for cardiac tissue scaffolds, based on different physical, chemical and biological modification strategies. Biological modification is essential to enhance cell attachment by incorporating cell recognition sites in or on the material. Analysis of the literature has also revealed that often a combination of different techniques is required to achieve optimal results. The significant progresses and the promising results achieved as documented in the analysed literature indicate that the area of CTE will continue to attract major research efforts in future.
Competing interests
We declare we have no competing interests.
Funding
The authors acknowledge partial funding from the EU FP-7 BIOSCENT project (ID 214539).
References
- 1.Kajstura J, et al. 2010. Myocyte turnover in the aging human heart. Circ. Res. 107, 1374–1386. ( 10.1161/CIRCRESAHA.110.231498) [DOI] [PubMed] [Google Scholar]
- 2.Improvement P, Goldberg LR. 2010. Heart failure. Ann. Intern. Med. 152, ITC61–15; quiz ITC616. [DOI] [PubMed] [Google Scholar]
- 3.Birks E, Tansley P, Hardy J, George R, Bowles C, Burke M, Banner N, Khaghani A, Yacoub M. 2006. Left ventricular assist device and drug therapy for the reversal of heart failure. N. Engl. J. Med. 335, 1873–1884. ( 10.1056/NEJMoa053063) [DOI] [PubMed] [Google Scholar]
- 4.Reffelmann T, Kloner RA. 2003. Cellular cardiomyoplasty—cardiomyocytes, skeletal myoblasts, or stem cells for regenerating myocardium and treatment of heart failure? Cardiovasc. Res. 58, 358–368. ( 10.1016/S0008-6363(02)00739-3) [DOI] [PubMed] [Google Scholar]
- 5.Laflamme M, Murry C. 2005. Regenerating the heart. Nat. Biotechnol. 23, 845–856. ( 10.1038/nbt1117) [DOI] [PubMed] [Google Scholar]
- 6.Leor J, Amsalem Y, Cohen S. 2005. Cells, scaffolds, and molecules for myocardial tissue engineering. Pharmacol. Ther. 105, 151–163. ( 10.1016/j.pharmthera.2004.10.003) [DOI] [PubMed] [Google Scholar]
- 7.Chen Q-Z, Harding SE, Ali NN, Lyon AR, Boccaccini AR. 2008. Biomaterials in cardiac tissue engineering: ten years of research survey. Mater. Sci. Eng. R Rep. 59, 1–37. ( 10.1016/j.mser.2007.08.001) [DOI] [Google Scholar]
- 8.Pons J, Huang Y, Takagawa J, Arakawa-Hoyt J, Ye J, Grossman W, Kan Y, Su H. 2009. Combining angiogenic gene and stem cell therapies for myocardial infarction. J. Gene Med. 11, 743–753. ( 10.1002/jgm.1362) [DOI] [PubMed] [Google Scholar]
- 9.Akins R. 2002. Can tissue engineering mend broken hearts? Circ. Res. 90, 120–122. [PubMed] [Google Scholar]
- 10.Gálvez-Montón C, Prat-Vidal C, Roura S, Soler-Botija C, Bayes-Genis A. 2013. Update: Innovation in cardiology (IV). Cardiac tissue engineering and the bioartificial heart. Rev. Esp. Cardiol. (Engl. Ed). 66, 391–399. ( 10.1016/j.recesp.2012.11.013) [DOI] [PubMed] [Google Scholar]
- 11.Jawad H, Ali NN, Lyon AR, Chen QZ, Harding SE, Boccaccini AR. 2007. Myocardial tissue engineering: a review. J. Tissue Eng. Regen. Med. 1, 327–342. ( 10.1002/term.46) [DOI] [PubMed] [Google Scholar]
- 12.Silvestri A, Boffito M, Sartori S, Ciardelli G. 2013. Biomimetic materials and scaffolds for myocardial tissue regeneration. Macromol. Biosci. 13, 984–1019. ( 10.1002/mabi.201200483) [DOI] [PubMed] [Google Scholar]
- 13.Giraud M-N, Guex AG, Tevaearai HT. 2012. Cell therapies for heart function recovery: focus on myocardial tissue engineering and nanotechnologies. Cardiol. Res. Pract. 2012, 971614 ( 10.1155/2012/971614) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rane AA, Christman KL. 2011. Biomaterials for the treatment of myocardial infarction: a 5-year update. J. Am. Coll. Cardiol. 58, 2615–2629. ( 10.1016/j.jacc.2011.11.001) [DOI] [PubMed] [Google Scholar]
- 15.Jawad H, Lyon A, Harding SE, Ali N, Boccaccini A. 2008. Myocardial tissue engineering. Brit. Med. Bull. 87, 31–47. ( 10.1093/bmb/ldn026) [DOI] [PubMed] [Google Scholar]
- 16.Bhatia S. 2010. Tissue engineering for clinical applications. Biotechnol. J. 5, 1309–1323. ( 10.1002/biot.201000230) [DOI] [PubMed] [Google Scholar]
- 17.Li RK, Jia ZQ, Weisel RD, Mickle DA, Choi A, Yau TM. 1999. Survival and function of bioengineered cardiac grafts. Circulation 100, II63–II69. ( 10.1161/01.CIR.100.15.e63) [DOI] [PubMed] [Google Scholar]
- 18.Leor J, Aboulafia-Etzion S, Dar A, Shapiro L, Barbash IM, Battler A, Granot Y, Cohen S. 2000. Bioengineered cardiac grafts: a new approach to repair the infarcted myocardium? Circulation 102, III56–III61. ( 10.1161/01.CIR.102.suppl_3.III-56) [DOI] [PubMed] [Google Scholar]
- 19.Kofidis T, Akhyari P, Wachsmann B, Boublik J, Mueller-Stahl K, Leyh R, Fischer S, Haverich A. 2002. A novel bioartificial myocardial tissue and its prospective use in cardiac surgery. Eur. J. Cardio-thoracic Surg. 22, 238–243. ( 10.1016/S1010-7940(02)00256-7) [DOI] [PubMed] [Google Scholar]
- 20.Zhong Z, Teo WE, Zhu X, Beuerman R, Ramakrishna S, Yung LYL. 2005. Formation of collagen− glycosaminoglycan blended nanofibrous scaffolds and their biological properties. Biomacromolecules 6, 2998–3004. ( 10.1021/bm050318p) [DOI] [PubMed] [Google Scholar]
- 21.Dai W, Wold LE, Dow JS, Kloner RA. 2005. Thickening of the infarcted wall by collagen injection improves left ventricular function in rats: a novel approach to preserve cardiac function after myocardial infarction. J. Am. Coll. Cardiol. 46, 714–719. ( 10.1016/j.jacc.2005.04.056) [DOI] [PubMed] [Google Scholar]
- 22.Christman KL, Vardanian AJ, Fang Q, Sievers RE, Fok HH, Lee RJ. 2004. Injectable fibrin scaffold improves cell transplant survival, reduces infarct expansion, and induces neovasculature formation in ischemic myocardium. J. Am. Coll. Cardiol. 44, 654–660. ( 10.1016/j.jacc.2004.04.040) [DOI] [PubMed] [Google Scholar]
- 23.Ryu JH, et al. 2005. Implantation of bone marrow mononuclear cells using injectable fibrin matrix enhances neovascularization in infarcted myocardium. Biomaterials 26, 319–326. ( 10.1016/j.biomaterials.2004.02.058) [DOI] [PubMed] [Google Scholar]
- 24.Grover GN, Rao N, Christman KL. 2014. Myocardial matrix–polyethylene glycol hybrid hydrogels for tissue engineering. Nanotechnology 25, 014011 ( 10.1088/0957-4484/25/1/014011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iyer RK, Chiu LLY, Radisic M. 2009. Microfabricated poly(ethylene glycol) templates enable rapid screening of triculture conditions for cardiac tissue engineering. J. Biomed. Mater. Res. A 89, 616–631. ( 10.1002/jbm.a.32014) [DOI] [PubMed] [Google Scholar]
- 26.Mathieu HJ. 2001. Bioengineered material surfaces for medical applications. Surf. Interface Anal. 32, 3–9. ( 10.1002/sia.995) [DOI] [Google Scholar]
- 27.Vogler EA, Siedlecki CA. 2009. Contact activation of blood–plasma coagulation. Biomaterials 30, 1857–1869. ( 10.1016/j.biomaterials.2008.12.041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Von Segesser LK. 1996. Heparin-bonded surfaces in extracorporeal membrane oxygenation for cardiac support. Ann. Thorac. Surg. 61, 330–335. ( 10.1016/0003-4975(95)01011-4) [DOI] [PubMed] [Google Scholar]
- 29.Bordenave L, Fernandez P, Rémy-Zolghadri M, Villars S, Daculsi R, Midy D. 2005. In vitro endothelialized ePTFE prostheses: clinical update 20 years after the first realization. Clin. Hemorheol. Microcirc. 33, 227–234. [PubMed] [Google Scholar]
- 30.Silvestri A, Sartori S, Boffito M, Mattu C, Di Rienzo AM, Boccafoschi F, Ciardelli G. 2014. Biomimetic myocardial patches fabricated with poly(ε-caprolactone) and polyethylene glycol-based polyurethanes. J. Biomed. Mater. Res. B, Appl. Biomater. 102, 1002–1013. ( 10.1002/jbm.b.33081) [DOI] [PubMed] [Google Scholar]
- 31.Guan J, Fujimoto KL, Sacks MS, Wagner WR. 2005. Preparation and characterization of highly porous, biodegradable polyurethane scaffolds for soft tissue applications. Biomaterials 26, 3961–3971. ( 10.1016/j.biomaterials.2004.10.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tjia J, Aneskievich B, Moghe P. 1999. Substrate-adsorbed collagen and cell secreted fibronectin concertedly induce cell migration on poly(lactide-glycolide) substrates. Biomaterials 20, 2223–2233. ( 10.1016/S0142-9612(99)00153-2) [DOI] [PubMed] [Google Scholar]
- 33.Khang G, Lee I, Lee H. 2001. Interaction of cells onto physicochemically treated PLGA surfaces. Tissue Eng. 7, 236. [Google Scholar]
- 34.Lavik E, Langer R. 2015. Tissue engineering: current state and perspectives. Appl. Microbiol. Biotechnol. 65, 1–8. [DOI] [PubMed] [Google Scholar]
- 35.Charlotte KW. 2007. Synthesis of functionalized biodegradable polyesters. Chem. Soc. Rev. 36, 1573–1580. ( 10.1039/b614342n) [DOI] [PubMed] [Google Scholar]
- 36.Lee JW, Serna F, Nickels J, Schmidt CE. 2006. Carboxylic acid-functionalized conductive polypyrrole as a bioactive platform for cell adhesion. Biomacromolecules 7, 1692–1695. ( 10.1021/bm060220q) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerhardt W, Noga D, Hardcastle K, García A, Collard D, Weck M. 2006. Functional lactide monomers: methodology and polymerization. Biomacromolecules 7, 1735–1742. ( 10.1021/bm060024j) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hird B, Eisenberg A. 1993. p-Carboxylation of linear high molecular-mass polystyrene. J. Polym. Sci. A, Polym. Chem. 31, 1377–1381. ( 10.1002/pola.1993.080310604) [DOI] [Google Scholar]
- 39.Beltrami AP, et al. 2003. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114, 763–776. ( 10.1016/S0092-8674(03)00687-1) [DOI] [PubMed] [Google Scholar]
- 40.Matsuura K, et al. 2004. Adult cardiac Sca-1-positive cells differentiate into beating cardiomyocytes. J. Biol. Chem. 279, 11 384–11 391. ( 10.1074/jbc.M310822200) [DOI] [PubMed] [Google Scholar]
- 41.Hench LL, Polak JM. 2002. Third-generation biomedical materials. Science 295, 1014–1017. ( 10.1126/science.1067404) [DOI] [PubMed] [Google Scholar]
- 42.Hersel U, Dahmen C, Kessler H. 2003. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials 24, 4385–4415. ( 10.1016/S0142-9612(03)00343-0) [DOI] [PubMed] [Google Scholar]
- 43.Ruoslahti E. 1996. RGD and other recognition sequences for integrins. Annu. Rev. Cell Dev. Biol. 12, 697–715. ( 10.1146/annurev.cellbio.12.1.697) [DOI] [PubMed] [Google Scholar]
- 44.Hattori S, Andrade JD, Hibbs JBJ, Gregonis DE, King RN. 1985. Fibroblast cell proliferation on charged hydroxyethyl methacrylate copolymers. J. Colloid Interface Sci. 104, 72–78. ( 10.1016/0021-9797(85)90010-4) [DOI] [Google Scholar]
- 45.Hildebrand HF P, Rocher P, Monchau F, Delcourt-Debruyne E. 2002. Kollagen – Aufbereitung und Eigenschaften als Biomaterial. Biomaterialien 3, 14–20. ( 10.1515/BIOMAT.2002.3.1.14) [DOI] [Google Scholar]
- 46.Serpooshan V, Julien M, Nguyen O, Wang H, Li A, Muja N, Henderson JE, Nazhat SN. 2010. Reduced hydraulic permeability of three-dimensional collagen scaffolds attenuates gel contraction and promotes the growth and differentiation of mesenchymal stem cells. Acta Biomater. 6, 3978–3987. ( 10.1016/j.actbio.2010.04.028) [DOI] [PubMed] [Google Scholar]
- 47.Lee CH, Singla A, Lee Y. 2001. Biomedical applications of collagen. Int. J. Pharm. 221, 1–22. ( 10.1016/S0378-5173(01)00691-3) [DOI] [PubMed] [Google Scholar]
- 48.Serpooshan V, Muja N, Marelli B, Nazhat SN. 2011. Fibroblast contractility and growth in plastic compressed collagen gel scaffolds with microstructures correlated with hydraulic permeability. J. Biomed. Mater. Res. A 96A, 609–620. ( 10.1002/jbm.a.33008) [DOI] [PubMed] [Google Scholar]
- 49.Venugopal JR, Prabhakaran MP, Mukherjee S, Ravichandran R, Dan K, Ramakrishna S. 2012. Biomaterial strategies for alleviation of myocardial infarction. J. R. Soc. Interface 9, 20110301 ( 10.1098/rsif.2011.0301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hein S, Schaper J. 2001. The extracellular matrix in normal and diseased myocardium. J. Nucl. Cardiol. 8, 188–196. ( 10.1067/mnc.2001.113331) [DOI] [PubMed] [Google Scholar]
- 51.Park H, Radisic M, Lim JO, Chang BH, Vunjak-Novakovic G. 2005. A novel composite scaffold for cardiac tissue engineering. Vitr. Cell. Dev. Biol. Anim. 41, 188–196. ( 10.1290/0411071.1) [DOI] [PubMed] [Google Scholar]
- 52.Bai XP, Zheng HX, Fang R, Wang TR, Hou XL, Li Y, Chen XB, Tian WM. 2011. Fabrication of engineered heart tissue grafts from alginate/collagen barium composite microbeads. Biomed. Mater. 6, 45002 ( 10.1088/1748-6041/6/4/045002) [DOI] [PubMed] [Google Scholar]
- 53.Hidalgo-Bastida L, Barry J, Everitt N, Rose F, Buttery L, Hall I, Claycomb W, Shakesheff K. 2007. Cell adhesion and mechanical properties of a flexible scaffold for cardiac tissue engineering. Acta Biomater. 3, 457–462. ( 10.1016/j.actbio.2006.12.006) [DOI] [PubMed] [Google Scholar]
- 54.Lundgren E, Terracio L, Mårdh S, Borg TK. 1985. Extracellular matrix components influence the survival of adult cardiac myocytes in vitro. Exp. Cell Res. 158, 371–381. ( 10.1016/0014-4827(85)90462-8) [DOI] [PubMed] [Google Scholar]
- 55.Choi S, Hong Y, Lee I, Huh D, Jeon T-J, Kim SM. 2013. Effects of various extracellular matrix proteins on the growth of HL-1 cardiomyocytes. Cells Tissues Organs 198, 349–356. ( 10.1159/000358755) [DOI] [PubMed] [Google Scholar]
- 56.Battista S, et al. 2005. The effect of matrix composition of 3D constructs on embryonic stem cell differentiation. Biomaterials 26, 6194–6207. ( 10.1016/j.biomaterials.2005.04.003) [DOI] [PubMed] [Google Scholar]
- 57.Santiago L, Nowak R, Peter Rubin J, Marra K. 2006. Peptide-surface modification of poly(caprolactone) with laminin-derived sequences for adipose-derived stem cell applications. Biomaterials 27, 2962–2969. ( 10.1016/j.biomaterials.2006.01.011) [DOI] [PubMed] [Google Scholar]
- 58.Genové E, Shen C, Zhang S, Semino C. 2005. The effect of functionalized self-assembling peptide scaffolds on human aortic endothelial cell function. Biomaterials 26, 3341–3351. ( 10.1016/j.biomaterials.2004.08.012) [DOI] [PubMed] [Google Scholar]
- 59.Ocalan M, Goodman S, Kühl U, Hauschka S, Von der Mark K. 1988. Laminin alters cell shape and stimulates motility and proliferation of murine skeletal myoblasts. Dev. Biol. 125, 158–167. ( 10.1016/0012-1606(88)90068-1) [DOI] [PubMed] [Google Scholar]
- 60.Baharvand H, Azarnia M, Parivar K, Ashtiani S. 2005. The effect of extracellular matrix on embryonic stem cell-derived cardiomyocytes. J. Mol. Cell. Cardiol. 38, 495–503. ( 10.1016/j.yjmcc.2004.12.011) [DOI] [PubMed] [Google Scholar]
- 61.McDevitt T, Angello J, Whitney M, Reinecke H, Hauschka S, Murry C, Stayton P. 2002. In vitro generation of differentiated cardiac myofibers on micropatterned laminin surfaces. J. Biomed. Mater. Res. 60, 472–479. ( 10.1002/jbm.1292) [DOI] [PubMed] [Google Scholar]
- 62.Chiono V, et al. 2014. Polyurethane-based scaffolds for myocardial tissue engineering. Interface Focus 4, 20130045 ( 10.1098/rsfs.2013.0045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McDevitt T, Woodhouse K, Hauschka S, Murry C, Stayton P. 2003. Spatially organized layers of cardiomyocytes on biodegradable polyurethane films for myocardial repair. J. Biomed. Mater. Res. A 66, 586–595. ( 10.1002/jbm.a.10504) [DOI] [PubMed] [Google Scholar]
- 64.Alperin C, Zandstra P, Woodhouse K. 2005. Polyurethane films seeded with embryonic stem cell-derived cardiomyocytes for use in cardiac tissue engineering applications. Biomaterials 26, 7377–7386. ( 10.1016/j.biomaterials.2005.05.064) [DOI] [PubMed] [Google Scholar]
- 65.LaNasa S, Bryant S. 2009. Influence of ECM proteins and their analogs on cells cultured on 2-D hydrogels for cardiac muscle tissue engineering. Acta Biomater. 5, 2929–2938. ( 10.1016/j.actbio.2009.05.011) [DOI] [PubMed] [Google Scholar]
- 66.Brandenberger R, Schmidt A, Linton J, Wang D, Backus C, Denda S, Müller U, Reichardt LF. 2001. Identification and characterization of a novel extracellular matrix protein nephronectin that is associated with integrin α8β1 in the embryonic kidney. J. Cell Biol. 154, 447–458. ( 10.1083/jcb.200103069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Patra C, Diehl F, Ferrazzi F, van Amerongen MJ, Novoyatleva T, Schaefer L, Muhlfeld C, Jungblut B, Engel FB. 2011. Nephronectin regulates atrioventricular canal differentiation via Bmp4-Has2 signaling in zebrafish. Development 138, 4499–4509. ( 10.1242/dev.067454) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Patra C, Ricciardi F, Engel F. 2012. The functional properties of nephronectin: an adhesion molecule for cardiac tissue engineering. Biomaterials 33, 4327–4335. ( 10.1016/j.biomaterials.2012.03.021) [DOI] [PubMed] [Google Scholar]
- 69.Datta A, Ghosh AK, Kundu SC. 2001. Differential expression of the fibroin gene in developmental stages of silkworm, Antheraea mylitta (Saturniidae). Comp. Biochem. Physiol. B, Biochem. Mol. Biol. 129, 197–204. ( 10.1016/S1096-4959(01)00377-3) [DOI] [PubMed] [Google Scholar]
- 70.Cameron VA, Ellmers LJ. 2003. Minireview: Natriuretic peptides during development of the fetal heart and circulation. Endocrinology 144, 2191–2194. [DOI] [PubMed] [Google Scholar]
- 71.Eppenberger-Eberhardt M, Flamme I, Kurer V, Eppenberger HM. 1990. Reexpression of alpha-smooth muscle actin isoform in cultured adult rat cardiomyocytes. Dev. Biol. 139, 269–278. ( 10.1016/0012-1606(90)90296-U) [DOI] [PubMed] [Google Scholar]
- 72.Van Bilsen M, Chien KR. 1993. Growth and hypertrophy of the heart: towards an understanding of cardiac specific and inducible gene expression. Cardiovasc. Res. 27, 1140–1149. ( 10.1093/cvr/27.7.1140) [DOI] [PubMed] [Google Scholar]
- 73.Schaub MC, Hefti MA, Harder BA, Eppenberger HM. 1997. Various hypertrophic stimuli induce distinct phenotypes in cardiomyocytes. J. Mol. Med. 75, 901–920. ( 10.1007/s001090050182) [DOI] [PubMed] [Google Scholar]
- 74.Heydarkhan-Hagvall S, Schenke-Layland K, Dhanasopon AP, Rofail F, Smith H, Wu BM, Shemin R, Beygui RE, MacLellan WR. 2008. Three-dimensional electrospun ECM-based hybrid scaffolds for cardiovascular tissue engineering. Biomaterials 29, 2907–2914. ( 10.1016/j.biomaterials.2008.03.034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang S, Huang Y, Yang X, Mei F, Ma Q, Chen G, Ryu S, Deng X. 2009. Gelatin nanofibrous membrane fabricated by electrospinning of aqueous gelatin solution for guided tissue regeneration. J. Biomed. Mater. Res. A 90, 671–679. ( 10.1002/jbm.a.32136) [DOI] [PubMed] [Google Scholar]
- 76.Zhang Y, Ouyang H, Chwee TL, Ramakrishna S, Huang ZM. 2005. Electrospinning of gelatin fibers and gelatin/PCL composite fibrous scaffolds. J. Biomed. Mater. Res. B Appl. Biomater. 72, 156–165. ( 10.1002/jbm.b.30128) [DOI] [PubMed] [Google Scholar]
- 77.Miskon A, Ehashi T, Mahara A, Uyama H, Yamaoka T. 2009. Beating behavior of primary neonatal cardiomyocytes and cardiac-differentiated P19.CL6 cells on different extracellular matrix components. J. Artif. Organs 12, 111–117. ( 10.1007/s10047-009-0449-4) [DOI] [PubMed] [Google Scholar]
- 78.Lanza R, Langer R, Vacanti JP. 2011. Principles of tissue engineering. San Diego, CA: Academic Press. [Google Scholar]
- 79.Pierschbacher M, Ruoslahti E. 1984. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature 309, 30–33. ( 10.1038/309030a0) [DOI] [PubMed] [Google Scholar]
- 80.Boateng S, Lateef S, Mosley W, Hartman T, Hanley L, Russell B. 2005. RGD and YIGSR synthetic peptides facilitate cellular adhesion identical to that of laminin and fibronectin but alter the physiology of neonatal cardiac myocytes. Am. J. Physiol. Cell Physiol. 288, C30–C38. [DOI] [PubMed] [Google Scholar]
- 81.Yeo Y, Geng W, Ito T, Kohane D, Burdick J, Radisic M. 2007. Photocrosslinkable hydrogel for myocyte cell culture and injection. J. Biomed. Mater. Res. B Appl. Biomater. 81, 312–322. ( 10.1002/jbm.b.30667) [DOI] [PubMed] [Google Scholar]
- 82.Sondermeijer H, See F, Seki T, Witkowski P, Woodland D, Hardy M. 2008. Biocompatible 3-dimensional RGD-modified alginate scaffold designed to enhance cell transplantation to infarcted myocardium. Circulation 118, S322. [Google Scholar]
- 83.Gandaglia A, et al. 2012. Cardiomyocytes in vitro adhesion is actively influenced by biomimetic synthetic peptides for cardiac tissue engineering. Tissue Eng. A 18, 725–736. ( 10.1089/ten.tea.2011.0254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tsur-Gang O, Ruvinov E, Landa N, Holbova R, Feinberg M, Leor J, Cohen S. 2009. The effects of peptide-based modification of alginate on left ventricular remodeling and function after myocardial infarction. Biomaterials 30, 189–195. ( 10.1016/j.biomaterials.2008.09.018) [DOI] [PubMed] [Google Scholar]
- 85.Yu J, Gu Y, Du K, Mihardja S, Sievers R, Lee R. 2009. The effect of injected RGD modified alginate on angiogenesis and left ventricular function in a chronic rat infarct model. Biomaterials 30, 751–756. ( 10.1016/j.biomaterials.2008.09.059) [DOI] [PubMed] [Google Scholar]
- 86.Schussler O, et al. 2009. Use of arginine–glycine–aspartic acid adhesion peptides coupled with a new collagen scaffold to engineer a myocardium-like tissue graft. Nat. Clin. Pr. Cardiovasc. Med. 6, 240–249. ( 10.1038/ncpcardio1451) [DOI] [PubMed] [Google Scholar]
- 87.Yu J, Du K, Fang Q, Gu Y, Mihardja S, Sievers R, Wu J, Lee R. 2010. The use of human mesenchymal stem cells encapsulated in RGD modified alginate microspheres in the repair of myocardial infarction in the rat. Biomaterials 31, 7012–7020. ( 10.1016/j.biomaterials.2010.05.078) [DOI] [PubMed] [Google Scholar]
- 88.Shachar M, Tsur-Gang O, Dvir T, Leor JCS, Shachar M, Tsur-Gang O, Dvir T, Leor J, Cohen S. 2011. The effect of immobilized RGD peptide in alginate scaffolds on cardiac tissue engineering. Acta Biomater. 7, 152–162. ( 10.1016/j.actbio.2010.07.034) [DOI] [PubMed] [Google Scholar]
- 89.Sapir Y, Kryukov O, Cohen S. 2011. Integration of multiple cell–matrix interactions into alginate scaffolds for promoting cardiac tissue regeneration. Biomaterials 32, 1838–1847. ( 10.1016/j.biomaterials.2010.11.008) [DOI] [PubMed] [Google Scholar]
- 90.Maitra N, Flink I, Bahl J, Morkin E. 2000. Expression of alpha and beta integrins during terminal differentiation of cardiomyocytes. Cardiovasc. Res. 47, 715–725. ( 10.1016/S0008-6363(00)00140-1) [DOI] [PubMed] [Google Scholar]
- 91.Ross R, Borg T. 2001. Integrins and the myocardium. Circ. Res. 88, 1112–1119. ( 10.1161/hh1101.091862) [DOI] [PubMed] [Google Scholar]
- 92.Kraehenbuehl T, Zammaretti P, Van der Vlies A, Schoenmakers R, Lutolf M, Jaconi M, Hubbell J. 2008. Three-dimensional extracellular matrix-directed cardioprogenitor differentiation: systematic modulation of a synthetic cell-responsive PEG-hydrogel. Biomaterials 29, 2757–2766. ( 10.1016/j.biomaterials.2008.03.016) [DOI] [PubMed] [Google Scholar]
- 93.Bauer S, Bauer R, Liu Z, Chen H, Goldstein L, Velazquez O. 2005. Vascular endothelial growth factor-C promotes vasculogenesis, angiogenesis, and collagen constriction in three-dimensional collagen gels. J. Vasc. Surg. 41, 699–707. ( 10.1016/j.jvs.2005.01.015) [DOI] [PubMed] [Google Scholar]
- 94.Zisch AH, et al. 2003. Cell-demanded release of VEGF from synthetic, biointeractive cell ingrowth matrices for vascularized tissue growth. FASEB J. 17, 2260–2262. ( 10.1096/fj.02-1041fje) [DOI] [PubMed] [Google Scholar]
- 95.Zisch A, Lutolf M, Hubbell J. 2003. Biopolymeric delivery matrices for angiogenic growth factors. Cardiovasc. Pathol. 12, 295–310. ( 10.1016/S1054-8807(03)00089-9) [DOI] [PubMed] [Google Scholar]
- 96.Huang Y, Kaigler D, Rice K, Krebsbach P, Mooney D. 2005. Combined angiogenic and osteogenic factor delivery enhances bone marrow stromal cell-driven bone regeneration. J. Bone Min. Res. 20, 848–857. ( 10.1359/JBMR.041226) [DOI] [PubMed] [Google Scholar]
- 97.Marra K, Defail A, Clavijo-Alvarez J, Badylak S, Taieb A, Schipper B, Bennett J, Rubin J. 2008. FGF-2 enhances vascularization for adipose tissue engineering. Plast. Reconstr. Surg. 121, 1153–1164. ( 10.1097/01.prs.0000305517.93747.72) [DOI] [PubMed] [Google Scholar]
- 98.Lin H, Chen B, Sun W, Zhao W, Zhao Y, Dai J. 2006. The effect of collagen-targeting platelet-derived growth factor on cellularization and vascularization of collagen scaffolds. Biomaterials 27, 5708–5714. ( 10.1016/j.biomaterials.2006.07.023) [DOI] [PubMed] [Google Scholar]
- 99.Zhao W, et al. 2008. Improved neovascularization and wound repair by targeting human basic fibroblast growth factor (bFGF) to fibrin. J. Mol. Med. 86, 1127–1138. ( 10.1007/s00109-008-0372-9) [DOI] [PubMed] [Google Scholar]
- 100.Elçin Y, Dixit V, Gitnick G. 2001. Extensive in vivo angiogenesis following controlled release of human vascular endothelial cell growth factor: implications for tissue engineering and wound healing. Artif. Organs 25, 558–565. ( 10.1046/j.1525-1594.2001.025007558.x) [DOI] [PubMed] [Google Scholar]
- 101.Andrae J, Gallini R, Betsholtz C. 2008. Role of plateletderived growth factors in physiology and medicine. Genes Dev. 22, 1276–1312. ( 10.1101/gad.1653708) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Perets A, Baruch Y, Weisbuch F, Shoshany G, Neufeld G, Cohen S. 2003. Enhancing the vascularization of three-dimensional porous alginate scaffolds by incorporating controlled release basic fibroblast growth factor microspheres. J. Biomed Mater. Res. A 65, 489–497. ( 10.1002/jbm.a.10542) [DOI] [PubMed] [Google Scholar]
- 103.Nian M, Lee P, Khaper N, Peter L. 2004. Inflammatory cytokines and postmyocardial infarction remodeling. Circ. Res. 94, 1543–1553. ( 10.1161/01.RES.0000130526.20854.fa) [DOI] [PubMed] [Google Scholar]
- 104.Minatoguchi S, et al. 2004. Acceleration of the healing process and myocardial regeneration may be important as a mechanism of improvement of cardiac function and remodeling by postinfarction granulocyte colony-stimulating factor treatment. Circulation 109, 2572–2580. ( 10.1161/01.CIR.0000129770.93985.3E) [DOI] [PubMed] [Google Scholar]
- 105.Takano H, Ohtsuka M, Akazawa H, Toko H, Harada M, Hasegawa H, Nagai T, Komuro I. 2003. Pleiotropic effects of cytokines on acute myocardial infarction: G-CSF as a novel therapy for acute myocardial infarction. Curr. Pharm. 9, 1121–1127. ( 10.2174/1381612033455008) [DOI] [PubMed] [Google Scholar]
- 106.Hiasa K, et al. 2004. Gene transfer of stromal cell-derived factor-1α enhances ischemic vasculogenesis and angiogenesis via vascular endothelial growth factor/endothelial nitric oxide synthase-related pathway: next-generation chemokine therapy for therapeutic neovascularization. Circulation 109, 2454–2461. ( 10.1161/01.CIR.0000128213.96779.61) [DOI] [PubMed] [Google Scholar]
- 107.Zou Y, et al. 2003. Leukemia inhibitory factor enhances survival of cardiomyocytes and induces regeneration of myocardium after myocardial infarction. Circulation 108, 748–753. ( 10.1161/01.CIR.0000081773.76337.44) [DOI] [PubMed] [Google Scholar]
- 108.Musarò A, et al. 2004. Stem cell-mediated muscle regeneration is enhanced by local isoform of insulin-like growth factor 1. Proc. Natl Acad. Sci. USA 101, 1206–1210. ( 10.1073/pnas.0303792101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Calvillo L, Latini R, Kajstura J, Leri A, Anversa P, Ghezzi P, Salio M, Cerami A, Brines M. 2003. Recombinant human erythropoietin protects the myocardium from ischemia–reperfusion injury and promotes beneficial remodeling. Proc. Natl Acad. Sci. USA 100, 4802–4806. ( 10.1073/pnas.0630444100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ladoux A, Frelin C. 1993. Hypoxia is a strong inducer of vascular endothelial growth factor mRNA expression in the heart. Biochem. Biophys. Res. Commun. 195, 1005–1010. ( 10.1006/bbrc.1993.2144) [DOI] [PubMed] [Google Scholar]
- 111.Maruyama K, et al. 1999. Interleukin-1 β upregulates cardiac expression of vascular endothelial growth factor and its receptor KDR/flk-1 via activation of protein tyrosine kinases. J. Mol. Cell Cardiol. 31, 607–617. ( 10.1006/jmcc.1998.0895) [DOI] [PubMed] [Google Scholar]
- 112.Chintalgattu V, Nair D, Katwa L. 2003. Cardiac myofibroblasts: a novel source of vascular endothelial growth factor (VEGF) and its receptors Flt-1 and KDR. J. Mol. Cell Cardiol. 35, 277–286. ( 10.1016/S0022-2828(03)00006-3) [DOI] [PubMed] [Google Scholar]
- 113.Koblizek T, Weiss C, Yancopoulos G, Deutsch U, Risau W. 1998. Angiopoietin-1 induces sprouting angiogenesis in vitro. Curr. Biol. 8, 529–532. ( 10.1016/S0960-9822(98)70205-2) [DOI] [PubMed] [Google Scholar]
- 114.Kutryk MJB, Stewart DJ. 2003. Angiogenesis of the heart. Microsc. Res. Technol. 60, 138–158. ( 10.1002/jemt.10252) [DOI] [PubMed] [Google Scholar]
- 115.Ozawa CR, Banfi A, Glazer NL, Thurston G, Springer ML, Kraft PE, McDonald DM, Blau HM. 2004. Microenvironmental VEGF concentration, not total dose, determines a threshold between normal and aberrant angiogenesis. J. Clin. Invest. 113, 516–527. ( 10.1172/JCI18420) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ohtsuka M, et al. 2004. Cytokine therapy prevents left ventricular remodeling and dysfunction after myocardial infarction through neovascularization. FASEB J. 18, 851–853. ( 10.1096/fj.03-0637fje) [DOI] [PubMed] [Google Scholar]
- 117.Orlic D, et al. 2001. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc. Natl Acad. Sci. USA 98, 10 344–10 349. ( 10.1073/pnas.181177898) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Torella D, et al. 2004. Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 overexpression. Circ. Res. 94, 514–524. ( 10.1161/01.RES.0000117306.10142.50) [DOI] [PubMed] [Google Scholar]
- 119.Winn N, Paul A, Musaró A, Rosenthal N. 2002. Insulin-like growth factor isoforms in skeletal muscle aging, regeneration, and disease. Cold Spring Harb. Symp. Quant. Biol. 67, 507–518. ( 10.1101/sqb.2002.67.507) [DOI] [PubMed] [Google Scholar]
- 120.Ito Y. 2008. Covalently immobilized biosignal molecule materials for tissue engineering. Soft Matter 4, 46–56. ( 10.1039/B708359A) [DOI] [PubMed] [Google Scholar]
- 121.Shen Y, Shoichet M, Radisic M. 2008. Vascular endothelial growth factor immobilized in collagen scaffold promotes penetration and proliferation of endothelial cells. Acta Biomater. 4, 477–489. ( 10.1016/j.actbio.2007.12.011) [DOI] [PubMed] [Google Scholar]
- 122.Losi P, et al. 2010. Tissue response to poly(ether)urethane–polydimethylsiloxane–fibrin composite scaffolds for controlled delivery of pro-angiogenic growth factors. Biomaterials 31, 5336–5344. ( 10.1016/j.biomaterials.2010.03.033) [DOI] [PubMed] [Google Scholar]
- 123.Tengood J, Kovach K, Vescovi P, Russell A, Little S. 2010. Sequential delivery of vascular endothelial growth factor and sphingosine 1-phosphate for angiogenesis. Biomaterials 31, 7805–7812. ( 10.1016/j.biomaterials.2010.07.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chiu L, Radisic M. 2010. Scaffolds with covalently immobilized VEGF and angiopoietin-1 for vascularization of engineered tissues. Biomaterials 31, 226–241. ( 10.1016/j.biomaterials.2009.09.039) [DOI] [PubMed] [Google Scholar]
- 125.Amsden B, Timbart L, Marecak D, Chapanian R, Tse M, Pang S. 2010. VEGF-induced angiogenesis following localized delivery via injectable, low viscosity poly(trimethylene carbonate). J. Control Release 145, 109–115. ( 10.1016/j.jconrel.2010.03.029) [DOI] [PubMed] [Google Scholar]
- 126.Chapanian R, Amsden B. 2010. Combined and sequential delivery of bioactive VEGF165 and HGF from poly(trimethylene carbonate) based photo-cross-linked elastomers. J. Control Release. 143, 53–63. ( 10.1016/j.jconrel.2009.11.025) [DOI] [PubMed] [Google Scholar]
- 127.Jia X, et al. 2011. Sustained release of VEGF by coaxial electrospun dextran/PLGA fibrous membranes in vascular tissue engineering. J. Biomater. Sci. Polym. Ed. 22, 1811–1827. ( 10.1163/092050610X528534) [DOI] [PubMed] [Google Scholar]
- 128.Silva E, Mooney D. 2010. Effects of VEGF temporal and spatial presentation on angiogenesis. Biomaterials 31, 1235–1241. ( 10.1016/j.biomaterials.2009.10.052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zieris A, Prokoph S, Levental K, Welzel P, Grimmer M, Freudenberg U, Werner C. 2010. FGF-2 and VEGF functionalization of starPEG-heparin hydrogels to modulate biomolecular and physical cues of angiogenesis. Biomaterials 31, 7985–7994. ( 10.1016/j.biomaterials.2010.07.021) [DOI] [PubMed] [Google Scholar]
- 130.Zieris A, Chwalek K, Prokoph S, Levental K, Welzel P, Freudenberg U, Werner C. 2011. Dual independent delivery of pro-angiogenic growth factors from starPEG-heparin hydrogels. J. Control Release 156, 28–36. ( 10.1016/j.jconrel.2011.06.042) [DOI] [PubMed] [Google Scholar]
- 131.Miyagi Y, Chiu L, Cimini M, Weisel R, Radisic M, Li R. 2010. Biodegradable collagen patch with covalently immobilized VEGF for myocardial repair. Biomaterials 32, 1280–1290. ( 10.1016/j.biomaterials.2010.10.007) [DOI] [PubMed] [Google Scholar]
- 132.Porter A, Klinge C, Gobin A. 2011. Biomimetic hydrogels with VEGF induce angiogenic processes in both hUVEC and hMEC. Biomacromolecules 12, 242–246. ( 10.1021/bm101220b) [DOI] [PubMed] [Google Scholar]
- 133.Singh S, Wu B, Dunn J. 2011. The enhancement of VEGF-mediated angiogenesis by polycaprolactone scaffolds with surface cross-linked heparin. Biomaterials 32, 2059–2069. ( 10.1016/j.biomaterials.2010.11.038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wu J, Zeng F, Huang X, Chung J, Konecny F, Weisel R, Li R. 2011. Infarct stabilization and cardiac repair with a VEGF-conjugated, injectable hydrogel. Biomaterials 32, 579–586. ( 10.1016/j.biomaterials.2010.08.098) [DOI] [PubMed] [Google Scholar]
- 135.Gao J, Liu J, Gao Y, Wang C, Zhao Y, Chen B, Xiao Z, Miao Q, Dai J. 2011. A myocardial patch made of collagen membranes loaded with collagen-binding human vascular endothelial growth factor accelerates healing of the injured rabbit heart. Tissue Eng. A 17, 2739–2747. ( 10.1089/ten.tea.2011.0105) [DOI] [PubMed] [Google Scholar]
- 136.Garbern J, Minami E, Stayton P, Murry C. 2011. Delivery of basic fibroblast growth factor with a pH-responsive, injectable hydrogel to improve angiogenesis in infarcted myocardium. Biomaterials 32, 2407–2416. ( 10.1016/j.biomaterials.2010.11.075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang T, Jiang X, Lin T, Ren S, Li X, Zhang X, Tang Q. 2009. The inhibition of postinfarct ventricle remodeling without polycythaemia following local sustained intramyocardial delivery of erythropoietin within a supramolecular hydrogel. Biomaterials 30, 4161–4167. ( 10.1016/j.biomaterials.2009.04.033) [DOI] [PubMed] [Google Scholar]
- 138.Spadaccio C, et al. 2011. A G-CSF functionalized scaffold for stem cells seeding: a differentiating device for cardiac purposes. J. Cell. Mol. Med. 15, 1096–1108. ( 10.1111/j.1582-4934.2010.01100.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fiedler U, Augustin H. 2006. Angiopoietins: a link between angiogenesis and inflammation. Trends Immunol. 27, 552–558. ( 10.1016/j.it.2006.10.004) [DOI] [PubMed] [Google Scholar]
- 140.Thurston G, Suri C, Smith K, McClain J, Sato T, Yancopoulos G, McDonald D. 1999. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science (80-.). 286, 2511–2514. ( 10.1126/science.286.5449.2511) [DOI] [PubMed] [Google Scholar]
- 141.Epstein S, Kornowski R, Fuchs S, Dvorak H. 2001. Angiogenesis therapy: amidst the hype, the neglected potential for serious side effects. Circulation. 104, 115–119. ( 10.1161/01.CIR.104.1.115) [DOI] [PubMed] [Google Scholar]
- 142.Peters M, Isenberg B, Rowley J, Mooney D. 1998. Release from alginate enhances the biological activity of vascular endothelial growth factor. J. Biomater. Sci. Polym. Ed. 9, 1267–1278. ( 10.1163/156856298X00389) [DOI] [PubMed] [Google Scholar]
- 143.Xu C, Police S, Rao N, Carpenter M. 2002. Characterization and enrichment of cardiomyocytes derived from human embryonic stem cells. Circ. Res. 91, 501–508. ( 10.1161/01.RES.0000035254.80718.91) [DOI] [PubMed] [Google Scholar]
- 144.Fukuda K. 2001. Development of regenerative cardiomyocytes from mesenchymal stem cells for cardiovascular tissue engineering. Artif Organs. 25, 187–193. ( 10.1046/j.1525-1594.2001.025003187.x) [DOI] [PubMed] [Google Scholar]
- 145.Fukuda K. 2002. Reprogramming of bone marrow mesenchymal stem cells into cardiomyocytes. C. R. Biol. 325, 1027–1038. ( 10.1016/S1631-0691(02)01524-X) [DOI] [PubMed] [Google Scholar]
- 146.Fukuda K. 2003. Use of adult marrow mesenchymal stem cells for regeneration of cardiomyocytes. Bone Marrow Transplant. 32, S25–S27. ( 10.1038/sj.bmt.1703940) [DOI] [PubMed] [Google Scholar]
- 147.Hakuno D, et al. 2002. Bone marrow-derived regenerated cardiomyocytes (CMG Cells) express functional adrenergic and muscarinic receptors. Circulation 105, 380–386. ( 10.1161/hc0302.102593) [DOI] [PubMed] [Google Scholar]
- 148.Rangappa S, Fen C, Lee E, Bongso A, Sim E. 2003. Transformation of adult mesenchymal stem cells isolated from the fatty tissue into cardiomyocytes. Ann. Thorac. Surg. 75, 775–779. ( 10.1016/S0003-4975(02)04568-X) [DOI] [PubMed] [Google Scholar]
- 149.Wobus AM, et al. 2014. Retinoic acid accelerates embryonic stem cell-derived cardiac differentiation and enhances development of ventricular cardiomyocytes. J. Mol. Cell. Cardiol. 29, 1525–1539. ( 10.1006/jmcc.1997.0433) [DOI] [PubMed] [Google Scholar]
- 150.Takahashi T, Lord B, Schulze PC, Fryer RM, Sarang SS, Gullans SR, Lee RT. 2003. Ascorbic acid enhances differentiation of embryonic stem cells into cardiac myocytes. Circulation 107, 1912–1916. ( 10.1161/01.CIR.0000064899.53876.A3) [DOI] [PubMed] [Google Scholar]
- 151.Ventura C, Maioli M. 2000. Opioid peptide gene expression primes cardiogenesis in embryonal pluripotent stem cells. Circ. Res. 87, 189–194. ( 10.1161/01.RES.87.3.189) [DOI] [PubMed] [Google Scholar]
- 152.McBurney MW, Jones-Villeneuve EM, Edwards MK, Anderson PJ. 1982. Control of muscle and neuronal differentiation in a cultured embryonal carcinoma cell line. Nature 299, 165–167. ( 10.1038/299165a0) [DOI] [PubMed] [Google Scholar]
- 153.Skerjanc IS, Petropoulos H, Ridgeway AG, Wilton S. 1998. Myocyte enhancer factor 2C and Nkx2–5 up-regulate each other's expression and initiate cardiomyogenesis in P19 Cells. J. Biol. Chem. 273, 34 904–34 910. ( 10.1074/jbc.273.52.34904) [DOI] [PubMed] [Google Scholar]
- 154.Jiao Y-PY, Cui F-ZF. 2007. Surface modification of polyester biomaterials for tissue engineering. Biomed. Mater. 2, R24–R37. ( 10.1088/1748-6041/2/4/R02) [DOI] [PubMed] [Google Scholar]
- 155.Oh SH, Lee JH. 2013. Hydrophilization of synthetic biodegradable polymer scaffolds for improved cell/tissue compatibility. Biomed. Mater. 8, 14101 ( 10.1088/1748-6041/8/1/014101) [DOI] [PubMed] [Google Scholar]
- 156.Croll T, O'Connor A, Stevens G, Cooper-White J. 2004. Controllable surface modification of poly(lactic-co-glycolic acid) (PLGA) by hydrolysis or aminolysis: I. Physical, chemical, and theoretical aspects. Biomacromolecules 5, 463–473. ( 10.1021/bm0343040) [DOI] [PubMed] [Google Scholar]
- 157.Cao Y, Croll T, Cooper-White J, O’ Connor A, Stevens G. 2004. Production and surface modification of polylactide-based polymeric scaffolds for soft-tissue engineering. In Biopolymer methods in tissue engineering, vol. 238 (eds Hollander A, Hatton P.), pp. 87–111. New York, NY: Humana Press. [DOI] [PubMed] [Google Scholar]
- 158.Chen J, Chu B, Hsiao B. 2006. Mineralization of hydroxyapatite in electrospun nanofibrous poly(l-lactic acid) scaffolds. J. Biomed. Mater. Res. A 79A, 307–317. ( 10.1002/jbm.a.30799) [DOI] [PubMed] [Google Scholar]
- 159.Yu H, Jang J, Kim T, Lee H, Kim H. 2009. Apatite-mineralized polycaprolactone nanofibrous web as a bone tissue regeneration substrate. J. Biomed. Mater. Res. A 88A, 747–754. ( 10.1002/jbm.a.31709) [DOI] [PubMed] [Google Scholar]
- 160.Nisbet D, Yu L, Zahir T, Forsythe J, Shoichet M. 2008. Characterization of neural stem cells on electrospun poly(ε-caprolactone) submicron scaffolds: evaluating their potential in neural tissue engineering. J. Biomater. Sci. Polym. Ed. 19, 623–634. ( 10.1163/156856208784089652) [DOI] [PubMed] [Google Scholar]
- 161.Zhu Y, Leong M, Ong W, Chan-Park M, Chian K. 2007. Esophageal epithelium regeneration on fibronectin grafted poly(l-lactide-co-caprolactone) (PLLC) nanofiber scaffold. Biomaterials 28, 861–868. ( 10.1016/j.biomaterials.2006.09.051) [DOI] [PubMed] [Google Scholar]
- 162.Yamagishi H, Crivello JV, Belfort G. 1995. Evaluation of photochemically modified poly(arylsulfone) ultrafiltration membranes. J. Memb. Sci. 105, 249–259. ( 10.1016/0376-7388(95)00064-J) [DOI] [Google Scholar]
- 163.Janorkar AV, Proulx SE, Metters AT, Hirt DE. 2006. Surface-confined photopolymerization of single- and mixed-monomer systems to tailor the wettability of poly(l-lactide) film. J. Polym. Sci. A, Polym. Chem. 44, 6534–6543. ( 10.1002/pola.21700) [DOI] [Google Scholar]
- 164.Kubies D, Machová L, Brynda E, Lukáš J, Rypáček F. 2003. Functionalized surfaces of polylactide modified by Langmuir–Blodgett films of amphiphilic block copolymers. J. Mater. Sci. Mater. Med. 14, 143–149. ( 10.1023/A:1022019813078) [DOI] [PubMed] [Google Scholar]
- 165.Chu P. 2002. Plasma-surface modification of biomaterials. Mater. Sci. Eng. R Rep. 36, 143–206. ( 10.1016/S0927-796X(02)00004-9) [DOI] [Google Scholar]
- 166.Inagaki N, Narushima K, Lim S. 2003. Effects of aromatic groups in polymer chains on plasma surface modification. J. Appl. Polym. Sci. 89, 96–103. ( 10.1002/app.12160) [DOI] [Google Scholar]
- 167.Wan Y, Qu X, Lu J, Zhu C, Wan L, Yang J, Bei J, Wang S. 2004. Characterization of surface property of poly(lactide-co-glycolide) after oxygen plasma treatment. Biomaterials 25, 4777–4783. ( 10.1016/j.biomaterials.2003.11.051) [DOI] [PubMed] [Google Scholar]
- 168.Barry JJA, Howard D, Shakesheff KM, Howdle SM, Alexander MR. 2006. Using a core-sheath distribution of surface chemistry through 3D tissue engineering scaffolds to control cell ingress. Adv. Mater. 18, 1406–1410. ( 10.1002/adma.200502719) [DOI] [Google Scholar]
- 169.Morent R, De Geyter N, Desmet T, Dubruel P, Leys C. 2011. Plasma surface modification of biodegradable polymers: a review. Plasma Process. Polym. 8, 171–190. ( 10.1002/ppap.201000153) [DOI] [Google Scholar]
- 170.Park K, Ju Y, Son J, Ahn K, Han D. 2007. Surface modification of biodegradable electrospun nanofiber scaffolds and their interaction with fibroblasts. J. Biomater. Sci. Polym. Ed. 18, 369–382. ( 10.1163/156856207780424997) [DOI] [PubMed] [Google Scholar]
- 171.Brown DA, Beygui RE, MacLellan WR, Laks H, Dunn JCY, Wu BM. 2005. Modulation of gene expression in neonatal rat cardiomyocytes by surface modification of polylactide-co-glycolide substrates. J. Biomed. Mater. Res. A 74, 419–429. ( 10.1002/jbm.a.30344) [DOI] [PubMed] [Google Scholar]
- 172.Tay CY, Yu H, Pal M, Leong WS, Tan NS, Ng KW, Leong DT, Tan LP. 2010. Micropatterned matrix directs differentiation of human mesenchymal stem cells towards myocardial lineage. Exp. Cell Res. 316, 1159–1168. ( 10.1016/j.yexcr.2010.02.010) [DOI] [PubMed] [Google Scholar]
- 173.Koh H, Yong T, Chan C, Ramakrishna S. 2008. Enhancement of neurite outgrowth using nano-structured scaffolds coupled with laminin. Biomaterials 29, 3574–3582. ( 10.1016/j.biomaterials.2008.05.014) [DOI] [PubMed] [Google Scholar]
- 174.Ma Z, He W, Yong T, Ramakrishna S. 2005. Grafting of gelatin on electrospun poly(caprolactone) nanofibers to improve endothelial cell spreading and proliferation and to control cell orientation. Tissue Eng. 11, 1149–1158. ( 10.1089/ten.2005.11.1149) [DOI] [PubMed] [Google Scholar]
- 175.Casper C, Yang W, Farach-Carson M, Rabolt J. 2007. Coating electrospun collagen and gelatin fibers with perlecan domain I for increased growth factor binding. Biomacromolecules 8, 1116–1123. ( 10.1021/bm061003s) [DOI] [PubMed] [Google Scholar]
- 176.Duan Y, Wang Z, Yan W, Wang S, Zhang S, Jia J. 2007. Preparation of collagen-coated electrospun nanofibers by remote plasma treatment and their biological properties. J. Biomater. Sci. Polym. Ed. 18, 1153–1164. ( 10.1163/156856207781554019) [DOI] [PubMed] [Google Scholar]
- 177.Lee K, Yoon K, Woo S, Choi I. 2003. Surface modification of poly(glycolic acid) (PGA) for biomedical applications. J. Pharm. Sci. 92, 933–937. ( 10.1002/jps.10556) [DOI] [PubMed] [Google Scholar]
- 178.Patel S, Kurpinski K, Quigley R, Gao H, Hsiao B, Poo M, Li S. 2007. Bioactive nanofibers: synergistic effects of nanotopography and chemical signaling on cell guidance. Nano Lett 7, 2122–2128. ( 10.1021/nl071182z) [DOI] [PubMed] [Google Scholar]
- 179.Chua K, Chai C, Lee P, Ramakrishna S, Leong K, Mao H. 2007. Functional nanofiber scaffolds with different spacers modulate adhesion and expansion of cryopreserved umbilical cord blood hematopoietic stem/progenitor cells. Exp. Hematol. 35, 771–781. ( 10.1016/j.exphem.2007.02.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lee S, Hsiue G, Chang P, Kao C. 1996. Plasma-induced graftedpolymerization of acrylic acid and subsequent grafting of collagen onto polymer film as biomaterials. Biomaterials 17, 1599–1608. ( 10.1016/0142-9612(95)00316-9) [DOI] [PubMed] [Google Scholar]
- 181.Bakowsky U, Schumacher G, Gege C, Schmidt R, Rothe U, Bendas G. 2002. Cooperation between lateral ligand mobility and accessibility for receptor recognition in selectin-induced cell rolling. Biochemistry 41, 4704–4712. ( 10.1021/bi0117596) [DOI] [PubMed] [Google Scholar]
- 182.Houseman B, Mrksich M. 2001. The microenvironment of immobilized Arg–Gly–Asp peptides is an important determinant of cell adhesion. Biomaterials 22, 943–955. ( 10.1016/S0142-9612(00)00259-3) [DOI] [PubMed] [Google Scholar]
- 183.Silva A, Richard C, Bessodes M, Scherman D, Merten O. 2009. Growth factor delivery approaches in hydrogels. Biomacromolecules 10, 9–18. ( 10.1021/bm801103c) [DOI] [PubMed] [Google Scholar]
- 184.Tessmar J, Göpferich A. 2007. Matrices and scaffolds for protein delivery in tissue engineering. Adv. Drug Deliv. Rev. 59, 274–291. ( 10.1016/j.addr.2007.03.020) [DOI] [PubMed] [Google Scholar]
- 185.Fan V, Tamama K, Au A, Littrell R, Richardson L, Wright J, Wells A, Griffith L. 2007. Tethered epidermal growth factor provides a survival advantage to mesenchymal stem cells. Stem Cells 25, 1241–1251. ( 10.1634/stemcells.2006-0320) [DOI] [PubMed] [Google Scholar]
- 186.Koch S, Yao C, Grieb G, Prével P, Noah E, Steffens G. 2006. Enhancing angiogenesis in collagen matrices by covalent incorporation of VEGF. J. Mater. Sci. Mater. Med. 17, 735–741. ( 10.1007/s10856-006-9684-x) [DOI] [PubMed] [Google Scholar]
- 187.Backer M, Patel V, Jehning B, Claffey K, Backer J. 2006. Surface immobilization of active vascular endothelial growth factor via a cysteine-containing tag. Biomaterials 27, 5452–5458. ( 10.1016/j.biomaterials.2006.06.025) [DOI] [PubMed] [Google Scholar]
- 188.Taguchi T, Kishida A, Akashi M, Maruyama I. 2000. Immobilization of human vascular endothelial growth factor (VEGF165) onto biomaterials: an evaluation of the biological activity of immobilized VEGF165. J. Bioact. Compat. Polym. 15, 309–320. ( 10.1106/U857-BGYG-UP8G-C6T3) [DOI] [Google Scholar]
- 189.Chiu LLY, Weisel RD, Li R-K, Radisic M, Chiu L, LY WRD, Li R-K. 2011. Defining conditions for covalent immobilization of angiogenic growth factors onto scaffolds for tissue engineering. J. Tissue Eng. Regen. Med. 5, 69–84. ( 10.1002/term.292) [DOI] [PubMed] [Google Scholar]
- 190.Hlady V, Buijs J. 1996. Protein adsorption on solid surfaces. Curr. Opin. Biotechnol. 7, 72–77. ( 10.1016/S0958-1669(96)80098-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Vogler EA. 2012. Protein adsorption in three dimensions. Biomaterials 33, 1201–1237. ( 10.1016/j.biomaterials.2011.10.059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Park H, Temenoff JS, Tabata Y, Caplan AI, Mikos AG. 2007. Injectable biodegradable hydrogel composites for rabbit marrow mesenchymal stem cell and growth factor delivery for cartilage tissue engineering. Biomaterials 28, 3217–3227. ( 10.1016/j.biomaterials.2007.03.030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Li B, Davidson JM, Guelcher SA. 2009. The effect of the local delivery of platelet-derived growth factor from reactive two-component polyurethane scaffolds on the healing in rat skin excisional wounds. Biomaterials 30, 3486–3494. ( 10.1016/j.biomaterials.2009.03.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Liu X, Won Y, Ma P. 2005. Surface modification of interconnected porous scaffolds. J. Biomed. Mater. Res. A 74, 84–91. ( 10.1002/jbm.a.30367) [DOI] [PubMed] [Google Scholar]
- 195.Park H, Temenoff JS, Holland TA, Tabata Y, Mikos AG. 2005. Delivery of TGF-β1 and chondrocytes via injectable, biodegradable hydrogels for cartilage tissue engineering applications. Biomaterials 26, 7095–7103. ( 10.1016/j.biomaterials.2005.05.083) [DOI] [PubMed] [Google Scholar]
- 196.Taipale J, Keski-Oja J. 1997. Growth factors in the extracellular matrix. FASEB J. 11, 51–59. [DOI] [PubMed] [Google Scholar]
- 197.Sivagnanam V, Song B, Vandevyver C, Gijs MAM. 2009. On-chip immunoassay using electrostatic assembly of streptavidin-coated bead micropatterns. Anal. Chem. 81, 6509–6515. ( 10.1021/ac9009319) [DOI] [PubMed] [Google Scholar]
- 198.Kim D, Karns K, Tia SQ, He M, Herr AE. 2012. Electrostatic protein immobilization using charged polyacrylamide gels and cationic detergent microfluidic western blotting. Anal. Chem. 84, 2533–2540. ( 10.1021/ac3000013) [DOI] [PubMed] [Google Scholar]
- 199.Bishop JR, Schuksz M, Esko JD. 2007. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature 446, 1030–1037. ( 10.1038/nature05817) [DOI] [PubMed] [Google Scholar]
- 200.Gama CI, Tully SE, Sotogaku N, Clark PM, Rawat M, Vaidehi N, Goddard WA, Nishi A, Hsieh-Wilson LC. 2006. Sulfation patterns of glycosaminoglycans encode molecular recognition and activity. Nat. Chem. Biol. 2, 467–473. ( 10.1038/nchembio810) [DOI] [PubMed] [Google Scholar]
- 201.Gallagher J. 2001. Heparan sulfate: growth control with a restricted sequence menu. J. Clin. Invest. 108, 357–361. ( 10.1172/JCI13713) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Jeon O, Kang S-W, Lim H-W, Hyung Chung J, Kim B-S. 2006. Long-term and zero-order release of basic fibroblast growth factor from heparin-conjugated poly(l-lactide-co-glycolide) nanospheres and fibrin gel. Biomaterials 27, 1598–1607. ( 10.1016/j.biomaterials.2005.08.030) [DOI] [PubMed] [Google Scholar]
- 203.Yamaguchi N, Kiick K. 2005. Polysaccharide–poly(ethylene glycol) star copolymer as a scaffold for the production of bioactive hydrogels. Biomacromolecules 6, 1921–1930. ( 10.1021/bm050003+) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Guo H, Cui G, Yang J, Wang C, Zhu J, Zhang L, Jiang J, Shao S. 2012. Sustained delivery of VEGF from designer self-assembling peptides improves cardiac function after myocardial infarction. Biochem. Biophys. Res. Commun. 424, 105–111. ( 10.1016/j.bbrc.2012.06.080) [DOI] [PubMed] [Google Scholar]
- 205.Tae G, Kim Y, Choi W, Kim M, Stayton P, Hoffman A. 2007. Formation of a novel heparin-based hydrogel in the presence of heparin-binding biomolecules. Biomacromolecules 8, 1979–1986. ( 10.1021/bm0701189) [DOI] [PubMed] [Google Scholar]
- 206.Tabata Y. 1998. Protein release from gelatin matrices. Adv. Drug Deliv. Rev. 31, 287–301. ( 10.1016/S0169-409X(97)00125-7) [DOI] [PubMed] [Google Scholar]
- 207.Layman H, Sacasa M, Murphy A, Murphy A, Pham S, Andreopoulos F. 2009. Co-delivery of FGF-2 and G-CSF from gelatin-based hydrogels as angiogenic therapy in a murine critical limb ischemic model. Acta Biomater. 5, 230–239. ( 10.1016/j.actbio.2008.07.024) [DOI] [PubMed] [Google Scholar]
- 208.Kanematsu A, Marui A, Yamamoto S, Ozeki M, Hirano Y, Yamamoto M, Ogawa O, Komeda M, Tabata Y. 2004. Type I collagen can function as a reservoir of basic fibroblast growth factor. J. Control. Release Off. J. Control. Release Soc. 99, 281–292. ( 10.1016/j.jconrel.2004.07.008) [DOI] [PubMed] [Google Scholar]
- 209.Lchek M, Bayer E. 1990. Introduction to avidin–biotin technology. Methods Enzym. 184, 5–13. [DOI] [PubMed] [Google Scholar]
- 210.Bogdanov AJ, Gordeeva L, Baibakov B, Margolis L, Torchilin V. 1991. Restoration of adhesive potentials of Ehrlich ascites carcinoma cells by modification of plasma membrane. J. Cell Physiol. 147, 182–190. ( 10.1002/jcp.1041470123) [DOI] [PubMed] [Google Scholar]
- 211.Bhat VD, Klitzman B, Koger K, Truskey GA, Reichert WM. 1998. Improving endothelial cell adhesion to vascular graft surfaces: clinical need and strategies. J. Biomater. Sci. Polym. Ed 9, 1117–1135. ( 10.1163/156856298X00686) [DOI] [PubMed] [Google Scholar]
- 212.Anamelechi C, Truskey G, Reichert W. 2005. Mylar and Teflon-AF as cell culture substrates for studying endothelial cell adhesion. Biomaterials 26, 6887–6896. ( 10.1016/j.biomaterials.2005.04.027) [DOI] [PubMed] [Google Scholar]
- 213.Mathur A, Chan B, Truskey G, Reichert W. 2003. High-affinity augmentation of endothelial cell attachment: long-term effects on focal contact and actin filament formation. J. Biomed. Mater. Res. A 66, 729–737. ( 10.1002/jbm.a.10581) [DOI] [PubMed] [Google Scholar]
- 214.Mathur A, Truskey G, Reichert W. 2003. Synergistic effect of high-affinity binding and flow preconditioning on endothelial cell adhesion. J. Biomed. Mater. Res. A 64, 155–163. ( 10.1002/jbm.a.10403) [DOI] [PubMed] [Google Scholar]
- 215.Bhat V, Truskey G, Reichert W. 1998. Fibronectin and avidin–biotin as a heterogeneous ligand system for enhanced endothelial cell adhesion. J. Biomed. Mater. Res. 41, 377–385. () [DOI] [PubMed] [Google Scholar]
- 216.Tsai W, Wang M. 2005. Effects of an avidin–biotin binding system on chondrocyte adhesion and growth on biodegradable polymers. Macromol. Biosci. 5, 214–221. ( 10.1002/mabi.200400144) [DOI] [PubMed] [Google Scholar]
- 217.Tsai W, Wang M. 2005. Effect of an avidin–biotin binding system on chondrocyte adhesion, growth and gene expression. Biomaterials 26, 3141–3151. ( 10.1016/j.biomaterials.2004.08.014) [DOI] [PubMed] [Google Scholar]
- 218.Kojima N, Matsuo T, Sakai Y. 2006. Hepatic cell attachment onto biodegradable polymer surfaces without toxicity using an avidin–biotin binding system. Biomaterials 27, 4904–4910. ( 10.1016/j.biomaterials.2006.05.026) [DOI] [PubMed] [Google Scholar]
- 219.Davis ME, Hsieh PCH, Takahashi T, Song Q, Zhang S, Kamm RD, Grodzinsky AJ, Anversa P, Lee RT. 2006. Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proc. Natl Acad. Sci. USA 103, 8155–8160. ( 10.1073/pnas.0602877103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Chew SY, Hufnagel TC, Lim CT, Kam WL. 2006. Mechanical properties of single electrospun drug-encapsulated nanofibres. Nanotechnology 17, 3880–3891. ( 10.1088/0957-4484/17/15/045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kim K, Luu Y, Chang C, Fang D, Hsiao B, Chu B, Hadjiargyrou M. 2004. Incorporation and controlled release of a hydrophilic antibiotic using poly(lactide-co-glycolide)-based electrospun nanofibrous scaffolds. J. Control Release 98, 47–56. ( 10.1016/j.jconrel.2004.04.009) [DOI] [PubMed] [Google Scholar]
- 222.Luu Y, Kim K, Hsiao B, Chu B, Hadjiargyrou M. 2003. Development of a nanostructured DNA delivery scaffold via electrospinning of PLGA and PLA-PEG block copolymers. J. Control Release 341–353. ( 10.1016/S0168-3659(03)00097-X) [DOI] [PubMed] [Google Scholar]
- 223.Chew S, Wen J, Yim E, Leong K. 2005. Sustained release of proteins from electrospun biodegradable fibers. Biomacromolecules 6, 2017–2024. ( 10.1021/bm0501149) [DOI] [PubMed] [Google Scholar]
- 224.Maretschek S, Greiner A, Kissel T. 2008. Electrospun biodegradable nanofiber nonwovens for controlled release of proteins. J. Control Release. 127, 180–187. ( 10.1016/j.jconrel.2008.01.011) [DOI] [PubMed] [Google Scholar]
- 225.Sanders EH, Kloefkorn R, Bowlin GL, Simpson DG, Wnek GE. 2003. Two-phase electrospinning from a single electrified jet: microencapsulation of aqueous reservoirs in poly(ethylene-co vinylacetate) fibers. Macromolecules 36, 3803–3805. ( 10.1021/ma021771l) [DOI] [Google Scholar]
- 226.Luong-Van E, Grondahl L, Chua KN, Leong KW, Nurcombe V, Cool SM. 2006. Controlled release of heparin from poly(ε-caprolactone) electrospun fibers. Biomaterials 27, 2042–2050. ( 10.1016/j.biomaterials.2005.10.028) [DOI] [PubMed] [Google Scholar]
- 227.Tabata Y. 2000. The importance of drug delivery systems in tissue engineering. Pharm. Sci. Technol. Today 3, 80–89. ( 10.1016/S1461-5347(00)00242-X) [DOI] [PubMed] [Google Scholar]
- 228.Kanematsu A, Yamamoto S, Ozeki M, Noguchi T, Kanatani I, Ogawa O, Tabata Y. 2004. Collagenous matrices as release carriers of exogenous growth factors. Biomaterials 25, 4513–4520. ( 10.1016/j.biomaterials.2003.11.035) [DOI] [PubMed] [Google Scholar]
- 229.Wachiralarpphaithoon C, Iwasaki Y, Akiyoshi K. 2007. Enzyme-degradable phosphorylcholine porous hydrogels cross-linked with polyphosphoesters for cell matrices. Biomaterials 28, 984–993. ( 10.1016/j.biomaterials.2006.10.024) [DOI] [PubMed] [Google Scholar]
- 230.Simón-Yarza T. 2012. Vascular endothelial growth factor-delivery systems for cardiac repair: an overview. Theranostics. 2, 541–552. ( 10.7150/thno.3682) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Hiemstra C, Zhong Z, Van Steenbergen M, Hennink W, Feijen J. 2007. Release of model proteins and basic fibroblast growth factor from in situ forming degradable dextran hydrogels. J. Control Release 122, 71–78. ( 10.1016/j.jconrel.2007.06.011) [DOI] [PubMed] [Google Scholar]
- 232.Iwakura A, Fujita M, Kataoka K, Tambara K, Sakakibara Y, Komeda M, Tabata Y. 2003. Intramyocardial sustained delivery of basic fibroblast growth factor improves angiogenesis and ventricular function in a rat infarct model. Hear. Vessel. 18, 93–99. ( 10.1007/s10380-002-0686-5) [DOI] [PubMed] [Google Scholar]
- 233.Hsieh PCH, Davis ME, Gannon J, MacGillivray C, Lee RT. 2006. Controlled delivery of PDGF-BB for myocardial protection using injectable self-assembling peptide nanofibers. J. Clin. Invest. 116, 237–248. ( 10.1172/JCI25878) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Fujita M, et al. 2005. Efficacy of photocrosslinkable chitosan hydrogel containing fibroblast growth factor-2 in a rabbit model of chronic myocardial infarction. J. Surg. Res. 126, 27–33. ( 10.1016/j.jss.2004.12.025) [DOI] [PubMed] [Google Scholar]
- 235.Cai S, Liu Y, Zheng Shu X, Prestwich G. 2005. Injectable glycosaminoglycan hydrogels for controlled release of human basic fibroblast growth factor. Biomaterials 26, 6054 ( 10.1016/j.biomaterials.2005.03.012) [DOI] [PubMed] [Google Scholar]
- 236.Hao X, et al. 2007. Angiogenic effects of sequential release of VEGF-A165 and PDGF-BB with alginate hydrogels after myocardial infarction. Cardiovasc. Res. 75, 178–185. ( 10.1016/j.cardiores.2007.03.028) [DOI] [PubMed] [Google Scholar]
- 237.Ruvinov E, Leor J, Cohen S. 2010. The effects of controlled HGF delivery from an affinity-binding alginate biomaterial on angiogenesis and blood perfusion in a hindlimb ischemia model. Biomaterials 31, 4573–4582. ( 10.1016/j.biomaterials.2010.02.026) [DOI] [PubMed] [Google Scholar]
- 238.Jeong B, Bae Y, Lee D, Kim S. 1997. Biodegradable block copolymers as injectable drug-delivery systems. Nature 388, 860–862. ( 10.1038/42218) [DOI] [PubMed] [Google Scholar]
- 239.Christman K, Fang Q, Yee M, Johnson K, Sievers R, Lee R. 2005. Enhanced neovasculature formation in ischemic myocardium following delivery of pleiotrophin plasmid in a biopolymer. Biomaterials 26, 1139–1144. ( 10.1016/j.biomaterials.2004.04.025) [DOI] [PubMed] [Google Scholar]
- 240.Kraehenbuehl T, Ferreira L, Hayward A, Nahrendorf M, van der Vlies A, Vasile E, Weissleder R, Langer R, Hubbell J. 2011. Human embryonic stem cell-derived microvascular grafts for cardiac tissue preservation after myocardial infarction. Biomaterials 32, 1102–1109. ( 10.1016/j.biomaterials.2010.10.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Ito Y, Hasuda H, Terai H, Kitajima T. 2005. Culture of human umbilical vein endothelial cells on immobilized vascular endothelial growth factor. J Biomed Mater Res A. 74, 659–665. ( 10.1002/jbm.a.30360) [DOI] [PubMed] [Google Scholar]
- 242.Davies N, Dobner S, Bezuidenhout D, Schmidt C, Beck M, Zisch AH, Zilla P. 2008. The dosage dependence of VEGF stimulation on scaffold neovascularisation. Biomaterials 29, 3531–3538. ( 10.1016/j.biomaterials.2008.05.007) [DOI] [PubMed] [Google Scholar]
- 243.Ennett A, Kaigler D, Mooney D. 2006. Temporally regulated delivery of VEGF in vitro and in vivo. J. Biomed. Mater. Res. A 79, 176–184. ( 10.1002/jbm.a.30771) [DOI] [PubMed] [Google Scholar]
- 244.Fischbach C, Mooney D. 2007. Polymers for pro- and anti-angiogenic therapy. Biomaterials 28, 2069–2076. ( 10.1016/j.biomaterials.2006.12.029) [DOI] [PubMed] [Google Scholar]
- 245.Péan J, Menei P, Morel O, Montero-Menei C, Benoit J. 2000. Intraseptal implantation of NGF-releasing microspheres promote the survival of axotomized cholinergic neurons. Biomaterials 21, 2097–2101. ( 10.1016/S0142-9612(00)00141-1) [DOI] [PubMed] [Google Scholar]
- 246.Biondi M, Ungaro F, Quaglia F, Netti P. 2008. Controlled drug delivery in tissue engineering. Adv. Drug Deliv. Rev. 60, 229–242. ( 10.1016/j.addr.2007.08.038) [DOI] [PubMed] [Google Scholar]
- 247.Lee J, Tan C, Lee S, Kim Y, Lee K. 2009. Controlled delivery of heat shock protein using an injectable microsphere/hydrogel combination system for the treatment of myocardial infarction. J. Control Release 137, 196–202. ( 10.1016/j.jconrel.2009.04.008) [DOI] [PubMed] [Google Scholar]
- 248.Ferreira L, Gerecht S, Fuller J, Shieh H, Vunjak-Novakovic G, Langer R. 2007. Bioactive hydrogel scaffolds for controllable vascular differentiation of human embryonic stem cells. Biomaterials 28, 2706–2717. ( 10.1016/j.biomaterials.2007.01.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Richardson T, Peters M, Ennett A, Mooney D. 2001. Polymeric system for dual growth factor delivery. Nat. Biotechnol. 19, 1029–1034. ( 10.1038/nbt1101-1029) [DOI] [PubMed] [Google Scholar]
- 250.Holland T, Tabata Y, Mikos A. 2005. Dual growth factor delivery from degradable oligo(poly(ethylene glycol) fumarate) hydrogel scaffolds for cartilage tissue engineering. J. Control Release 101, 111–125. ( 10.1016/j.jconrel.2004.07.004) [DOI] [PubMed] [Google Scholar]
- 251.Holland T, Bodde E, Cuijpers V, Baggett L, Tabata Y, Mikos A, Jansen J. 2007. Degradable hydrogel scaffolds for in vivo delivery of single and dual growth factors in cartilage repair. Osteoarthr. Cartil. 15, 187–197. ( 10.1016/j.joca.2006.07.006) [DOI] [PubMed] [Google Scholar]
- 252.Sun Z, Zussman E, Yarin A, Wendorff J, Greiner A. 2003. Compound core–shell polymer nanofibers by co-electrospinning. Adv. Mater. 15, 1929–1932. ( 10.1002/adma.200305136) [DOI] [Google Scholar]
- 253.Zhang Y, Wang X, Feng Y, Li J, Lim C, Ramakrishna S. 2006. Coaxial electrospinning of (fluorescein isothiocyanate-conjugated bovine serum albumin)-encapsulated poly(ε-caprolactone) nanofibers for sustained release. Biomacromolecules 7, 1049–1057. ( 10.1021/bm050743i) [DOI] [PubMed] [Google Scholar]
- 254.Wall ST, Walker JC, Healy KE, Ratcliffe MB, Guccione JM. 2006. Theoretical impact of the injection of material into the myocardium: a finite element model simulation. Circulation 114, 2627–2635. ( 10.1161/CIRCULATIONAHA.106.657270) [DOI] [PubMed] [Google Scholar]
- 255.Landa N, Miller L, Feinberg MS, Holbova R, Shachar M, Freeman I, Cohen S, Leor J. 2008. Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation 117, 1388–1396. ( 10.1161/CIRCULATIONAHA.107.727420) [DOI] [PubMed] [Google Scholar]
- 256.Davis ME, Motion JPM, Narmoneva DA, Takahashi T, Hakuno D, Kamm RD, Zhang S, Lee RT. 2005. Injectable self-assembling peptide nanofibers create intramyocardial microenvironments for endothelial cells. Circulation 111, 442–450. ( 10.1161/01.CIR.0000153847.47301.80) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Dubois G, Segers VFM, Bellamy V, Sabbah L, Peyrard S, Bruneval P, Hagge AA, Lee RT, Menasch P. 2008. Self-assembling peptide nanofibers and skeletal myoblast transplantation in infarcted myocardium. J. Biomed. Mater. Res. B, Appl. Biomater. 87, 222–228. ( 10.1002/jbm.b.31099) [DOI] [PubMed] [Google Scholar]
- 258.Lin YD, et al. 2010. Intramyocardial peptide nanofiber injection improves postinfarction ventricular remodeling and efficacy of bone marrow cell therapy in pigs. Circulation 122(Suppl. 11), S132–141. ( 10.1161/CIRCULATIONAHA.110.939512) [DOI] [PubMed] [Google Scholar]
- 259.Kim JH, Jung Y, Kim SH, Sun K, Choi J, Kim HC, Park Y, Kim SH. 2011. The enhancement of mature vessel formation and cardiac function in infarcted hearts using dual growth factor delivery with self-assembling peptides. Biomaterials 32, 6080–6088. [DOI] [PubMed] [Google Scholar]
- 260.Mosbach K, Ramström O. 1996. The emerging technique of molecular imprinting and its future impact on biotechnology. Biotechnology 14, 163–170. ( 10.1038/nbt0296-163) [DOI] [Google Scholar]
- 261.Shea K. 1994. Molecular imprinting of synthetic network polymers: the de novo synthesis of macromolecular binding and catalytic sites. Trends Polym. Sci. 2, 166–173. [Google Scholar]
- 262.Steinke J, Sherrington D, Dunkin I. 1995. Imprinting of synthetic polymers using molecular templates. Adv. Polym. Sci. 123, 80–125. [Google Scholar]
- 263.Mosbach K. 1994. Molecular imprinting. Trends Biochem. Sci. 19, 9–14. ( 10.1016/0968-0004(94)90166-X) [DOI] [PubMed] [Google Scholar]
- 264.Wulff G, Knorr K. 2002. Stoichiometric noncovalent interaction in molecular imprinting. Bioseparation 10, 257–276. ( 10.1023/A:1021585518592) [DOI] [PubMed] [Google Scholar]
- 265.Wang H, Kobayashi T, Fujii N. 1996. Molecular imprint membranes prepared by the phase inversion precipitation technique. Langmuir 12, 4850–4856. ( 10.1021/la960243y) [DOI] [Google Scholar]
- 266.Wulff G. 1995. Molecular imprinting in cross-linked materials with the aid of molecular templates—a way towards artificial antibodies. Angew. Chem. Int. Ed. Engl. 34, 1812–1832. ( 10.1002/anie.199518121) [DOI] [Google Scholar]
- 267.Svenson J, Nicholls IA. 2001. On the thermal and chemical stability of molecularly imprinted polymers. Anal. Chim. Acta 435, 19–24. ( 10.1016/S0003-2670(00)01396-9) [DOI] [Google Scholar]
- 268.Sellergren B, Shea K. 1993. Chiral ion exchange chromatography: correlation between solute retention and a theoretical ion-exchange model using imprinted polymers. J. Chromatogr. A 654, 17–28. ( 10.1016/0021-9673(93)83061-V) [DOI] [PubMed] [Google Scholar]
- 269.Kempe M, Mosbach K. 1995. Separation of amino acids, peptides and proteins on molecularly imprinted stationary phases. J. Chromatogr. A 691, 317–323. ( 10.1016/0021-9673(94)00820-Y) [DOI] [PubMed] [Google Scholar]
- 270.Hart B, Shea K. 2001. Synthetic peptide receptors: molecularly imprinted polymers for the recognition of peptides using peptide–metal interactions. J. Am. Chem. Soc. 123, 2072–2073. ( 10.1021/ja005661a) [DOI] [PubMed] [Google Scholar]
- 271.Cormack PAG, Mosbach K. 1999. Molecular imprinting: recent developments and the road ahead. React. Funct. Polym. 41, 115–124. ( 10.1016/S1381-5148(99)00024-3) [DOI] [Google Scholar]
- 272.Cristallini C, Ciardelli G, Barbani N, Giusti P. 2004. Acrylonitrile–acrylic acid copolymer membrane imprinted with uric acid for clinical uses. Macromol. Biosci. 4, 31–38. ( 10.1002/mabi.200300026) [DOI] [PubMed] [Google Scholar]
- 273.Silvestri D, Borrelli C, Giusti P, Cristallini C, Ciardelli G. 2005. Polymeric devices containing imprinted nanospheres: a novel approach to improve recognition in water for clinical uses. Anal. Chim. Acta 542, 3–13. ( 10.1016/j.aca.2004.12.005) [DOI] [Google Scholar]
- 274.Rosellini E, Barbani N, Giusti P, Ciardelli G, Cristallini C. 2010. Novel bioactive scaffolds with fibronectin recognition nanosites based on molecular imprinting technology. J. Appl. Polym. Sci. 118, 3236–3244. ( 10.1002/app.32622) [DOI] [Google Scholar]
- 275.Rosellini E, Barbani N, Giusti P, Ciardelli G, Cristallini C. 2010. Molecularly imprinted nanoparticles with recognition properties towards a laminin H–Tyr–Ile–Gly–Ser–Arg–OH sequence for tissue engineering applications. Biomed. Mater. 5, 11 ( 10.1088/1748-6041/5/6/065007) [DOI] [PubMed] [Google Scholar]
- 276.Rachkova A, Norihiko M. 2001. Towards molecularly imprinted polymers selective to peptides and proteins. The epitope approach. Biochim. Biophys. Acta 1544, 255–266. ( 10.1016/S0167-4838(00)00226-0) [DOI] [PubMed] [Google Scholar]
- 277.Akiyama SK, Yamada KM. 1987. Fibronectin. Adv. Enzymol. Relat. Areas Mol. Biol. 59, 1–57. ( 10.1002/9780470123058.ch1) [DOI] [PubMed] [Google Scholar]
- 278.Humphries MJ, Obara M, Olden K, Yamada KM. 1989. Role of fibronectin in adhesion, migration, and metastasis. Cancer Invest. 7, 373–393. ( 10.3109/07357908909039866) [DOI] [PubMed] [Google Scholar]
- 279.Larsen M, Artym VV, Green JA, Yamada KM. 2006. The matrix reorganized: extracellular matrix remodeling and integrin signaling. Curr. Opin. Cell. Biol. 18, 463–471. ( 10.1016/j.ceb.2006.08.009) [DOI] [PubMed] [Google Scholar]
- 280.Colognato H, Yurchenco PD. 2000. Form and function: the laminin family of heterotrimers. Dev. Dyn. 218, 213–234. () [DOI] [PubMed] [Google Scholar]
- 281.Miner JH, Yurchenco PD. 2004. Laminin functions in tissue morphogenesis. Annu. Rev. Cell Dev. Biol. 20, 255–284. ( 10.1146/annurev.cellbio.20.010403.094555) [DOI] [PubMed] [Google Scholar]