Abstract
Background
Visceral adiposity and obstructive sleep apnoea (OSA) may be independently associated with daytime sleepiness/low performance, insulin resistance, hypercytokinaemia, and/or hypertension. The objectives of this study are to simultaneously test these associations at baseline and after 3 months of continuous positive airway pressure (CPAP) therapy.
Materials and methods
Sixteen obese men with OSA; 13 non-apnoeic, obese controls, and 15 non-obese controls were monitored in the sleep laboratory for four consecutive nights. Objective measures of daytime sleepiness and performance, serial 24 h plasma measures of interleukin-6 (IL-6), tumour necrosis factor-α (TNF-α), TNF receptor 1 (TNF-r1) and adiponectin, fasting blood glucose and insulin, visceral adiposity and blood pressure were obtained. Sleep apnoeics were re-assessed using the same protocol after 3 months of CPAP.
Results
At baseline, IL-6, TNF-r1, and insulin resistance were highest in OSA patients, intermediate in obese controls, and lowest in non-obese controls (P < 0·05). Visceral fat was significantly greater in sleep apnoeics than obese controls and predicted insulin resistance and IL-6 levels, whereas OSA predicted TNF-r1 levels (P < 0·05). CPAP decreased daytime sleepiness and blood pressure (P < 0·05), but did not affect fasting glucose or insulin or around the clock adiponectin, IL-6, TNF-α, or TNF-r1 levels.
Conclusions
In obese sleep apnoeics, visceral fat is strongly associated with insulin resistance and inflammation. CPAP decreases sleepiness and moderates hypertension but does not affect visceral adiposity, insulin resistance, hypoadiponectinaemia or hypercytokinaemia, all of which are independent risk factors for cardiovascular disease and diabetes.
Keywords: CPAP, inflammation, sleep apnoea, visceral fat
Introduction
Obstructive sleep apnoea (OSA) is a prevalent disorder among middle-aged, obese men and is associated with considerable morbidity, including excessive daytime sleepiness, hypertension and cardiovascular disease, as well as cardiovascular and accident-related mortality [1,2]. Since 1997, we and others have shown that OSA in obese men is associated with visceral adiposity, hyperinsulinaemia, and elevation of circulating pro-inflammatory cytokines [3–8].
Visceral fat is strongly associated with metabolic abnormalities including insulin resistance [9] and its tissue secretes a higher level of pro-inflammatory cytokines, i.e, interleukin-6 (IL-6), compared to subcutaneous fat [10]. In addition, it appears that visceral adiposity, and not just obesity, is what predicts who among the obese is at risk for sleep apnoea, and has been proposed as a principal culprit progressively leading to that disorder [4,8]. However, there is a paucity of studies on the role of visceral adiposity in the pathogenesis of sleep apnoea and in metabolic aberrations associated with this sleep disorder.
Continuous positive airway pressure (CPAP) is the most widely accepted treatment for OSA, with beneficial effects on daytime sleepiness and high blood pressure in patients with moderate or severe OSA [11,12]. It appears that these beneficial effects are mediated by the effects of CPAP on the activation of the stress system, including both the sympathetic system and the hypothalamic–pituitary–adrenal axis, present in this condition [13,14]. There are very limited data on the effects of CPAP on visceral adiposity and inflammatory aberrations in the obese with OSA, whereas its effects on insulin resistance are inconsis tent [6,8]. Such information is important given that all these conditions are independent risk factors that affect health adversely and increase cardiovascular morbidity and mortality [9,15,16].
The purpose of this study was to examine the separate association of visceral adiposity or sleep apnoea with daytime sleepiness/low performance, insulin resistance, inflammation and hypertension at baseline and after 3 months of CPAP therapy. To accomplish this, we conducted a controlled study that included three groups, i.e. obese with sleep apnoea, obese without sleep apnoea, and non-obese controls. We studied these three groups at baseline with night time polysomnography; objective and subjective measures of daytime sleepiness and performance; blood pressure recordings; serial 24-h plasma measures of the pro-inflammatory cytokines IL-6, tumour necrosis factor alpha (TNF-α) and tumour necrosis factor receptor 1 (TNF-r1); single morning measures of fasting glucose and insulin; serial 24-h plasma measures of the insulin sensitivity-promoting hormone adiponectin; and visceral fat adiposity evaluated by computerized tomography (CT).
Materials and methods
Study population
The study was completed by 16 obese, middle-aged men with sleep apnoea, 13 non-apnoeic, age and body mass index (BMI) comparable to obese controls, and 15 age comparable non-obese controls.
The subjects were recruited from the Sleep Disorders Clinic and through advertisements from the community.
To qualify for the study, apnoeic patients had to have apnoea of sufficient severity to warrant recommendation for treatment [4]. Control subjects who demonstrated an apnoea/hypopnoea index (A/HI) of more than five events h−1 of sleep were excluded from the study. Also, apnoeics and control subjects with a diagnosis of type 2 diabetes mellitus or who were receiving treatment with psychotropics, steroids, sympathomimetics, or sympatholytics, including β-blockers, were excluded from the study. The study was approved by the Institutional Review Board, and each subject signed a written consent form.
Procedures
Sleep laboratory
A thorough medical assessment, including physical examination, routine laboratory tests and sleep history, was completed for each patient and control subject. Blood pressure was measured in the evening and was the average of three consecutive readings during a 5 min period following 10 min of rest in the supine position. All potential participants were screened in the sleep laboratory for 1 night for 8 h employing standard polysomnographic procedures [17]. The subjects who met the inclusion criteria were monitored in the sleep laboratory for 4 consecutive nights (1 adaptation and 3 baseline nights). The patients with sleep apnoea were reassessed with the same protocol after at least 3 months of closely monitored nightly use of CPAP. The sleep records were scored independently of any knowledge of the experimental conditions according to standardized criteria [17]. Also, the respiratory data were quantified as previously described [4].
Assessment of daytime sleepiness and performance
Multiple Sleep Latency Test (MSLT)
During the fourth day (day of blood sampling), the subjects’ levels of sleepiness and alertness were evaluated using MSLT [18]. In our study, we allowed six 20 min opportunities to sleep at 0900, 1200, 1500, 1700, 1900 and 2100 h. Onset of sleep was defined as attaining any sleep stage for a duration of one epoch (30 s) or longer. The MSLT was terminated 20 min after lights out if there had been no sleep or after two consecutive epochs of Stage 2 sleep.
Subjective levels of sleepiness
These were assessed using a 10 cm visual analogue scale that ranged from 0 (extremely sleepy) to 10 (not sleepy at all) and the Stanford Sleepiness Scale (a seven point question: ‘How sleepy do you feel right now?’), which were administered every hour. Also, at baseline, subjects completed the Epworth Sleepiness Scale (ESS). Psychomotor Vigilance Task (PVT). PVT is a test of behavioural alertness [19] that was administered every hour from 0800 to 2200 during the blood draw days.
CPAP use
All 16 patients with sleep apnoea used CPAP for 3 months. The optimal nasal CPAP pressure was determined during a full night polysomnographic study as the pressure necessary to abolish all respiratory events and snoring, secondary arousals, and episodes of SaO2 desaturation during REM sleep and in the supine position. To assure adherence, we monitored closely the CPAP use on a daily basis by calculating the time that the patient was breathing through the machine and not just the time the machine was on (Auto Set T, Smart Start, ResMed Corp. Paway, CA, USA). Furthermore, a respiratory therapist visited the home of each patient weekly for the first 4 weeks and monthly thereafter to provide us with information regarding CPAP usage (number of hours used each day), pressure setting, and mask leakage. As regular users of CPAP were considered, those who used the apparatus for ≥ 4 h per night for ≥5 nights out of 7 nights [20]; 15 out of the 16 patients met the criteria of regular users. The average nightly use of CPAP on the nights that CPAP was used was 5·3 ± 0·4 h. The average CPAP use per night, independent of whether CPAP was used or not, was 4·6 ± 0·4 h. When we used, as a criterion of satisfactory compliance, CPAP use per night ≥4 h, 6 out of the 16 patients showed ‘low adherence’ to therapy.
Twenty-four hour blood sampling
Twenty-four hour blood sampling was performed serially, every 30 min, on the fourth day in the sleep laboratory at baseline and repeated in the patients with sleep apnoea after 3 months of regular nightly use of CPAP (for more details, see [21]).
Assays
Plasma collected from the indwelling catheter was transferred to an EDTA-containing tube, refrigerated until centrifugation (within 3 h) and frozen at −70 °C until assayed. Concentrations of TNF-α, TNF-r1, IL-6 and adiponectin were measured in all diurnal samples while, in addition, in all three groups, single blood samples for measurement of fasting blood glucose and insulin were drawn in the morning following the overnight sleep recording. All samples were processed in the same manner. Plasma TNF-α, TNF-r1 and IL-6 concentrations were measured by enzyme-linked immunosorbent assay (ELISA) [Research & Development (R & D) Systems, Minneapolis, MN, USA] [21]. The intra- and interassay coefficients of variation were from 3·1–6·1% and 7·5–10·4%, for TNF-α, 3·6 to 5·0 and 3·7 to 8·8% for TNF-r1 and from 3·2–8·5% and 3·5–8·7% for IL-6. The lower detection limits for TNF-α, TNF-r1 and IL-6 were 0·18, 7·8 and 0·094 pg mL−1, respectively. Adiponectin was measured by a commercially available radioimmunoassay (RIA) (Linco Research Inc., St. Charles, MO, USA). The intra- and interassay coefficients were 1·78 to 3·59 and 6·90 to 9·25, respectively, and the minimal detection limit was 1·0 ngr mL−1. Samples were run in duplicate and standards in triplicate. Plasma insulin was measured by specific RIA. The intra- and interassay coefficients of variation for insulin were from 3·5–4·6% and 4·5–7·0%, respectively.
Computerized tomography scanning
The distribution of abdominal fat (visceral versus subcutaneous fat) in sleep apnoeics and their obese controls was quantified with CT scanning as previously described [4].
Statistical analyses
For comparisons of the subject characteristics among the three groups and within the apnoea group, we used the analysis of variance, the 2-sample Student’s t-test, and the paired t-test, where appropriate. We note that the results of the analyses were essentially the same if nonparametric tests were applied (not shown). The baseline sleep variables were calculated based on the mean values from the two consecutive baseline nights (nights 2 and 3).
For mean comparisons of the hormonal/cytokine data, we calculated the 24-h, daytime (0800–2200) and night time (2300–0700) means for each study participant. Comparisons among the three groups at baseline were performed using analysis of covariance (ANCOVA) to control for age and race. The linear trend test was performed to assess the dose–response relation among the sleep apnoeics, obese, and non-obese groups. Because IL-6 plasma levels are affected by the blood drawing technique, i.e. IL-6-values are higher at the end of the 24-h blood draw compared to the beginning [22,23], we proceeded with a ‘detrended’ analysis. Specifically, we estimated the overall blood drawing effect as the difference between the IL-6-value of the average of 0700 and 0730 the next day and the average of 0800 and 0830 on the previous day. Then we assumed that its effect was increasing in a linear fashion with time and detrended the IL-6-values accordingly. Specifically, the mean difference between the beginning and the end of the blood draw was added to each original IL-6-value in a linear increment manner. Insulin resistance was expressed in two ways: one was the glucose-to-insulin ratio, and the other was the homeostasis model assessment (HOMA) index. For the calculation of HOMA, we used the formula as suggested by Matthews et al. [24].
For the MSLT, PVT, and subjective sleep data, we used repeated measurements analysis to compare the overall differences, as well as differences by time among the three groups and within the apnoea groups. The results were adjusted for age, race and the average percent sleep time at baseline.
Lastly, relations between sleep variables, respiratory data, BMI, CT measures and hormonal/cytokine values were modelled using multiple regression analysis. The results of parametric values are expressed as the mean ± SE, except for age and BMI, whose values are expressed as the mean ± SD. The critical statistical confidence level selected for all analyses was P < 0·05.
Results
Sleep, respiratory, and blood pressure data
Sleep apnoeics, compared both to obese and non-obese controls, slept worse (Table 1). There were no significant differences between obese and non-obese controls in any of the sleep variables. Following the use of CPAP for 3 months, there was a significant improvement of sleep, respiratory and blood pressure variables (Table 2).
Table 1.
Demographic, sleep and respiratory data in sleep apnoeics, obese controls and non-obese controls
| Sleep apnoeics (n = 16) | Obese controls (n = 13) | Non-obese controls (n = 15) | |
|---|---|---|---|
| Age (years) | 48·1 ± 5·6 | 45·6 ± 8·3 | 40·9 ± 12·8 |
| BMI | 37·5 ± 4·7 | 35·4 ± 3·2† | 26·8 ± 2·5 |
| Blood pressure | |||
| Systolic | 141·7 ± 3·4* | 140·1 ± 3·2 | 129·9 ± 2·8 |
| Diastolic | 87·7 ± 2·6 | 83·8 ± 3·8 | 82·7 ± 2·0 |
| Mean | 105·7 ± 2·8 | 102·6 ± 3·0 | 98·3 ± 2·0 |
| Sleep latency (min) | 12·8 ± 2·2 | 17·5 ± 3·0 | 15·6 ± 3·6 |
| Wake time after sleep onset (min) | 70·5 ± 6·9 | 57·8 ± 7·3 | 64·9 ± 11·9 |
| Total wake time | 79·2 ± 7·4 | 72·2 ± 8·3 | 79·2 ± 11·8 |
| % Sleep time | 83·5 ± 1·5 | 85·0 ± 1·7 | 83·5 ± 2·5 |
| % Stage 1 | 26·6 ± 3·6*‡ | 8·6 ± 0·8 | 7·6 ± 1·0 |
| % Stage 2 | 57·5 ± 3·4*‡ | 69·6 ± 1·6 | 67·4 ± 1·3 |
| % Slow wave | 3·1 ± 1·5 | 4·3 ± 1·0 | 6·6 ± 1·8 |
| % REM | 12·8 ± 1·5*‡ | 17·5 ± 1·3 | 18·4 ± 1·1 |
| REM latency | 121.5 ± 22·4 | 95·3 ± 14·3 | 85·9 ± 7·3 |
| Apnoea/hypopnoea index | 53·3 ± 7·0*‡ | 2·7 ± 0·7 | 1·6 ± 0·4 |
| Minimum O2 saturation | 72·4 ± 2·1*‡ | 88·1 ± 1·0 | 88·3 ± 2·0 |
Data are presented as the mean ± SE, except age and body mass index (BMI) mean ± SD.
P< 0·05 sleep apnoeics vs. non-obese controls.
P < 0·05 obese controls vs. non-obese controls.
P < 0·05 sleep apnoeics vs. obese controls.
Table 2.
Sleep and respiratory data in sleep apnoeics at baseline and after treatment with CPAP for 3 months
| Pre CPAP treatment (n = 16) | Post CPAP treatment (n = 16) | |
|---|---|---|
| BMI | 37·5 ± 4·7 | 37·5 ± 4·9 |
| Blood pressure | ||
| Systolic | 141·7 ± 3·4 | 135·3 ± 3·5 |
| Diastolic | 87·7 ± 2·6* | 79·9 ± 1·9 |
| Mean | 105·7 ± 2·8* | 98·4 ± 2·0 |
| Sleep latency (min) | 12·8 ± 2·2 | 14·3 ± 2·7 |
| Wake time after sleep onset (min) | 70·5 ± 6·9* | 53·1 ± 6·9 |
| Total wake time | 79·2 ± 7·4 | 65·9 ± 8·5 |
| % Sleep time | 83·5 ± 1·5 | 86·3 ± 1·8 |
| % Stage 1 | 26·6 ± 3·6** | 9·4 ± 1·0 |
| % Stage 2 | 57·5 ± 3·4* | 67·0 ± 1·9 |
| % Slow wave | 3·1 ± 1·5 | 3·1 ± 1·2 |
| % REM | 12·8 ± 1·5** | 20·4 ± 1·9 |
| REM Latency | 121·5 ± 22·4* | 81·4 ± 8·7 |
| Apnoea/hypopnoea index saturation | 53·3 ± 7·0** | 4·5 ± 1·9 |
| Minimum O2 | 72·4 ± 2·1** | 85·9 ± 1·6 |
Data are presented as the mean ± SE, except age and body mass index (BMI) mean ± SD.
P < 0·05.
P < 0·01.
CPAP, continuous positive airway pressure.
Daytime sleepiness and performance
MSLT
At baseline, there were no significant differences among the three groups either in terms of the mean sleep latencies or at individual time points (mean values: 11·9 ± 1·1 vs. 13·6 ± 0·9 vs. 10·8 ± 1·0 min for non-obese controls, obese controls, and sleep apnoeics, respectively). After three months of CPAP use, there was a significant increase of mean MSLT sleep latency (mean difference: 1·8 ± 0·8 min, P < 0·05).
Subjective sleepiness
At baseline, using the Stanford Sleepiness Scale, apnoeics were sleepier than obese controls and non-obese controls (2·8 ± 0·2 vs. 2·2 ± 0·2 vs. 1·9 ± 0·1, P = 0·05 for sleep apnoeics vs. obese controls; P < 0·01 for sleep apnoeics vs. non-obese controls). The same pattern was observed with the visual-analogue scale (6·6 ± 0·4 vs. 7·3 ± 0·5 vs. 7·9 ± 0·3; overall P = 0·11 and P < 0·05 for sleep apnoeics vs. non-obese controls). Sleep apnoeics scored significantly higher on the Epworth Sleepiness Scale than obese or non-obese controls (12·1 ± 1·1 vs. 7·8 ± 1·6 vs. 7·8 ± 1·0, P < 0·05, respectively). After three months of CPAP use, patients with sleep apnoea scored less sleepy on the visual-analogue scale (difference: 0·8 ± 0·3, P < 0·05) and the SSS (difference: 0·4 ± 0·2, P = 0·09).
PVT
At baseline, there was a non-significant dose–response pattern in all four PVT variables among the three groups (sleep apnoeics performing the worst, obese controls in-between and non-obese controls the best). CPAP use did not improve PVT performance.
Twenty-four-hour plasma IL-6, Tnf-α, and TNF-r1 pre- and post-CPAP
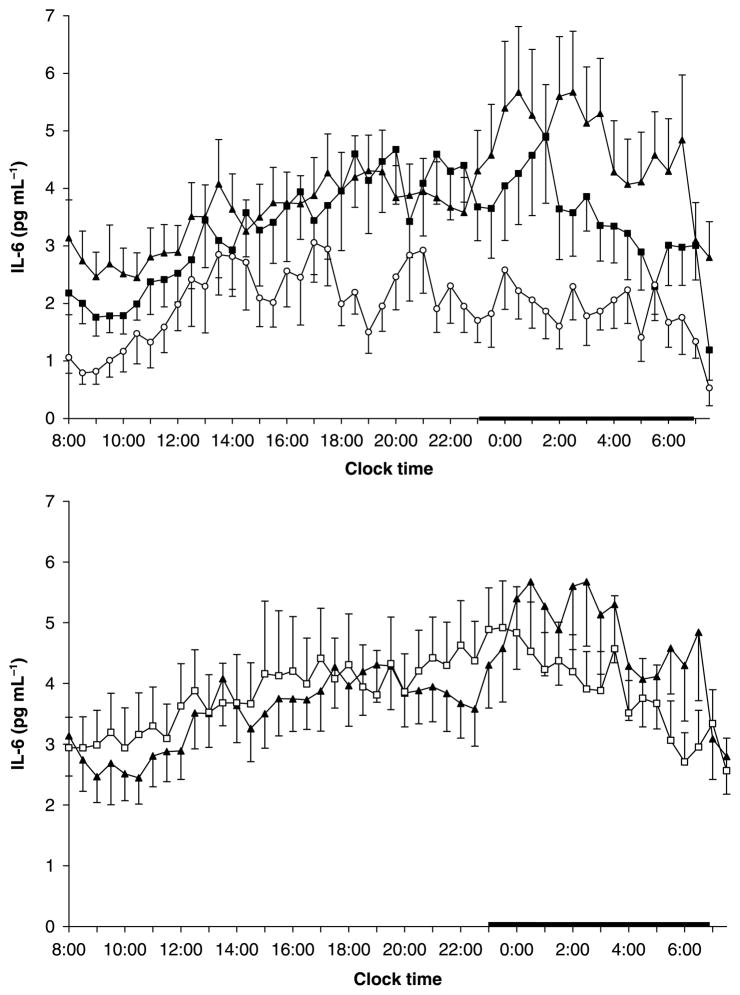
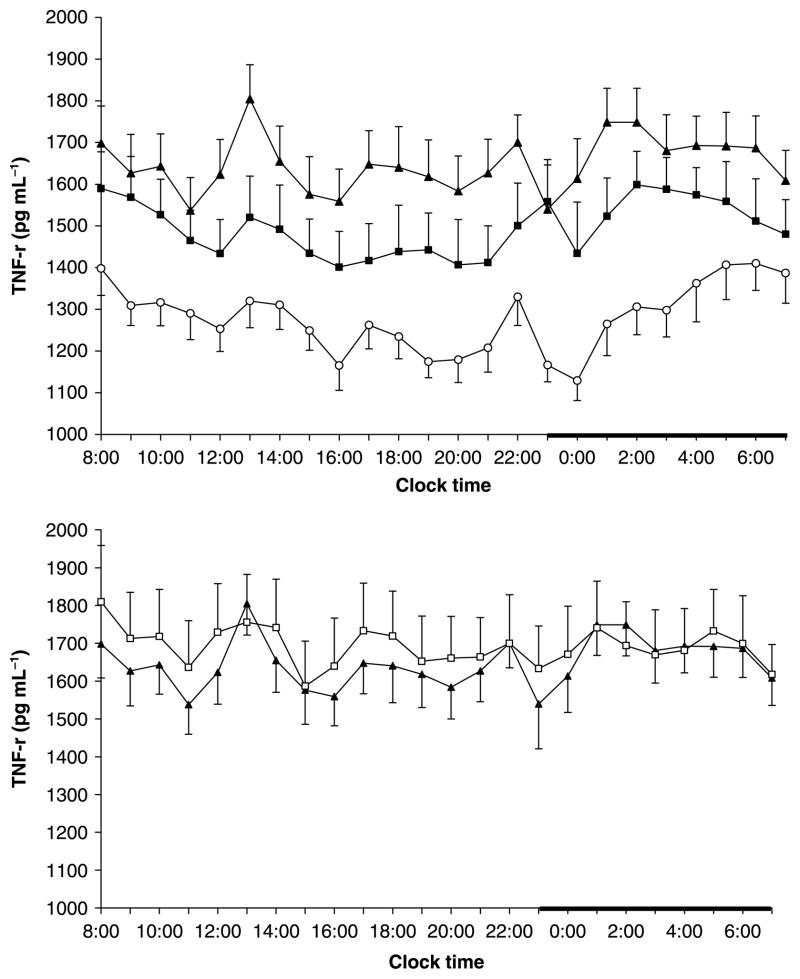
Twenty-four hour mean values (non-detrended) of IL-6 were significantly higher in sleep apnoea patients and obese controls versus non-obese controls (4·5 ± 0·7, vs. 4·4 ± 0·5, vs. 2·4 ± 0·4, respectively, P < 0·05). There was a significant dose–response pattern in the morning values (0800–1000) of IL-6 among sleep apnoea patients, obese controls and non-obese controls (2·8 ± 0·6, vs. 2·1 ± 0·3, vs. 0·8 ± 0·2, respectively, linear trend P < 0·05). These differences were attenuated later in the 24-h period, possibly due to the well known effects of blood drawing technique on IL-6 values [22,23]. Analysis of the 24-h detrended IL-6 values showed that the IL-6 mean levels were highest in sleep apnoeics, intermediate in obese controls and lowest in non-obese controls (4·1 ± 0·8 vs. 3·5 ± 0·5 vs. 1·6 ± 0·3 pg mL−1, linear trend, P < 0·05) (Fig. 1). A similar dose–response pattern was noted for 24-h mean values of TNF-r1 among the three groups (1641·4 ± 78·1 vs. 1489·5 ± 88·8 vs. 1307·3 ± 57·2, linear trend, P < 0·01) (Fig. 2). A similar pattern was observed for the mean 24-h TNF-α values with close to significant linear trend (1·7 ± 0·3 vs. 1·5 ± 0·2 vs. 1·4 ± 0·2 pg mL−1, linear trend P = 0·09).
Figure 1.
Twenty-four-hour secretory pattern of Interleukin 6 (IL-6) in non-obese (n = 15) (○) and obese controls (n = 13) (■) and in patients with sleep apnoea (n = 16) (▲)[top] and before and after 3 months of CPAP treatment: pre CPAP (▲), post CPAP (□)[bottom]. The thick bar on the abscissa represents the sleep recording period. IL-6-values represent detrended data.
Figure 2.
Twenty-four-hour secretory pattern of TNF-r1 in non-obese (n = 15) (○) and obese controls (n = 13) (■) and in patients with sleep apnoea (n = 16) (▲) [top] and before and after 3 months of CPAP treatment: pre CPAP (▲), post CPAP (□) [bottom]. The thick bar on the abscissa represents the sleep recording period.
The 3 month use of CPAP did not affect the plasma concentrations of IL-6 (4·5 ± 0·7 vs. 4·7 ± 0·7 pg mL−1 original and 4·0 ± 0·7 vs. 3·8 ± 0·6 pg mL−1 detrended), TNF-r1 (1645·9 ± 78·1 vs. 1691·6 ± 114·7 pg mL−1), or TNF-α (1·7 ± 0·3 vs. 1·8 ± 0·3 pg mL−1) in the group of sleep apnoeic men (Figs 1 and 2).
Visceral versus generalized obesity and biochemical indices of insulin resistance pre- and post-CPAP
There were no significant differences between sleep apnoeics and obese controls in terms of total or subcutaneous fat. In contrast, sleep apnoeics had significantly higher amounts of visceral fat than their obese controls overall, as well as at L4, L5, and a trend at L3 levels (P < 0·01, < 0·05 and < 0·1, respectively; numerical data are shown in Table 3). The use of CPAP did not affect the amount of either fat (Table 3). Visceral but not subcutaneous fat was significantly correlated with indices of sleep apnoea (rxy = 0·62, P < 0·01 for A/HI and rxy = 0·57, P < 0·01 for minimum SaO2).
Table 3.
Abdominal fat distribution at lumbar spine level 4 (L4) in sleep apnoeics pre- and post 3 month CPAP treatment and obese controls
| Sleep apnoeics | Obese controls (n = 13) | ||
|---|---|---|---|
| Pre CPAP (n = 16) | Post CPAP (n = 16) | ||
| BMI (kg m−2) | 37·5 ± 4·7 | 37·5 ± 4·9 | 35·4 ± 3·2 |
|
| |||
| Total body fat area (cm3) | 641·8 ± 44·8 | 642·2 ± 42·9 | 565·1 ± 35·4 |
|
| |||
| Subcutaneous fat area (cm3) | 305·1 ± 32·3 | 310·5 ± 26·1 | 315·5 ± 30·6 |
|
| |||
| Visceral fat area (cm3) | 336·7 ± 28·3 | 331·7 ± 27·6 | 249·6 ± 24·1* |
Data are presented as the mean ± SE, except BMI mean ± SD.
P < 0·05 sleep apnoeics vs. obese controls.
A close to significant dose–response pattern was observed for mean fasting blood glucose levels in sleep apnoeics versus obese and non-obese controls (96·6 ± 5·8 vs. 94·9 ± 5·2 vs. 81·0 ± 2·2, linear trend, P = 0·06). Mean plasma insulin levels were highest in sleep apnoeics, intermediate in obese controls, and lowest in non-obese controls (18·2 ± 2·1 vs. 15·4 ± 2·4 vs. 6·8 ± 1·4, linear trend, P < 0·01). The same order was observed in terms of glucose/insulin ratio (6·4 ± 0·9 vs. 8·0 ± 1·1 vs. 14·4 ± 1·5 linear trend, P < 0·01) and HOMA index (4·6 ± 0·8 vs. 3·6 ± 0·6 vs. 1·4 ± 0·3, linear trend P < 0·01).
The use of CPAP did not affect fasting glucose, insulin, glucose/insulin ratio, or HOMA index in the group of sleep apnoeic patients (Table 4).
Table 4.
Insulin resistance variables in sleep apnoeics pre- and post 3 month CPAP treatment
| Pre- (n = 16) | Post- (n = 16) | |
|---|---|---|
| Fasting blood sugar (mg dL−1) | 96·6 ± 5·8 | 93·3 ± 3·0 |
| Fasting insulin (mcU mL−1) | 18·2 ± 2·1 | 18·7 ± 26 |
| Glucose/insulin ratio | 6·4 ± 0·9 | 7·1 ± 1·1 |
| HOMA | 4·6 ± 0·8 | 4·3 ± 0·7 |
| 24-h adiponectin (μg mL−1) | 9·0 ± 1·5 | 10·4 ± 3·4 |
Data are presented as the mean ± SE.
HOMA, homeostasis model assessment.
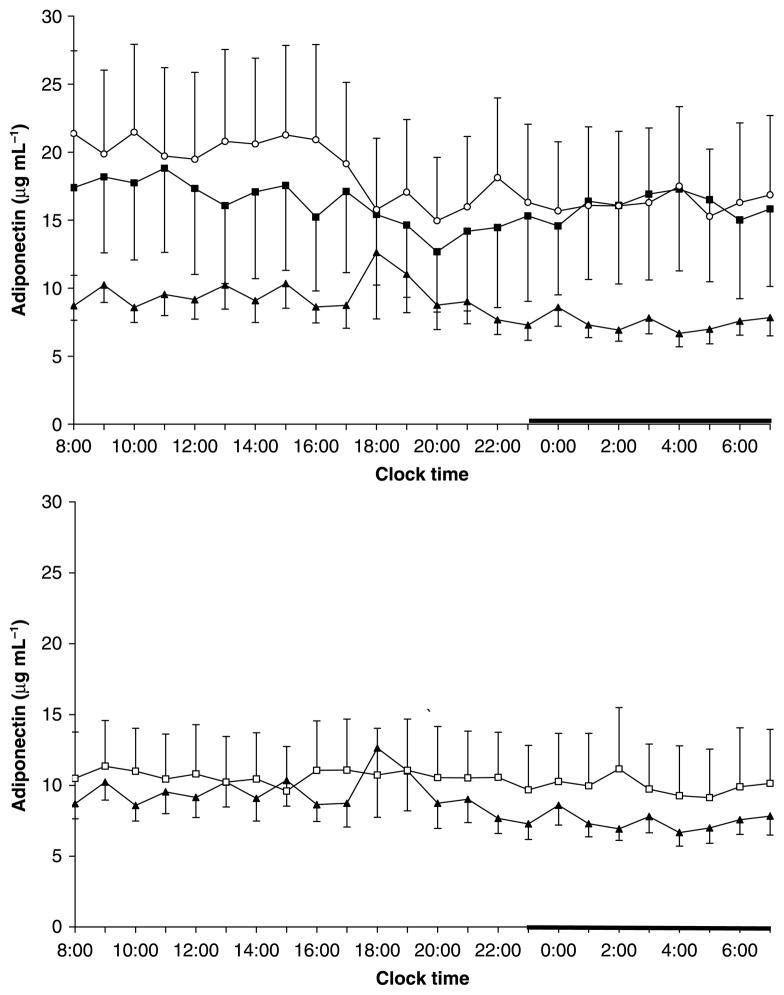
24-h adiponectin levels showed a tendency to be lower in sleep apnoeics than in non-obese controls, with obese having intermediate values (9·0 ± 1·5 vs. 16·5 ± 6·2 vs. 18·9 ± 5·8, trend P = 0·2) (Fig. 3). CPAP did not affect 24-h plasma levels of adiponectin (Table 4, Fig. 3).
Figure 3.
Twenty-four-hour secretory pattern of adiponectin in non-obese (n = 15) (○) and obese controls (n = 13) (■) and in patients with sleep apnoea (n = 16) (▲) [top] and before and after 3 months of CPAP treatment: pre CPAP (▲), post CPAP (□)[bottom]. The thick bar on the abscissa represents the sleep recording period.
CPAP compliance: ‘low’ versus ‘high’ adherence to therapy
In order to explore further the potential confounding factor of compliance to CPAP therapy, we divided the apnoea group into ‘high’ and ‘low’ adherence based on a criterion of CPAP use per night ≥ 4 h. Ten of the patients fell into the category of ‘high’ adherence and six into the category of ‘low’ adherence. There was no significant or trend difference between pre- and post-CPAP values in the two subgroups in terms of the inflammatory markers or insulin resistance indices. Visceral fat was the only variable in which the CPAP effect was significant at P < 0·2. In the high adherence group, there was a reduction of total visceral fat (the sum across all five CT slices) by 48·2 ± 31·5 cm3, P = 0·15, whereas in the low adherence group, there was an increase by 57·4 ± 39·9 cm3, P = 0·17. Interestingly, the high adherence group had a slight reduction of BMI (37·76 vs. 37·36), whereas the low adherence group showed a slight increase (37·01 vs. 37·84).
Types of fat and sleep apnoea as predictors of plasma cytokine concentrations and indices of insulin resistance
Visceral fat but not subcutaneous fat was significantly associated with IL-6 levels (partial R2 = 16·7%). For every 10 cm3 increase of visceral fat, the secretion of IL-6 is increased by 0·1 pg mL−1 (95% CI: 0·04 pg mL−1 to 0·23 pg mL−1; P < 0·01). Sleep apnoea, but not any of the two types of fat, was associated with an increase of TNF-r1 levels by 239·82 pg mL−1 (95% CI: 45·51 pg mL−1 to 434·13 pg mL−1; P < 0·05; partial R2 = 16·6%). Glucose/insulin ratio and HOMA index were predicted by visceral and subcutaneous fat, but not sleep apnoea (Glucose/insulin ratio: partial R2 = 44·4% for visceral fat and 11·8% for subcutaneous fat; HOMA: partial R2 = 35·0% for visceral fat and 6·4% for subcutaneous fat). The glucose/insulin ratio was decreased by 0·32% (95% CI: −5·4% to −1·1%; P < 0·01) per 10 cm3 increase of visceral fat, and by 0·22% (95% CI: −3·6% to −0·7%; P < 0·01) per 10 cm3 increase of subcutaneous fat. Similarly, the HOMA index was increased by 0·11 (95% CI: 0·003 to 0·019; P < 0·01; partial R2 = 35·0%) per 10 cm3 increase of visceral fat, and by 0·06 (95% CI: 0·001 to 0·011; P < 0·05; partial R2 = 6·4%) per 10 cm3 increase of subcutaneous fat. Neither type of fat nor sleep apnoea predicted to a significant degree adiponectin levels.
Discussion
Our study demonstrated that (a) visceral fat is strongly and independently associated with insulin resistance and inflammation in obese men with sleep apnoea. This association appears to be stronger than sleep apnoea per se with insulin resistance indices and IL-6; and (b) the therapeutic use of CPAP for three months does not improve low-grade inflammation, insulin resistance, or visceral adiposity in obese men with sleep apnoea. Because all these conditions are independent risk factors that affect health adversely and increase cardiovascular morbidity and mortality [9,15,16], the use of CPAP in obese men should be combined with other measures known to improve inflammation, insulin resistance and visceral adiposity.
Around the clock IL-6 and TNF-r1 peripheral levels and insulin resistance indices were highest in the patients with sleep apnoea, whereas in the obese controls, these values were intermediate between those of sleep apnoeics and non-obese controls. Also, among obese individuals, it was visceral fat rather than generalized obesity that predisposed to the development of sleep apnoea. These findings are consistent with our first report in 1997 as well as most of the subsequent reports by us and others that sleep apnoea, after controlling for obesity, is associated with visceral obesity, inflammatory cytokine elevation and hyperinsulinaemia [3–7,25–29]. In addition, the peripheral levels of adiponectin, an adipokine that promotes insulin sensitivity, tended to be lower in obese apnoeics compared to non-obese controls, consistent with previous reports [30]. That the adiponectin differences did not reach statistical significance is most likely due to the high variability of the peripheral levels.
CPAP did not improve visceral adiposity, insulin resistance or low-grade inflammation. Our negative findings on insulin and adiponectin are consistent with most, but not all, studies that have assessed the effects of CPAP on these metabolic indices [6,8,30]. The conflicting results among the studies could be attributed to some extent to differences in sample sizes, measurement techniques, duration of CPAP treatment, lack of objective adherence data, or lack of a placebo control group. However, a more parsimonious explanation for these seemingly discrepant findings is degree of obesity, a long recognized confounder in the relationship between insulin resistance and OSA. For example, in a study that used ‘the gold standard’ technique for insulin resistance, i.e. euglycaemic clamp technique, a beneficial effect was reported primarily in non-obese subjects [25]. Furthermore, two recent placebo-controlled studies [31,32] with relatively large samples, and with one of them using the euglycaemic clamp technique [31], failed to show any improvement in obese patients with sleep apnoea. A similar pattern is noted in the few studies that have assessed the effects of CPAP on inflammation with positive findings again being reported in non-obese apnoeics [7,26,27,33,34]. These findings combined suggest that CPAP is effective in reversing inflammation/metabolic abnormalities in non-obese, but not in obese apnoeics. It is possible that obese apnoeics are different from non-obese in terms of underlying pathophysiology and symptom profile. Indeed, non-obese apnoeics are characterized by less daytime sleepiness and more frequent presence of anatomic abnormalities than obese apnoeics [35,36]. In turn, the metabolic/inflammatory aberrations in obese apnoeics might play a primary role in the pathogenesis of apnoea, whereas in the non-obese it might be a secondary phenomenon to the apnoea per se. Further studies focusing on non-obese apnoeics should test this hypothesis.
A major confounder in the relations between inflammation/metabolic aberrations and sleep apnoea is abdominal fat distribution and, particularly, visceral adiposity. It is well established that visceral fat is strongly associated with insulin resistance and production of pro-inflammatory cytokines, i.e. IL-6 [9,10 (Fang personal communication)]. Also, it appears that visceral fat is a primary abnormality in sleep apnoea. In 2000 we reported that obese people with sleep apnoea have significantly higher visceral fat than BMI, age, and gender-matched controls. Our new study confirms these early findings and further suggests that CPAP does not have a significant effect on visceral adiposity. The lack of a significant effect of CPAP on visceral adiposity, in our study, may explain the negative findings on indices such as IL-6 and HOMA, that were primarily associated with visceral fat. It is interesting to note that there is a paucity of studies on the effects of CPAP on visceral adiposity in sleep apnoeics with the exception of one early study that did not include controls and reported beneficial effects of CPAP in non-obese apnoeics [5]. Such information may shed light on the inconsistencies of the aforementioned studies on inflammation and insulin resistance. Future studies on obese and non-obese apnoeics, who are stratified according to the degree of visceral adiposity, might provide important new insights into this topic.
There are other possible explanations for our findings. One possibility is that obesity might have masked the ability to detect changes in these parameters, particularly insulin resistance that may be subject to a ‘ceiling’ effect in obese individuals. However, in our study, sleep apnoea, independent of obesity, was associated with these inflammatory/metabolic aberrations. Another possibility is that the duration of the use of CPAP was too short to allow metabolic improvement or that the compliance to CPAP therapy was low. However, a 3-month period has been shown to be adequate for correction of other abnormalities, i.e. catecholamines [35], cortisol [13], and TNF-α [7,26,27] elevation, and some trend for improvement, even if not significant, should have been detected after 3 months of systematic use of CPAP. Also, high adherence to CPAP therapy, using more stringent criteria than those currently recommended [20], did not change the effect of CPAP on these variables with the exception of a slight reduction of visceral fat in the group with high adherence. Furthermore, it is possible that our study did not detect any changes post-CPAP because of the small sample size. However, first, the sample size of our study is not different from most of the previously published studies [5–7,26,30,34]. Second, our study included two control groups that demonstrated the previously reported dose–response difference of the outcome variables among non-obese, obese, and obese with sleep apnoea. Third, our study was comprehensive and thorough in terms of outcome variables studied by including all previously reported inflammation and metabolic alterations in sleep apnoea i.e. pro-inflammatory cytokines, insulin resistance and abdominal fat distribution; serial 24-h, every thirty minutes, blood sampling for hormones/cytokines; all day sampling of objective sleepiness and performance using MSLT and PVT; and state-of-the-art measures i.e. abdominal CT scan, instead of simple anthropometric measures i.e. waist circumference. Finally, post-CPAP, all three cytokines were slightly increased compared to pre-CPAP, whereas the insulin resistance/visceral fat variables showed no changes. Thus, even a much higher sample size should not have been able to detect a clinically meaningful difference.
We should note that CPAP was effective in this group of apnoeic patients to reduce plasma cortisol levels and bring them close to those of obese controls [13]. This is consistent with findings of previous studies that have shown a beneficial effect of CPAP on the other limb of the stress system, i.e. the sympathetic system [14,37]. The normalizing effect of CPAP on the stress system, by eliminating chronic intermittent hypoxia and repetitive microawakenings, may explain the beneficial effect of CPAP on blood pressure, as well as sleepiness and fatigue that were also observed in this study. However, this protection is incomplete given the independent association of insulin resistance and inflammation markers, e.g. IL-6 with cardiovascular morbidity and mortality [9,15,16].
CPAP, in contrast to its effect on MSLT, did not significantly improve the performance (PVT) of patients with sleep apnoea. The dissociation between MSLT and PVT responses may suggest that the two tests are measuring different CNS functions and that PVT sensitivity may be affected by factors such as motivation and duration of the session [21,38].
In conclusion, (a) visceral adiposity is strongly and independently associated with insulin resistance and inflammation in obese sleep apnoeics; and (b) CPAP may improve blood pressure and sleepiness in obese men with sleep apnoea. However, it does not affect low-grade inflammation, insulin resistance or visceral adiposity. Given that these abnormalities independently affect health and longevity adversely, other therapeutic measures, e.g. weight loss, exercise, pharmacological agents such as drugs that improve insulin sensitivity and reduce visceral fat should be included in the management of sleep apnoea.
Acknowledgments
This study was partially funded by National Institutes of Health Grants R01 HL64415, RR010732, and RR016499. Also, Young’s Medical Equipment, a division of Air Products Healthcare, provided the CPAP devices and the compliance monitoring throughout the study.
References
- 1.Vgontzas AN, Kales A. Sleep and its disorders. Annu Rev Med. 1999;50:387–400. doi: 10.1146/annurev.med.50.1.387. [DOI] [PubMed] [Google Scholar]
- 2.Partinen M, Hublin C. Epidemiology of sleep disorders. In: Kryger M, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4. Philadelphia, PA: Saunders Elsevier; 2005. pp. 626–47. [Google Scholar]
- 3.Vgontzas AN, Papanicolaou DA, Bixler EO, Kales A, Tyson K, Chrousos G. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. J Clin Endocrinol Metab. 1997;82:1313–6. doi: 10.1210/jcem.82.5.3950. [DOI] [PubMed] [Google Scholar]
- 4.Vgontzas AN, Papanicolaou DA, Bixler EO, Hopper K, Lin HM, Kales A, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab. 2000;85:1151–8. doi: 10.1210/jcem.85.3.6484. [DOI] [PubMed] [Google Scholar]
- 5.Chin K, Shimizu K, Nakamura T, Narai N, Masuzaki H, Ogawa Y, et al. Changes in intra-abdominal visceral fat and serum leptin levels in patients with obstructive sleep apnea syndrome following nasal continuous positive airway pressure therapy. Circulation. 1999;100:706–12. doi: 10.1161/01.cir.100.7.706. [DOI] [PubMed] [Google Scholar]
- 6.Punjabi NM, Polotsky VY. Disorders of glucose metabolism in sleep apnea. J Appl Physiol. 2005;99:1998–2007. doi: 10.1152/japplphysiol.00695.2005. [DOI] [PubMed] [Google Scholar]
- 7.Ryan S, Taylor C, McNicholas W. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep Apnea Syndrome. Circulation. 2005;112:2660–7. doi: 10.1161/CIRCULATIONAHA.105.556746. [DOI] [PubMed] [Google Scholar]
- 8.Vgontzas AN, Bixler EO, Chrousos GP. Sleep apnea is a manifestation of the metabolic syndrome. Sleep Med Rev. 2005;9:211–24. doi: 10.1016/j.smrv.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Chrousos GP. The role of stress and the hypothalamic-pituitary-adrenal axis in the pathogenesis of the metabolic syndrome: neuro-endocrine and target tissue-related causes. Int J Obes Relat Metab Disord. 2000;24:550–5. doi: 10.1038/sj.ijo.0801278. [DOI] [PubMed] [Google Scholar]
- 10.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot different and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83:847–50. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- 11.Patel S, White D, Malhotra A, Stanchina M, Ayas N. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med. 2003;163:565–71. doi: 10.1001/archinte.163.5.565. [DOI] [PubMed] [Google Scholar]
- 12.Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, Mullins R, Jenkinson C, Stradling JR, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnea: a randomized parallel trial. Lancet. 2002;359:204–10. doi: 10.1016/S0140-6736(02)07445-7. [DOI] [PubMed] [Google Scholar]
- 13.Vgontzas AN, Pejovic S, Zoumakis E, Lin HM, Bentley CM, Bixler EO, et al. Hypothalamic-pituitary-adrenal axis activity in obese men with and without sleep apnea: effects of continuous positive airway pressure therapy. J Clin Endocrinol Metab. 2007;92:4199–207. doi: 10.1210/jc.2007-0774. [DOI] [PubMed] [Google Scholar]
- 14.Waradckar NV, Sinoway LI, Zwillich CW, Leuenberger UA. Influence of treatment on muscle sympathetic nerve activity in sleep apnea. Am J Respir Crit Care Med. 1996;153:1333–8. doi: 10.1164/ajrccm.153.4.8616563. [DOI] [PubMed] [Google Scholar]
- 15.Harris TM, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger HW, Jr, et al. Associations of elevated interleukin-6 and c reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–12. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 16.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–72. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 17.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Washington DC: US Government Printing Office; 1968. NIMH Publication 204. [Google Scholar]
- 18.Richardson GS, Carskadon MA, Flagg W, van den Hoed J, Dement WC, Mitler MM. Excessive daytime sleepiness in man: multiple sleep latency measurement in narcoleptic and control subjects. Electroencephalogr Clin Neurophysiol. 1978;45:621–7. doi: 10.1016/0013-4694(78)90162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;27:117–26. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 20.Dinges D, Weaver T. Effects of modafinil on sustained attention performance and quality of life in OSA patients with residual sleepiness while being treated with nCPAP. Sleep Med. 2003;4:393–402. doi: 10.1016/s1389-9457(03)00108-4. [DOI] [PubMed] [Google Scholar]
- 21.Vgontzas AN, Pejovic S, Zoumakis E, Lin HM, Bixler EO, Basta M, et al. Daytime napping after a night of sleep loss decreases sleepiness, improves performance, and causes beneficial changes in cortisol and interleukin-6 secretion. Am J Physiol Endocrinol Metab. 2006;292:253–61. doi: 10.1152/ajpendo.00651.2005. [DOI] [PubMed] [Google Scholar]
- 22.Haack M, Kraus T, Schuld A, Dalal M, Koethe D, Pollmächer T. Diurnal variations of interleukin-6 plasma levels are confounded by blood drawing procedures. Psychoneuroendocrinology. 2002;27:921–31. doi: 10.1016/s0306-4530(02)00006-9. [DOI] [PubMed] [Google Scholar]
- 23.Vgontzas AN, Bixler EO, Lin HM, Prolo P, Trakada G, Chrousos G. IL-6 and its circadian secretion in humans. Neuroimmunomodulation. 2005;12:131–40. doi: 10.1159/000084844. [DOI] [PubMed] [Google Scholar]
- 24.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 25.Harsch I, Schahin S, Radespiel-Troger M, Weintz O, Jahreiss H, Fuchs FS, et al. Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2004;169:156–62. doi: 10.1164/rccm.200302-206OC. [DOI] [PubMed] [Google Scholar]
- 26.Minoguchi K, Tazaki T, Yokoe T, Minoguchi H, Watanabe Y, Yamamoto M, et al. Elevated production of tumor necrosis factor-α by monocytes in patients with obstructive sleep apnea syndrome. Chest. 2004;126:1473–9. doi: 10.1378/chest.126.5.1473. [DOI] [PubMed] [Google Scholar]
- 27.Ryan S, Taylor CT, McNicholas WT. Predictors of elevated nuclear factor-κβ-dependent genes in obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2006;174:824–30. doi: 10.1164/rccm.200601-066OC. [DOI] [PubMed] [Google Scholar]
- 28.Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165:670–6. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]
- 29.Punjabi NM, Sorkin JD, Katzel LI, Goldberg A, Schwartz A, Smith PL. Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med. 2002;165:677–82. doi: 10.1164/ajrccm.165.5.2104087. [DOI] [PubMed] [Google Scholar]
- 30.Harsch A, Wallaschofski H, Koebnick C, Pour Schahin S, Hahn EG, Ficher JH, et al. Adiponectin in patients with obstructive sleep apnea syndrome: course and physiological relevance. Respiration. 2004;71:580–6. doi: 10.1159/000081758. [DOI] [PubMed] [Google Scholar]
- 31.West SD, Nicoll DJ, Wallace TM, Matthews DR, Stradling JR. Effect of CPAP on insulin resistance and HbA1c in men with obstructive sleep apnoea and type 2 diabetes. Thorax. 2007;62:969–74. doi: 10.1136/thx.2006.074351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coughlin SR, Mawdsley L, Mugarza JA, Wilding J, Calverley P. Cardiovascular and metabolic effects of CPAP in obese men with OSA. Eur Respir J. 2007;29:720–7. doi: 10.1183/09031936.00043306. [DOI] [PubMed] [Google Scholar]
- 33.Harsch I, Koebnick C, Wallaschofski H, Schahin SP, Hahn EG, Ficker JH, et al. Restin levels in patients with obstructive sleep apnea syndrome – the link to subclinical inflammation. Med Sci Monit. 2004;10:CR510–5. [PubMed] [Google Scholar]
- 34.Yokoe T, Minoguchi K, Matsuo H, Oda N, Minoguchi H, Yoshino G, et al. Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation. 2003;107:1129–34. doi: 10.1161/01.cir.0000052627.99976.18. [DOI] [PubMed] [Google Scholar]
- 35.Bixler EO, Vgontzas AN, Lin HM, Calhoun SL, Vela-Bueno A, Kales A. Excessive daytime sleepiness in a general population sample: the role of sleep apnea, age, obesity, diabetes, and depression. J Clin Endocrinol Metab. 2005;90:4510–5. doi: 10.1210/jc.2005-0035. [DOI] [PubMed] [Google Scholar]
- 36.Smith PL, Schwartz AR. Biomechanics of the upper airway during sleep. In: Pack AL, editor. Sleep Apnea: Pathogenesis, Diagnosis, and Treatment. New York: Marcel Dekker; 2002. pp. 31–52. [Google Scholar]
- 37.Mills PJ, Kennedy BP, Loredo JS, Dimsdale JE, Ziegler MG. Effects of nasal continuous positive airway pressure and oxygen supplementation on norepinephrine kinetics and cardiovascular responses in obstructive sleep apnea. J Appl Physiol. 2006;100:343–8. doi: 10.1152/japplphysiol.00494.2005. [DOI] [PubMed] [Google Scholar]
- 38.Belenky G, Wesensten NJ, Thorne DR, Thomas ML, Sing HC, Redmond DP, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res. 2003;12:1–12. doi: 10.1046/j.1365-2869.2003.00337.x. [DOI] [PubMed] [Google Scholar]