Abstract
Recent studies reveal that cocaine experience results in persistent neuroadaptive changes within glutamate (Glu) synapses in brain areas associated with drug reward. However, it remains unclear whether cocaine affects Glu release in drug-naive animals and how it is altered by drug experience. By using high-speed amperometry with enzyme-based and enzyme-free biosensors in freely moving rats, we show that an initial intravenous cocaine injection at a low self-administering dose (1 mg/kg) induces rapid, small and transient Glu release in the nucleus accumbens shell (NAc), which with subsequent injections rapidly becomes a much stronger, two-component increase. Using cocaine-methiodide, cocaine’s analogue that does not cross the blood-brain barrier, we confirm that the initial cocaine-induced Glu release in the NAc has a peripheral neural origin. Unlike cocaine, Glu responses induced by cocaine-methiodide rapidly habituate following repeated exposure. However, after cocaine experience this drug induces cocaine-like Glu responses. Hence, the interoceptive actions of cocaine, which essentially precede its direct actions in the brain, play a critical role in experience-dependent alterations in Glu release, cocaine-induced neural sensitization and may contribute to cocaine addiction.
Keywords: electrochemistry, enzyme-based glutamate biosensors, glutamate release, experience-dependent neuroplasticity of glutamate neurotransmission, peripheral actions of cocaine
INTRODUCTION
The development of cocaine addiction in humans requires repeated experience with the drug. While repeated exposure to cocaine results in widespread changes in the brain and behavior, the mechanisms underlying these alterations and the processes by which they occur remain poorly understood. In addition to morphological (Robinson and Kolb 2004) and biochemical changes (Berke and Hyman 2000) induced by repeated cocaine exposure in rats, recent in vitro electrophysiological data reveal long-lasting changes in synaptic strength in brain areas associated with drug reward, particularly in the mesocorticolimbic dopamine (DA) and corticostriatal glutamate (Glu) systems (Hyman et al. 2006; Kalivas and O’Brien 2008; Luscher and Malenka 2011; Ungless et al. 2001). These drug-induced neuroadaptations within Glu synapses correlate with the enhancement of DA responses and the development of locomotor sensitization (Borgland et al. 2004; Ungless et al. 2001). Despite increasing evidence of synaptic plasticity within the Glu system at the postsynaptic receptor level, our knowledge on cocaine-induced changes in Glu release, another dynamic component of Glu transmission, remains more limited.
In the nucleus accumbens (NAc), the primary target of mesocorticolimbic DA neurons, Glu levels have been shown to increase after a cocaine challenge, but only in rats with extensive drug exposure that also show a sensitized locomotor response to the drug (Pierce et al. 1996). Glu levels also moderately increase in the NAc and ventral tegmental area (VTA) during cocaine self-administration, but larger Glu elevations have been found during the extinction of lever-pressing behavior (Suto et al. 2010; You et al. 2007). While these data indicate that cocaine-induced effects on Glu release may be experience-dependent and more tightly correlated with drug-seeking and drug-taking behavior, they also suggest that changes in Glu release are a slow process requiring extensive experience. However, these and other studies involving cocaine (Ferrario et al. 2008; You et al. 2001) have used in vivo microdialysis, which despite recent advances in rapid detection (Perry et al. 2009), has low temporal resolution. These technical limitations place significant constraints on revealing rapid Glu fluctuations, possibly explaining the inability to detect any changes in NAc Glu following an acute cocaine injection in drug-naive rats (Venton et al., 2006).
High temporal resolution is particularly important for Glu measurements because of the rapid nature of Glu transmission and exceptionally fast neural responses induced by cocaine. Intravenous (iv) cocaine in awake, freely moving rats induces cortical EEG desynchronization, robust increases in EMG activity, and excitation of most accumbal and VTA neurons with second-scale onset latencies (Brown and Kiyatkin 2008; Kiyatkin and Brown 2007; Kiyatkin and Smirnov 2010). The second-scale latencies of these cocaine-induced neural responses also suggest a peripheral, neural trigger. Cocaine-methiodide (cocaine-M), a peripherally acting analogue that cannot cross the blood-brain barrier (BBB) (Hemby et al. 1994; Shriver and Long 1971; You et al. 2007), also induces equally rapid cortical EEG desynchronization, EMG activation (Kiyatkin and Smirnov 2010), excitation of NAc neurons (Kiyatkin and Brown 2007), and cocaine-like physiological effects (Brown and Kiyatkin 2006). All of these excitatory neural responses to cocaine implicate Glu release as their possible cause, but it remains unknown whether in fact this release occurs, how rapid it is, and what mechanisms underlie this central response.
In the present study, enzyme-based, Glu-selective biosensors coupled with high-speed amperometry were used to examine experience-dependent changes in NAc extracellular Glu induced by iv cocaine in freely moving rats. This study builds upon our previous work, which established the reliability of this technique and described physiological fluctuations in NAc Glu induced by natural arousing stimuli (Kiyatkin et al. 2013; Wakabayashi and Kiyatkin 2012). With proper controls, this technique allows second-to-second measurement resolution, a critical advantage for detecting rapid and relatively small drug- and behavior-associated Glu fluctuations.
Three major goals were formulated for this study. First: to clarify the initial pharmacological effects of cocaine on Glu release in drug-naive conditions and their possible experience-dependent changes, we examined NAc Glu responses induced by repeated iv cocaine injections at a low, behaviorally relevant dose (1 mg/kg) in awake, freely moving rats. Since the behavioral effects of cocaine are enhanced or sensitized following repeated exposure (Robinson and Berridge 1993), we combined electrochemical recordings with the monitoring of locomotion. Second: to clarify the possible role of the peripheral, interoceptive actions of cocaine in mediating its effects on Glu release, we examined how NAc Glu levels are affected by cocaine-M. If the rapid, second-scale central effects of regular, BBB-permeable cocaine hydrochloride are mediated by Glu release in the NAc via the drug’s peripheral actions, cocaine-M, which acts only in the periphery, could effectively induce a similarly rapid Glu release. However, since cocaine-M lacks central actions, its acute effects and changes following repeated exposure may differ from those of regular cocaine. Third and finally: if the peripheral actions of cocaine are involved in the development of experience-dependent changes in Glu responses, cocaine-M may elicit a conditioned, cocaine-like Glu response when given after cocaine experience. To verify this mechanism, we examined the effects of cocaine-M on NAc Glu levels in cocaine-experienced animals.
METHODS
Subjects and surgery
Data from 61 male Long-Evans rats (Charles River Laboratories) weighing 440±40 g at the time of surgery were used in this study. Rats were individually housed in a climate-controlled animal colony maintained on a 12-12 light-dark cycle, with food and water available ad libitum. All procedures were approved by the NIDA-IRP Animal Care and Use Committee and complied with the Guide for the Care and Use of Laboratory Animals (NIH Publication 865-23) and the UK ARRIVE guidelines.
Details of surgical preparations for electrochemical experiments were described in detail elsewhere (Wakabayashi and Kiyatkin, 2012). Briefly, under general anesthesia (Equithesin 0.33 ml/100 g, ip; active ingredients: sodium pentobarbital, 32.5 mg/kg and chloral hydrate, 145 mg/kg), each rat was unilaterally implanted with BASi cannulae (Bioanalytical Systems, West Lafayette, IN) for future insertions of the electrochemical sensor in the NAc shell. Target coordinates were: AP +1.2 mm, ML ±0.8 mm and DV +7.6 mm according to coordinates of Paxinos and Watson (1998). The guide cannula hubs were fixed to the skull with a head mount constructed from acrylic that was secured using stainless steel bone screws. During the surgery, each rat was also implanted with a chronic jugular catheter, which ran subcutaneously to the head mount and was secured to the head assembly. Rats were allowed a minimum of 4 days of post-operative recovery; jugular catheters were flushed daily with 0.2 ml heparinized saline (15 units/ml) containing gentamicin (0.08 mg/ml).
In vitro calibration and testing of electrochemical sensors
Commercially produced glutamate oxidase-based Glu sensors (Pinnacle Technology, Lawrence, KS) were used in this study. Each sensor is prepared from platinum-iridium wires of 180 μm diameter, with a sensing cavity of ~1 mm length on its tip. The active electrode is incorporated with an integrated Ag/AgCl reference electrode. On the active surface of Glu sensors, glutamate oxidase converts Glu to α-ketoglutarate and H2O2, which is detected as an amperometric oxidation current generated by a +0.6 V applied potential (Hu et al. 1994). The contribution of ascorbic acid to the measured current is competitively reduced by co-localizing ascorbic acid oxidase on the active surface of the sensor that converts ascorbic acid to non-electroactive dehydroascorbate and water. In addition, a negatively charged Nafion polymer layer under the enzyme layer serves to exclude endogenous anionic compounds.
To further reduce the contribution of chemical and physical interferents to electrochemical recordings, we also used enzyme-free Glu0 sensors that were identically constructed but lacked glutamate oxidase. When used in vivo, these sensors are exposed to the same physical and chemical environment as Glu sensors but are fully insensitive to Glu. Therefore, the difference between currents detected by Glu and Glu0 sensors provides the best possible method for revealing actual changes in extracellular Glu levels. Electrochemical recordings employing Glu and Glu0 sensors were conducted in different groups of rats to eliminate the possibility of electrical cross-talk between sensors during an in vivo recording (see Kiyatkin et al. 2013). The currents from both types of sensors were passed to a computer via a potentiostat (Model 3104, Pinnacle Technology). All electrochemical data were sampled at 1 Hz using the PAL software utility (Version 1.5.0, Pinnacle Technology).
Both Glu and Glu0 sensors were calibrated in vitro immediately before and after each in vivo experiment. In vitro calibrations were conducted in PBS (pH 7.3; 23°C) by incrementally increasing the concentration of Glu (Sigma-Aldrich) from 0 to 2, 4, 6, and 10 μM followed by a single addition of ascorbate (250 μM). Since the current response to Glu directly depends upon temperature (Kiyatkin et al. 2013), all sensitivity values were corrected for 37°C (+84%). While Glu sensors used in this study (n=36) varied slightly in their in vitro Glu sensitivity [mean 0.35±0.02 (23°C); 0.64±0.03 (37°C) nA/1 μM], they produced incremental, highly linear (r=0.99) increases in current with increases in [Glu]. Glu sensors also showed current increases with addition of ascorbate (mean 0.75±0.07 nA/250 μM); their average ascorbate:Glu selectivity ratio was 1:150. Electrodes with low sensitivity to Glu (<0.11 nA/1 μM) and/or low selectivity against ascorbate (<1:50) were not used. Post-recording calibrations of Glu sensors (~8 hrs after pre-recording calibration) revealed a ~28% decrease in their sensitivity to Glu (0.25±0.02 nA/1 μM). This decrease in sensitivity may be in part due to fouling of the active surface (Kulagina et al. 1999). As expected, Glu0 sensors (n=23) were fully insensitive to Glu, but showed current responses to ascorbate that were typically slightly smaller than those in Glu sensors. These parameters remained virtually unchanged during post-recording in vitro tests. As shown previously, Glu and Glu0 sensors are equally temperature sensitive and show similar dynamics of current changes following long-term in vitro recording.
Experimental protocol
All behavioral procedures including habituation, drug pretreatment and electrochemical recordings occurred in an electrically insulated chamber located in a larger sound-attenuating cabinet under dim red illumination while an air-filter provided white noise. The cage was equipped with four infrared motion detectors connected to a computer running MED-PC IV (Med Associates, Burlington, VT), which were used for monitoring locomotion during pretreatment and test sessions. Rats were habituated to the testing environment for a minimum of 6 hrs/day for 2 consecutive days before and one day after surgery.
Electrochemical testing
On the day of electrochemical recording, rats were minimally anesthetized (<2 min) with isoflurane and a calibrated Glu or Glu0 sensor was inserted into the brain through the guide cannula. The rat was then placed into the testing chamber and the sensor was connected to the potentiostat via an electrically shielded cable and a multi-channel electrical swivel. Additionally, the injection port of the jugular catheter was connected to a plastic catheter extension that passed through a liquid swivel to outside the recording chamber, allowing stress- and cue-free drug delivery. Testing began a minimum of 150 min post-implant when the baseline current had relatively stabilized (see Results). During the session, rats were exposed to several drug treatments, which differed for each experiment (see below). To verify sensor functionality and specificity of drug-induced Glu responses, one hour prior to the first drug injection, all rats received a 3-min tail-touch, a mild somatosensory stimulus with known behaviorally activating effects and phasic Glu release (Wakabayashi and Kiyatkin, 2012). At the end of each session, rats were deeply anesthetized (iv Equithesin 0.7-1.0 ml, over 2 min) and the biosensor was removed for post-recording calibration.
Drugs and treatment protocols
For the three major goals of this study (see Introduction), we conducted three consecutive experiments to evaluate changes in NAc Glu levels induced by (1) repeated injections of cocaine, (2) repeated injections of cocaine-M, and (3) repeated injections of cocaine-M after cocaine exposure (see Supplemental Fig. S1 for a schematic of our experimental protocols). All drugs were delivered iv while the rats were in quiet resting conditions.
In Experiment 1, we examined the effects of cocaine injections on NAc Glu responses. Since electrochemical recordings are limited to one daily session, two equal groups of rats (n=15 each) were used in this experiment. The first group of drug-naive rats received two injections of cocaine (HCl) at a dose (1 mg/kg, in 0.2 ml saline, over 20 s) optimal for drug self-administration in rats (Pickens and Thompson 1968) that induces clear behavioral, physiological and neuronal effects (Brown and Kiyatkin 2006, 2008; Smirnov and Kiyatkin 2010). The cocaine injections were given 90 min apart. This time interval is 7-8 times longer than the half-life of iv cocaine (Tsibulsky and Norman 1999) and is sufficient for both Glu and Glu0 currents to return to a baseline (Wakabayashi and Kiyatkin 2012). The second group of rats was pretreated with two injections of cocaine on a previous day (1 mg/kg) and used to examine the effects of a third and fourth cocaine injection during electrochemical recording. In Experiment 2, we examined the pattern of Glu responses induced by repeated injections of cocaine-M. In this case, drug-naive rats (n=13) received four consecutive injections of cocaine-M at an equimolar dose (1.33 mg/kg, in 0.2 ml saline, over 20 s) with 90-min inter-injection intervals. All injections of cocaine-M were conducted in one session because there were no significant drug effects observed longer than 20 min post-injection in either Glu levels or behavior. In Experiment 3, we examined how Glu responses to cocaine-M are affected by previous cocaine exposure. Rats used in this experiment (n=16) were pre-treated with cocaine (1 mg/kg, four injections over two consecutive days, similar to Exp. 1) and, after one free day, were given three injections of cocaine-M (1.33 mg/kg, 90 min apart) during an electrochemical recording. In additional 3 rats, we examined changes in Glu electrochemical currents induced by repeated iv injections of saline (0.2 ml over 20 s) delivered under stress- and cue-free conditions.
Histological verification
After recording, the rats were transcardially perfused with PBS followed by 10% formalin and brains were removed for subsequent verification. Sensor placements were verified on 45 μm brain slices using the stereotaxic atlas of Paxinos and Watson (1998). All rats that did not have catheter patency at the end of the experiment, had incorrect sensor placements, or had technical complications during recording were removed from further analysis. Results of sensor placement verification are shown in Fig. S2.
Data analysis
As shown previously, electrochemical currents generated by Glu sensors in vivo are affected by non-specific factors, particularly fluctuations in brain temperature, the presence of other oxidisable substances (i.e., ascorbate, DA, urate), and a slow downward trend in baseline typical of long-term amperometric recordings (Kiyatkin et al. 2013). To exclude these influences, recordings obtained with Glu sensors were compared with similar recordings conducted with Glu0 sensors (see Fig. S3A). Using this approach, we first determined differences in electrochemical currents detected by Glu and Glu0 sensors after each drug injection. Slow current changes were analyzed with 1-min bins from 5 min before to 55 min after each injection, and rapid current changes were analyzed with 4-s bins from 30 s before to 360 s after each injection (Fig. S3B-C). Since the baseline currents slightly varied in amplitude between individual electrodes and showed a gradual within-session decrease, the absolute drug-induced current changes were transformed into relative changes, taking a basal value before each injection (30 s for slow and 8 s for rapid time-course analyses) as 0 nA. Current data were normalized to the average sensitivity of the Glu sensors used. To reveal the Glu contribution, we calculated current differentials as changes generated by individual Glu sensors minus the mean changes generated by Glu0 sensors. These data were calibrated in nM concentrations based on in vitro sensor sensitivity adjusted by the known temperature coefficient.
Based on differences in background currents generated by Glu and Glu0 sensors, we also evaluated basal Glu levels in animals of each group (Fig. S4). Although electrochemical currents detected by both types of sensors decreased during the session, they differed significantly at each time point, and the between-sensor differences remained relatively similar during the session. By transferring these current differences into concentration values, we found that basal levels of extracellular Glu in the NAc are about 1.5 μM, with no significant between-group differences (Fig. S4C). These values are well within the range (1-3 μM) obtained for NAc using microdialysis (Hershey and Kennedy 2013; Vizi et al. 2010).
Two-way repeated-measures (RM) ANOVA was used for evaluating differences in the drug-induced current dynamics produced by Glu and Glu0 sensors. Since both Glu and Glu0 currents were analyzed as a change from 0 nA baseline, the length of the drug effect on [Glu] was determined as the average duration when either the two currents were different (significant Glu vs. Glu0 current main effect) or when the Glu current was changing with respect to the Glu0 current (significant Current × Time interaction). To further examine dynamic changes in [Glu], one-way RM ANOVAs were conducted on calculated [Glu] values to find time periods where there was a significant post-injection main effect and individual bins were compared with baseline using Fisher PLSD post-hoc test. For within-session group comparisons, we determined the amount of Glu released after each drug injection as the area under the curve calculated for both rapid (4-s bins for the first min post-injection) and slow (1-min bins for the duration of the effect) components of the [Glu] response. Locomotor data were analyzed with 1-min resolution using one-way RM ANOVA. For between-group comparisons, we compared the amount of Glu released per minute to normalize for different durations of drug action. One-, two-way, and mixed-factor ANOVAs with Fisher PLSD post-hoc tests were also used to examine within- and between-group differences where appropriate. Since cocaine injections 3 and 4 occurred on a different day, comparisons with cocaine-M injections 3 and 4 were conservatively analyzed as planned comparisons with an appropriate alpha correction for multiple comparisons. For clarity, statistical details are presented in the figure legends.
RESULTS
Cocaine-induced NAc Glu responses are rapidly enhanced (sensitized) following repeated drug exposure
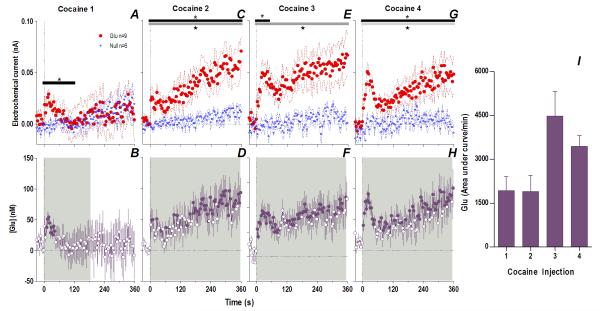
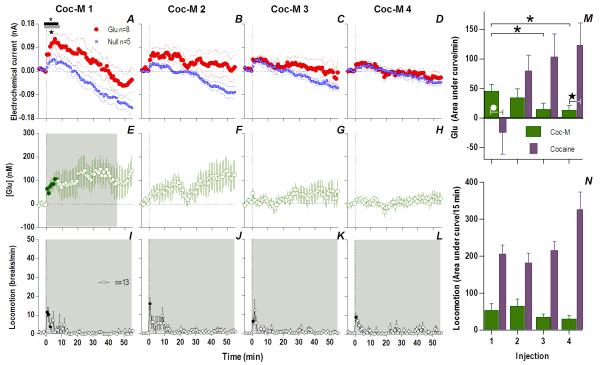
When analyzed at the second time scale, iv cocaine given to awake, drug-naive rats induced a significant increase in NAc Glu levels for ~120 s post-injection (Fig. 1A, 118 s, Current × Time interaction F30,390=1.57 p<0.04). This increase was very rapid (latency to first significant bin 10 s), peaked (54.1±7.3 nM or ~4% above ~1.4 μM baseline) close to the end of the 20-s injection, and disappeared at ~60 s from the injection start (Fig. 1B). In control rats repeatedly injected with saline, this increase was absent, and Glu currents slightly decreased at the same time scale (Fig. 2), suggesting that the procedure of injection itself did not affect Glu levels. Despite relatively large data samples, no significant changes in Glu levels were detected after the first cocaine injection when analyzed at the minute timescale (see Fig. 3A, E). Despite a locomotor response (Fig. 3I), electrochemical currents detected by Glu and Glu0 sensors showed similar dynamics in this case, with no differences within the entire 60-min post-injection interval.
Fig. 1. An immediate, highly phasic Glu release induced by cocaine and its dramatic changes following repeated exposure.
Left top row shows mean (±SEM) changes in currents detected by Glu (red) and Glu0 (blue) sensors for each injection. Horizontal bars on this and all subsequent figures show the duration of significant main effect between Glu/Glu0 currents (black), or a significant Current × Time interaction (gray). The first injection (A) showed only a brief (118 s) interaction (F30,390=1.57 p<0.04) which progressively became greater and longer with each subsequent injection (C. 2: Glu/Glu0 [358 s, F1,13=7.99], interaction [358 s, F90,1170=1.68]; E. 3: Glu/Glu0 [358 s, F1,13=8.375], interaction [46s, F12,156=1.92]; G. 4: Glu/Glu0 [358 s, F1,13=9.00], interaction [358 s, F90,1170=1.34], all p<0.05). Left bottom row shows mean (±SEM) change in [Glu] (purple). Each successive injection resulted in a greater and more prolonged [Glu] response, where the shaded area in this and all subsequent figures shows duration of main effect (1-way RM ANOVA: B. 1: 186 s, F47,376=1.41; D. 2: 358 s, F90,720=2.69; F. 3: 358 s, F90,720=1.63 H. 4: 358 s, F90,720=2.49, all p<0.05). Filled symbols are values significantly different from baseline (PLSD post-hoc). Vertical hatched lines in this and all subsequent figures show the injection start. Right panel (I) compares the area under the curve of [Glu] for the first min after each injection. The total [Glu] released for this period differed significantly between Day 1 (Injection 1-2) and Day 2 (Injection 3-4), (mixed-factor ANOVA F1,16=12.18 p<0.05).
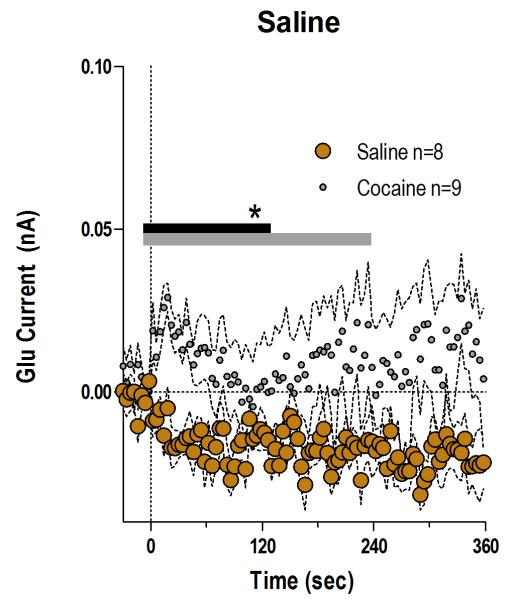
Fig. 2. Saline injection fails to affect NAc Glu levels.
Mean (±SEM) changes in electrochemical currents detected by Glu sensors after the iv saline injection (brown; n=8). The changes induced by the initial injection of cocaine (gray, n=9) are shown for comparison. There was a significant main effect between cocaine and saline (gray bar; 230 s/n=59, F1,15=4.55 p<0.05) or a significant Injection × Time interaction (black bar; 122 s, F31,465=1.48, p<0.05).
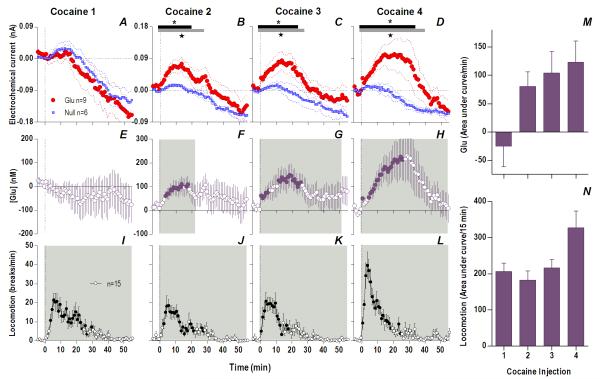
Fig. 3. The appearance of an increasing and persistent Glu release following repeated cocaine exposure.
Top left row shows mean (±SEM) current changes detected by Glu (red) and Glu0 (blue) sensors. Middle left row shows mean (±SEM) change in [Glu] (purple). While the first cocaine injection resulted in no significant difference in Glu/Glu0 currents (A) and [Glu] (E), each successive injection resulted in a greater and more prolonged difference in Glu/Glu0 currents [2: Glu/Glu0 (gray bars; 27 min, F1,13=4.68), interaction (black bars; 19 min, F19,247=1.69); 3: Glu/Glu0 (27 min, F1,13=4.68), interaction (23 min, F23,299=1.67); 4: Glu/Glu0 (39 min, F1,13=4.73), interaction (33 min, F33,429=1.55), all p<0.05], and greater [Glu] release (2: 23 min, F23,184=1.69; 3: 51 min, F51,416=1.38; 4: 56 min, F56,440=2.59), all p<0.05. Bottom left row shows mean (±SEM) changes in locomotion (shaded area, duration of main effect 1: F56,784=6.43; 2: F56,784=6.74; 3: F56,784=7.29; 4: F56,784=12.79, all p<0.001). Filled symbols are values significantly larger than baseline (PLSD post-hoc). M summarizes mean increases in [Glu] across rats for the significant duration of drug effect and N shows locomotion for 15 min post-injection (as area under curve). There were significant increases in [Glu] (mixed-factor ANOVA: Day, F1,16=4.89, Injection × Day, F1,16=4.64,) and locomotion (Day, F1,28=5.61, Injection × Day F1,28=5.02) all p<0.05.
However, Glu responses induced by subsequent cocaine injections changed dramatically in both rapid and slow time scales (Fig. 1 and 3). While the initial component of the Glu response persisted with each subsequent injection, a slower, more tonic increase in Glu levels appeared. This increase was evident at both time scales, becoming progressively larger and more prolonged with each subsequent injection. Compared to the first cocaine injection where no tonic changes were found, the fourth injection induced a large, sustained increase in [Glu] that peaked at 20-30 min (~225 nM or ~17% above baseline) and continued for up to 50-60 min post-injection. While the amount of Glu at the slow time scale was larger with each injection (Fig. 3M), the rapid component of the Glu response remained relatively stable (Fig. 2I). Cocaine-induced locomotor responses also changed modestly following repeated cocaine injections (Fig. 3I-L), primarily as a more rapid onset of locomotion with each successive injection. Some increases in overall locomotion were also observed by the last, fourth injection (Fig. 3N). Although these data suggest rapid sensitization of Glu responses, it is possible that these effects are related to a generalized change in responsiveness due to previous drug exposure. To control for this possibility, we evaluated how Glu responses induced by tail-touch, a mild non-drug somatosensory stimulus, are affected by cocaine pretreatment. When analyzed at both slow and rapid time scale, Glu responses elicited by tail-touch remained similar in drug-naive and cocaine-experienced rats (Fig. S5), suggesting that the enhanced Glu responses observed with cocaine are selective to the drug.
Therefore, cocaine administration is accompanied by very rapid, transient and relatively small changes in NAc [Glu] when the rat is drug-naive but a relatively small number of repeated cocaine injections results in robust changes in drug-induced Glu release. The phasic Glu increase typical of the initial injection is supplemented by a more tonic, increasingly larger, and prolonged Glu increase that parallels more gradual increases in drug-induced locomotor activation.
Peripherally acting cocaine analogue induces stronger NAc Glu release but its effects rapidly habituate following repeated exposure
The rapidity of the initial phasic Glu response induced by cocaine appears inconsistent with a direct drug action on brain neurons, considering the time it takes for the drug to reach the brain, cross the BBB, diffuse to central receptive sites, and exert its effects on central neurons. Alternatively, this rapid Glu response could occur due to direct cocaine action on peripherally located neural substrates and be related to fast neural transmission into the NAc via visceral sensory pathways. To directly test this mechanism, we used BBB-impermeable cocaine-M, which can only mimic the peripheral actions of regular cocaine.
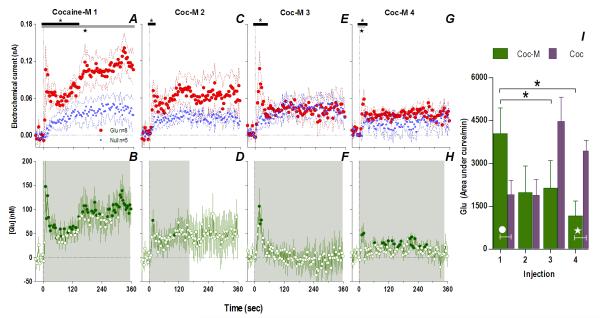
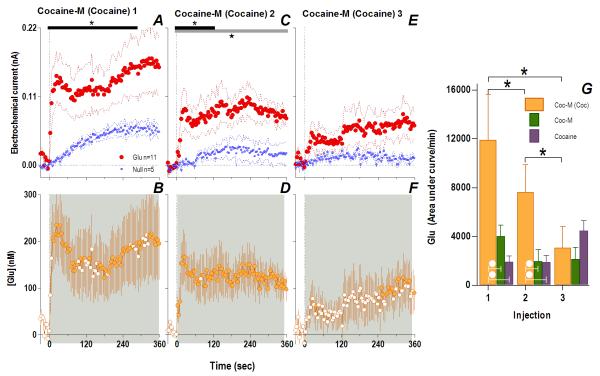
Consistent with this hypothesis, the effects of cocaine-M differed markedly from those of regular cocaine. Similar to cocaine, the first cocaine-M injection induced different changes in Glu and Glu0 currents at the rapid time scale (Fig. 4A), indicating a drug effect on [Glu] for at least 360 s (Glu/Glu0, F1,990=6.61, p<0.03), with dynamic changes occurring ~140 s post-injection (Current × Time interaction F35,358=1.48, p<0.05). In terms of [Glu], the increase was equally rapid (10 s latency) but stronger (~148.0±53.9 nM or ~10% above ~1.6 μM baseline) than that induced by the first cocaine injection (one-tailed Student t-test t15=1.82, p<0.05). Although this initial peak persisted after each subsequent injection, its amplitude and duration progressively decreased (Fig. 4I). Rapid weakening of the Glu response was also clearly evident at 1-min time scale, when Glu increase was significant only for ~7 min after the first drug injection (Fig. 5). Unlike cocaine, cocaine-M induced only weak and transient increases in locomotion (Fig. 5I-L,N) after each drug injection.
Fig. 4. Peripherally acting cocaine-methiodide induces rapid Glu release that rapidly habituates following repeated exposure.
While cocaine-M initially resulted in different Glu (red)/Glu0 (blue) currents (A. Glu/Glu0 main effect [gray bar: 358 s/n=91, F1,11=6.61], interaction [black bar: 138 s, F35,385=1.48], both p<0.05) and rapid [Glu] release (B. shaded area, duration of main effect [358 s, F90,630=1.48 p<0.001]), this effect rapidly habituated with each next injection (Glu/Glu0 interaction 2: [18 s, F5,55=2.54]; 3: [46s, F12,132=1.90]; 4: [26 s, F7,77=2.21] all p<0.05) so that [Glu] release was less (1: [358 s, F90,630=1.48]; 2: [166 s, F42,294=1.43]; 3: [358 s, F90,630=2.08] 4: [350 s, F88,616=1.31] all p<0.05). Filled symbols are values significantly different from baseline (PLSD post-hoc). Right panel (I) summarizes this decreased effect of cocaine-M as the area under the curve of [Glu] for the first min after each injection (green bars, 1-way RM ANOVA, F3,21=3.93 p<0.05, * indicates cocaine-M injections that were significantly different, p<0.05). Similar data for cocaine (purple bars) are shown for comparison. Although the first two injections across treatments did not show overall significant differences (2-way RM ANOVA, ns), this is due to opposite changes with each drug. For the 1st injection, [Glu] was larger for cocaine-M than cocaine HCl (white circle, t15=2.15 p<0.03) but for the last injection changes were opposite (white star, planned comparison t15=3.64 with Bonferroni correction for multiple comparisons, α=0.0125, p<0.002).
Fig. 5. Repeated injections of cocaine-M show marked habituation in tonic Glu release.
While the first cocaine-M injection (A) resulted in different Glu (red)/Glu0 (blue) currents, this effect was short (Glu/Glu0 [gray bar]: 8 min, F1,11=5.26, interaction [black bar]: 7 min, F7,77=2.32, both p<0.05) and not significant for later injections (B-D). [Glu] release was significant only for the first cocaine-M injection (individual points significantly different from baseline (filled symbols) within the period of a main effect [shaded area, 46 min, F46,322=1.43 p<0.05]). M summarizes mean increases in [Glu] per minute for the significant duration of drug effect; effect of cocaine-M decreased with each injection (F3,21=3.47 p<0.04, asterisks show individual significant decreases, p<0.05). When compared with cocaine, there was a significant Treatment × Injection interaction between the first two injections of cocaine-M and cocaine (2-way RM ANOVA, F1,15=13.6 p<0.05), injection was almost significant p=0.07. Glu response to the 1st injection of cocaine-M was greater than that for cocaine (white circle; p<0.05, PLSD post-hoc test). Star shows a significant difference between the 4th injection of cocaine and cocaine-M (planned comparison t15=2.70 with Bonferroni correction for multiple comparisons, α=0.0125, p<0.008). Bottom left row shows mean (±SEM) changes in locomotion. While cocaine-M induced a weak increase in locomotion (main effect: 1: F56,672=2.73; 2: F56,672=2.75; 3: F56,672=2.53, 4: F56,672=2.0, all p<0.05), this response did not differ across injections (N). Cocaine-induced locomotion is shown for comparison.
Therefore, it appears that the rapid component of NAc Glu release induced by cocaine is triggered by the drug’s actions in the peripheral nervous system. While Glu release induced by cocaine-M at the same equimolar dose in drug-naive conditions was stronger than that induced by regular cocaine, it rapidly habituated following repeated exposure. While supporting our hypothesis that the peripheral actions of cocaine contribute significantly to rapid NAc Glu release, direct actions of this drug in the brain appear to be essential to induce locomotor activation and progressive enhancement of Glu responses following its repeated exposure.
Glu responses induced by cocaine-M markedly differ after cocaine experience
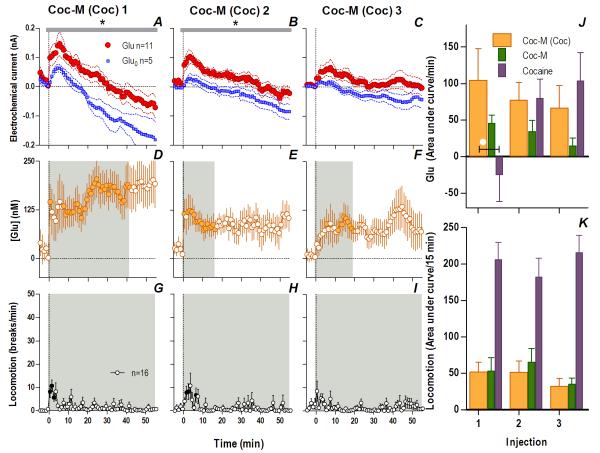
If the peripheral actions of cocaine that always precede the slower, more prolonged direct drug actions in the brain induce rapid, phasic NAc Glu release, these two actions could interact following repeated drug exposure, thus contributing to experience-dependent sensitization of Glu release. To test this hypothesis, we examined NAc Glu responses to cocaine-M in cocaine-experienced rats. These rats were given the same drug treatment as in Experiment 1 and they showed similar increases in cocaine-induced locomotor activation following repeated injections (Fig. S6). Consistent with our expectations, cocaine-experienced rats showed dramatically altered Glu responses to cocaine-M. In these rats, an initial cocaine-M injection induced a rapid change in the Glu vs. Glu0 current for 282 s post-injection (Fig. 6A; Current × Time interaction, F71,994=1.31 p<0.05), indicating a rapid and strong rise in [Glu] (14 s latency, peak at 235.1±68.7 nM or 14% above baseline; Fig. 6B) that exceeded those induced by the initial injections of either cocaine (54.1± 7.3 nM, p<0.01) or cocaine-M (147.2± 53.9 nM) in drug-naive rats. Differences in Glu/Glu0 current persisted for an hour after the injection (Fig. 7A, F1,14=6.80, p<0.03) indicating a prolonged [Glu] rise that remained significant for ~40 min (Fig. 7D).
Fig. 6. Cocaine-methiodide in cocaine-experienced rats induces enhanced Glu release: second scale.
Cocaine-M injections in cocaine-experienced rats resulted in significantly different Glu (red)/Glu0 (blue) currents for the first two injections (1: interaction [black bar, 282 s, F71,994=1.31]; 2: Glu/Glu0 [gray bar, 358 s/n=91, F1,14=5.60], interaction [118 s, F30,420=1.49], all p<0.05) showing significant increases in [Glu] (shaded area, duration of main effect: 1: 358 s, F90,900=2.48; 2: 350 s, F88,880=1.29; 3: 358 s, F90,900=2.42, all p<0.05). Filled symbols are values significantly different from baseline. G compares the area under the curve of [Glu] for the 1st min post-injection across cocaine-treated rats given cocaine-M (orange), where there was a significant decrease with each successive injection (1-way RM ANOVA, F2,20=12.1 p<0.001, *, PLSD post hoc, all p<0.05). Comparison between the first two injections of cocaine-M in experienced rats (orange), acute cocaine-M (green) and cocaine (purple) revealed that Glu differed by both the injection number (2-way RM ANOVA, F1,25=7.04) and by treatment group (F2,25=4.64, both p<0.02). Injection × Group interaction (p=0.09) trended towards significance. Cocaine-experienced rats had a significantly elevated Glu response to cocaine-M compared to drug-naive rats and those that received cocaine (white circles, 1-way ANOVA, 1: F2,27=4.50, Injection 2: F2,27=4.31, both p<0.03; PLSD post-hoc p<0.05). A planned comparison for the 3rd injection revealed no significant differences (all ns).
Fig. 7. Cocaine-methiodide in cocaine-experienced rats induces enhanced Glu release: minute scale.
Cocaine-M injections in cocaine-experienced rats resulted in significantly different Glu (red)/Glu0 (blue) currents for the first two injections at the minute scale (1: Glu/Glu0 [gray bar, 56 min, F1,14=7.03]; 2: Glu/Glu0 (56 min, F1,14=6.04), all p<0.03; 3: ns) showing [Glu] (orange, shaded area, duration of main effect, 1: 42 min, F42,420=1.43; 2: 17 min, F17,170=1.73; 3: 20 min, F20,200=1.73, all p<0.03). Filled symbols are values significantly different from baseline. J summarizes mean (±SEM) increases in [Glu] across rats for the significant duration of drug effect, no differences within the cocaine-experienced group to cocaine-M injections (orange) were found (ns). Comparing Glu responses to the first two injections of cocaine-M in experienced rats, with acute cocaine-M (green) and cocaine (purple) revealed an Injection × Treatment interaction (F2,25=4.46 p<0.03); the Glu response to the first cocaine-M injection in cocaine-experienced rats differed from that of rats receiving cocaine for the first time (white circle, F2,27=3.45, p<0.05). While there was a locomotor response to cocaine-M in cocaine-experienced rats (shaded area, duration of main effect, 56 min all injections: 1: F56,840=2.76; 2: F56,840=2.04; 3: F56,840=1.39, all injections p<0.05) this response did not differ across injections (K, ns). Cocaine and acute cocaine-M locomotion is shown for comparison.
Glu responses to cocaine-M in cocaine-experienced rats also remained significantly elevated after the second injection but steadily decreased after the third (Fig. 6-7). Although there is some indication of an elevated [Glu] response to the third injection (Fig. 6F, 7F), this result should be viewed with caution since the Glu and Glu0 currents at both time scales did not differ significantly after this injection (Fig. 6C, 7E). At the rapid timescale, cocaine-M in cocaine-experienced rats evoked a greater Glu response for two successive injections than either cocaine-M in naive rats or cocaine itself (Fig. 6G). At the slow timescale, cocaine-M induced an enhanced tonic Glu release that differed from the acute response to the drug, sharing some similarities with the Glu response induced by cocaine. In contrast to profound Glu responses, these rats showed minimal and relatively stable locomotor responses to repeated cocaine-M injections (Fig. 7G-I, K). Since cocaine-M mimics only peripheral actions of cocaine, the enhanced Glu release induced by this drug in cocaine-experienced rats could be viewed as a conditioned Glu response to a cocaine-related interoceptive cue, indicating the importance of this action in the development of neurochemical sensitization.
DISCUSSION
Recent evidence suggests that repeated cocaine administration induces persistent neuroadaptive changes in the brain circuits involved in drug reinforcement. While postsynaptic receptor changes within Glu synapses are suggested to play a critical role in the development of cocaine-induced neuroadaptations and possibly cocaine addiction (Kalivas et al. 2009; Schmidt and Pierce 2010), the influence of cocaine on rapid-scale Glu release has remained generally unknown. In this study, we used high-speed electrochemical monitoring of extracellular levels of Glu in the NAc in awake, freely moving rats to determine whether passive injections of cocaine at a low, self-administering dose affect Glu release, whether and how cocaine experience influences this release, and what mechanisms may underlie these changes.
Glu measurements in behaving animals
While rapid time-course resolution is a critical advantage of electrochemical techniques over microdialysis, multiple tests and controls are essential to make these measurements reliable. The enzyme-based Glu sensors used in this study had sufficiently high sensitivity and selectivity to detect relatively small and rapid fluctuations in Glu levels. However, as shown in our previous studies (Kiyatkin et al. 2013; Wakabayashi and Kiyatkin 2012), reliable Glu measurements in freely moving rats require the use of Glu0 sensors that are equally sensitive to all chemical and physical interferents except Glu. This approach allows us to exclude the influence of most non-specific physical and chemical contributors to the electrochemical currents detected by Glu sensors, which accounts for up to 90-95% of the total current changes induced by natural arousing stimuli (Kiyatkin et al. 2013). Using this approach, we revealed that the basal extracellular Glu levels in the NAc (1.0-1.5 μM) are lower than usually reported in electrochemical studies but well within the range of the most accurate microdialysis estimates obtained with no-net flux analysis (Hershey and Kennedy 2013; Vizi et al. 2010) and are within the affinity of extrasynaptic NMDA Glu receptors (0.4-1.7 μM; Zito and Scheuss 2009). While these physiological Glu fluctuations are smaller in amplitude (50-200 nM, or 5-20% over baseline), they are much more phasic than those detected in microdialysis studies.
Phasic Glu release as a primary neural response induced by iv cocaine in drug-naive conditions
By using this high temporal resolution, we found that the first cocaine injection in drug-naive rats induces a very rapid (6-8 s latency), relatively small (~50 nM) and transient (~60 s) rise in Glu levels. Despite this second-scale effect and clear evidence of locomotor activation, no significant changes in NAc Glu levels were found when the data were analyzed on a minute scale, a time resolution similar to microdialysis. Thus, differences in temporal resolution between these approaches may explain why this phasic Glu release has remained undetected. Similar short latencies were reported in awake rats for other neural effects of iv cocaine, particularly cortical EEG desynchronization and EMG activation (Kiyatkin and Smirnov, 2010), excitation of NAc neurons (Kiyatkin and Brown 2007), activation of both DA and non-DA VTA neurons (Brown and Kiyatkin 2008), and electrochemically detected NAc DA release (Aragona et al. 2008). Considering Glu release as the primary means of neuronal excitation (Krnjevic 1970) and exclusive Glu sensitivity of ventral striatal neurons (Kiyatkin and Rebec 1999), similar onset latencies of both NAc Glu release and activation of NAc neurons induced by iv cocaine may suggest an underlying causal relationship between the two effects.
It is challenging to explain these second-scale neural effects based exclusively on cocaine’s direct action in the brain. The drug must first travel from the venous to arterial circulation to reach brain vessels, cross the BBB before diffusing to receptors and interacting with them to induce direct neural effects. Alternatively, this short-latency NAc Glu release and other excitatory neural effects could result from cocaine’s actions on peripherally located neural substrates (i.e. multiple subtypes of ionic channels expressed on afferents of sensory nerves) that induce an ascending neural signal to the CNS. The peripheral origin of the initial, fast phase of cocaine-induced Glu release was confirmed in this study by using BBB-impermeable cocaine-M. Like cocaine, cocaine-M induced equally rapid and even stronger increases in NAc Glu levels when used at an equimolar dose in drug-naive rats. Cocaine-M also induces similarly rapid and somewhat stronger EEG desynchronization (Kiyatkin and Smirnov 2010) and NAc neuronal excitation (Kiyatkin and Brown 2007), lending further support to the idea that Glu release may underlie these neural effects.
The ability of cocaine to induce a second-scale, excitatory Glu signal transmitted from the periphery via visceral sensory pathways may help explain the discrepancy with the drug’s relatively slower and more delayed direct central actions. Although cocaine’s interactions with brain monoamine transporters appear essential for mediating its reinforcing properties (Ritz et al. 1987; Wise and Bozarth 1987), DA uptake inhibition in the NAc ex vivo (when any contribution of DA release is excluded) is only detected at high doses (5- 10 mg/kg, iv; Pogun et al. 1991; Stathis et al. 1995; Wang et al. 2007) and 3-5 minutes after the iv injection (Stathis et al. 1995; Wang et al. 2007). Even when using a more sensitive approach by combining DA iontophoresis with fast-cyclic voltammetric DA detection, iv cocaine at self-administering doses has weak effects on DA uptake, appearing with 1-2 min onset latencies (Kiyatkin et al. 2000; see, however, Mateo et al, 2004). Thus, these rapid excitatory neural effects are difficult to explain based solely on cocaine’s eventual interaction with brain monoamine transporters, and they could constitute an additional component of cocaine’s overall action on the brain. Indeed, these peripheral actions may be also relevant for other sites of cocaine administration (i.e. lung alveoli, nasal and oral cavities), which are densely innervated by afferents of sensory nerves and also capable of rapidly transmitting cocaine’s neural signal to the CNS.
Experience-dependent changes in NAc Glu responses induced by cocaine
The second novel finding of this study was the appearance of an enhanced Glu response to cocaine after relatively short drug experience. This effect was evident during the second drug injection within the first session and became progressively larger by the third and fourth injections of the next session. Second-scale analysis revealed that while the initial, phasic component of the Glu response persisted with each cocaine injection, an additional, tonic phase appeared and became larger with each consecutive injection. Importantly, these robust changes were selective to cocaine; Glu responses to tail-touch, an arousing non-drug stimulus, were not affected by cocaine treatment.
While the initial, phasic and persistent rise in Glu levels induced by each cocaine injection is likely due to the drug’s peripheral actions and impulse-dependent Glu release, the cause and mechanisms of the slowly developing and prolonged tonic increases in Glu (100-200 nM) are less clear. Although increases in extracellular Glu levels are usually viewed as its spillover from synapses (Nichols and Attwell, 1990), Glu is also released from boutons that do not make synaptic contacts, and reaches high-affinity extrasynaptic Glu receptors by diffusion. Glu is also released by glial cells (van der Zeyden et al. 2008; Vizi et al. 2010) to interact with extrasynaptic Glu receptors located on other glial cells and neurons (Zito and Scheuss 2009). Whereas synaptic Glu is regulated by Glu transporters acting at high concentrations (0.1-1.0 mM) on a rapid time scale to rapidly remove Glu from the synaptic cleft and prevent its spillover in the extracellular space (Kanai and Hediger 2003; Moussawi et al. 2011), Glu in the extracellular space is regulated by high-affinity transporters which operate at much lower levels (1-3 μM) and slower time scales (Gegelashvili and Schousboe 1998; Kanai et al. 2013; Vizi et al. 2010), thus allowing more prolonged but lower-magnitude fluctuations. Our estimates of basal extracellular Glu levels (~1.5 μM) correspond well to the range of basal extracellular Glu levels typically detected by no-net flux microdialysis (Hershey and Kennedy 2013). Whereas synaptic Glu transmission is involved in rapid information transfer between neurons, the functional role of non-synaptic Glu transmission remains less clear. However, Glu fluctuations at the levels found in this study are sufficient to affect high-affinity NMDA receptors and modulate the activity of multiple neural and possibly glial cells via Ca2+ release (Vizi et al. 2010; Zito and Scheuss 2009), and thus potentially able to induce long-lasting neuroadaptations (Kalivas 2009). While longer and more extensive cocaine exposure, especially following self-administration, may result in more profound changes in Glu transmission, including alterations in release, pre and post-synaptic receptor number and subtypes, our study suggests very rapid and significant alterations in the dynamic components of Glu release occur during the initial stages of drug experience.
Regardless of their mechanism, our data suggest that repeated cocaine exposure results in rapid sensitization of Glu release. Importantly, this neurochemical sensitization occurs somewhat more rapidly and robustly than increases in cocaine-induced locomotion, supporting the view that behavioral sensitization is an index of the neuroadaptive changes in the brain in response to drug (Robinson and Berridge 1993; Robinson and Berridge 2000). However, it should be noted that motor activation with each consecutive cocaine injection occurred more rapidly, a more sensitive measure of locomotor sensitization.
Critical role of interoceptive actions of cocaine in neural sensitization
While associative learning is viewed as an important contributor to drug-induced neural sensitization (Di Chiara 1998; Kalivas and Stewart 1991) and the development of drug-taking behavior (Wise and Bozarth 1987), it is usually considered to be the result of an interaction between environmental (exteroceptive) stimuli that precede drug intake and the drug per se. However, this stimulus-drug interaction could be more complex in the case of cocaine, which has both fast peripheral and slower central actions, each mediated via different neural substrates (ionic channels expressed on afferents of sensory nerves at the sites of administration and monoamine transporters, respectively) and each triggering distinct neural effects with different time-courses. Following repeated drug exposure, these distinct neural effects may interact based on principles of Pavlovian conditioning. As a result of this interaction, the initial, peripherally triggered actions of cocaine may acquire some conditioned interoceptive stimulus properties, thus contributing to its sensitized neural effects. Interestingly, our data also suggests that the central actions of cocaine may be necessary to maintain or even enhance the phasic Glu response triggered by the peripheral actions of cocaine. Therefore, experience-dependent sensitization could be viewed as a consequence of a within-drug conditioning: a dynamic interaction between peripherally driven (sensory or interoceptive) and centrally mediated (rewarding) actions of cocaine.
Since cocaine-M cannot reach the brain and mimics only some of the peripheral aspects of cocaine’s action, this drug was an important tool to verify this hypothesis. While the initial administration of cocaine-M in drug-naive animals induced equally rapid but stronger Glu release than the first injection of cocaine HCl, this effect rapidly habituated with subsequent injections, a pattern typical of simple somato-sensory stimuli (Hendry et al. 1999; Sandler and Tsitolovsky 2008; Stancak 2006). Unlike cocaine, cocaine-M did not elicit true locomotor activation, but did mimic cocaine in its ability to induce an acute motor response during the injection. However, the response to cocaine-M was drastically different in rats with cocaine experience. In this case, this drug induces powerful Glu release, significantly exceeding that induced by either cocaine-M or cocaine in drug-naive conditions. Thus, it appears that cocaine-M acts in cocaine-experienced animals as a conditioned drug stimulus, inducing conditioned Glu release. With subsequent injections, Glu responses to cocaine-M decreased but remained consistently larger than those induced by cocaine-M in naive rats. This finding is in agreement with classic observations (Skinner 1933) suggesting the ability of conditioned stimuli to retain their activating potential for some time after termination of their pairing with the unconditioned stimulus.
Conclusions and functional implications
In this study, we demonstrate that cocaine at a low, self-administering dose induces rapid, transient NAc Glu release that is consistently enhanced following relatively short drug experience. We show that cocaine-induced Glu release has distinct peripheral and central sources and substantiate the critical role of peripheral actions of cocaine in mediating acute Glu release and its experience-dependent sensitization.
While the direct interaction of cocaine with monoamine transporters is essential in mediating its reinforcing properties, the ability of cocaine to engage visceral sensory systems, induce rapid neural effects independent of its direct central actions, and establish a within-drug conditioned association appears to be an important factor in determining its addictive potential. The ability of peripheral sensory actions of cocaine to acquire new signaling significance may explain the experience-dependent changes in the psycho-emotional and physiological effects of cocaine in humans. Similar to the well-established shift of neuronal activation from the primary reinforcer to the earliest reinforcer-predicting stimulus during learning (Schultz 1998), the initially neutral or even unpleasant sensory effects of cocaine may rapidly become the most desired, rewarding events of drug consumption. This ability of cocaine’s peripheral sensory actions to acquire new signaling properties through repeated pairing with its central actions may contribute to the mechanism attributing incentive motivational significance to interoceptive drug cues (Robinson and Berridge 1993) and help explain the persistent nature of human cocaine addiction and the limited success achieved so far in its pharmacological correction.
While this study was focused on cocaine, a similar mechanism of within-drug conditioning may apply to other addictive drugs. We have recently shown that, like cocaine, iv nicotine given to drug-naive rats at a low, self-administering dose induces rapid EEG desynchronization, EMG activation and weak locomotor activation; all these effects are substantially reduced by hexamethonium, a selective blocker of peripheral nicotinic receptors, and their immediate components are mimicked by peripherally acting nicotine analog (Lenoir and Kiyatkin 2011; Lenoir et al., 2013). Similar to this study, the effects of this methiodide nicotine analog consistently decreased following its repeated exposure but re-appeared again when the drug was used after nicotine experience. Hence, the findings of this study of cocaine may generalize to other drugs of abuse, and implicate the critical involvement of Glu release triggered via visceral sensory systems in mediating the acute neural effects of drugs and their consistent changes following repeated drug experience.
Supplementary Material
Acknowledgements
We would like to thank Drs. A. Bonci, C. Lupica, and T. Robinson for helpful suggestions regarding this manuscript. We also appreciate editorial assistance of Ms. Stephanie Myal. This study was supported by the Intramural Research Program of NIDA-IRP.
Abbreviations
- BBB
blood-brain barrier
- cocaine-M
cocaine methiodide
- DA
dopamine
- Glu
glutamate
- NAc
nucleus accumbens
- VTA
ventral tegmental area
Footnotes
Conflict of interests The Authors declare no competitive interests in relation to the work described.
Author contributions: EAK supervised the project. KTW and EAK performed experiments, wrote the manuscript and both have approved the submission of this version.
REFERENCES
- Aragona BJ, Cleaveland NA, Stuber GD, Day JJ, Carelli RM, Wightman RM. Preferential enhancement of dopamine transmission within the nucleus accumbens shell by cocaine is attributable to a direct increase in phasic dopamine release events. J. Neurosci. 2008;28:8821–31. doi: 10.1523/JNEUROSCI.2225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–32. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Malenka RC, Bonci A. Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: electrophysiological and behavioral correlates in individual rats. J. Neurosci. 2004;24:7482–90. doi: 10.1523/JNEUROSCI.1312-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PL, Kiyatkin EA. The role of peripheral Na+ channels in triggering the central excitatory effects of intravenous cocaine. Eur. J. Neurosci. 2006;24:1182–92. doi: 10.1111/j.1460-9568.2006.05001.x. [DOI] [PubMed] [Google Scholar]
- Brown PL, Kiyatkin EA. Sensory effects of intravenous cocaine on dopamine and non dopamine ventral tegmental area neurons. Brain Res. 2008;1218:230–49. doi: 10.1016/j.brainres.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G. A motivational learning hypothesis of the role of mesolimbic dopamine in compulsive drug use. J. Psychoparmacol. 1998;12:54–67. doi: 10.1177/026988119801200108. [DOI] [PubMed] [Google Scholar]
- Ferrario CR, Shou M, Samaha AN, Watson CJ, Kennedy RT, Robinson TE. The rate of intravenous cocaine administration alters c-fos mRNA expression and the temporal dynamics of dopamine, but not glutamate, overflow in the striatum. Brain Res. 2008;1209:151–6. doi: 10.1016/j.brainres.2008.02.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegelashvili G, Achousboe Cellular distribution and kinetic properties of high-affinity glutamate transporters. Brain Res. Bul. 1998;45:233–8. doi: 10.1016/s0361-9230(97)00417-6. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Jones GH, Hubert GW, Neill DB, Justice JB. Assessment of the relative contribution of peripheral and central components in cocaine place conditioning. Pharmacol. Biochem. Behav. 1994;47:973–9. doi: 10.1016/0091-3057(94)90306-9. A. [DOI] [PubMed] [Google Scholar]
- Hendry SC, Hsiao SS, Brown MC. Fundamentals of sensory systems. In: Zigmond MJ, Landis SC, Roberts JL, Squire LR, editors. Fundamental Neuroscience. Academic Press; San Diego: 1999. pp. 657–70. [Google Scholar]
- Hershey ND, Kennedy RT. In vivo calibration of microdialysis using infusion of stable-isotope labeled neurotransmitters. ACS Chem. Neurosci. 2013;4:729–36. doi: 10.1021/cn300199m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Mitchell KM, Albahadily FN, Michaelis EK, Wilson GS. Direct measurement of glutamate release in the brain using a dual enzyme-based electrochemical sensor. Brain Res. 1994;659:117–25. doi: 10.1016/0006-8993(94)90870-2. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu. Rev. Neurosci. 2006;29:565–98. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat. Rev. Neurosci. 2009;10:561–72. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Lalumiere RT, Knackstedt L, Shen H. Glutamate transmission in addiction. Neuropharmacology. 2009;56(Suppl 1):169–73. doi: 10.1016/j.neuropharm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, O’Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33:166–80. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res. Rev. 1991;16:223–44. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Hediger MA. The glutamate and neutral amino acid transporter family: physiological and pharmacological implications. Eur. J. Pharmacol. 2003;479:237–47. doi: 10.1016/j.ejphar.2003.08.073. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Clemenson B, Simonin A, Leuenberger M, Lochner M, Weisstanner M, Hegiger MA. The SLC1 high-affinity glutamate and neutral amino acid transporter family. Mol. Asp, Med. 2013;34:108–20. doi: 10.1016/j.mam.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Rebec GV. Modulation of striatal neuronal activity by glutamate and GABA: iontophoresis in awake, unrestrained rats. Brain Res. 1999;822:88–106. doi: 10.1016/s0006-8993(99)01093-8. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Kiyatkin DE, Rebec GV. Phasic inhibition of dopamine uptake by intravenous cocaine in freely moving rats. Neuroscience. 2000;98:729–41. doi: 10.1016/s0306-4522(00)00168-8. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Brown PL. I.v. cocaine induces rapid, transient excitation of striatal neurons via its action on peripheral neural elements: single-cell, iontophoretic study in awake and anesthetized rats. Neuroscience. 2007;148:978–95. doi: 10.1016/j.neuroscience.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA, Smirnov MS. Rapid EEG desynchronization and EMG activation induced by intravenous cocaine in freely moving rats: a peripheral, nondopamine neural triggering. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;298:R285–300. doi: 10.1152/ajpregu.00628.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA, Wakabayashi KT, Lenoir M. Physiological fluctuations in brain temperature as a factor affecting electrochemical evaluations of extracellular glutamate and glucose in behavioral experiments. ACS Chem. Neurosci. 2013;4:652–65. doi: 10.1021/cn300232m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjevic K. Glutamate and gamma-aminobutyric acid in brain. Nature. 1970;228:119–24. doi: 10.1038/228119a0. [DOI] [PubMed] [Google Scholar]
- Kulagina NV, Shankar L, Michael AC. Monitoring glutamate and ascorbate in the extracellular space of brain tissue with electrochemical microsensors. Anal. Chem. 1999;71:5093–100. doi: 10.1021/ac990636c. [DOI] [PubMed] [Google Scholar]
- Lenoir M, Kiyatkin EA. Critical role of peripheral actions of intravenous nicotine in mediating its central effects. Neuropsychopharmacology. 2011;36:2125–38. doi: 10.1038/npp.2011.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir M, Tang JS, Woods AS, Kiyatkin EA. Rapid sensitization of physiological, neuronal, and locomotor effects of nicotine: Critical role of peripheal drug actions. J. Neurosci. 2013;33:9937–49. doi: 10.1523/JNEUROSCI.4940-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–63. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Riegel A, Nair S, Kalivas PW. Extracellular glutamate: functional compartments operate in different concentration ranges. Front. Syst. Neurosci. 2011;5:94. doi: 10.3389/fnsys.2011.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls D, Attwell D. The release and uptake of excitatory amino acids. Trends Pharmacol. Sci. 1990;11:462–68. doi: 10.1016/0165-6147(90)90129-v. [DOI] [PubMed] [Google Scholar]
- Paxinos G. The rat brain in stereotaxic coordinates. 4th ed Academic Press; San Diego: 1998. [Google Scholar]
- Perry M, Li Q, Kennedy RT. Review of recent advances in analytical techniques for the determination of neurotransmitters. Anal. Chem. Acta. 2009;653:1–22. doi: 10.1016/j.aca.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens R, Thompson T. Cocaine-reinforced behavior in rats: effects of reinforcement magnitude and fixed-ratio size. J. Pharmacol. Exp. Ther. 1968;161:122–29. [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J. Neurosci. 1996;16:1550–60. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogun S, Scheffel U, Kuhar MJ. Cocaine displaces [3H]WIN 35,428 binding to dopamine uptake sites in vivo more rapidly than mazindol or GBR 12909. Eur. J. Pharmacol. 1991;198:203–5. doi: 10.1016/0014-2999(91)90622-w. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–23. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res. Rev. 1993;18:247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95(Suppl 2):S91–117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):3–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Sandler U, Tsitolovsky L. Neural Cell Behavior and Fuzzy Logic. Springer; 2008. p. 478. [Google Scholar]
- Schmidt HD, Pierce RC. Cocaine-induced neuroadaptations in glutamate transmission: potential therapeutic targets for craving and addiction. Ann. N. Y. Acad. Sci. 2010;1187:35–75. doi: 10.1111/j.1749-6632.2009.05144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J. Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Shriver DA, Long JP. A pharmacologic comparison of some quaternary derivatives of cocaine. Arch. Int. Pharmacodyn. Ther. 1971;189:198–208. [PubMed] [Google Scholar]
- Skinner BF. The abolishment of a discrimination. Proc. Natl. Acad. Sci. USA. 1933;19:825–28. doi: 10.1073/pnas.19.9.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stancak A. Cortical oscillatory changes occurring during somatosensory and thermal stimulation. Prog. Brain Res. 2006;159:237–52. doi: 10.1016/S0079-6123(06)59016-8. [DOI] [PubMed] [Google Scholar]
- Stathis M, Scheffel U, Lever SZ, Boja JW, Carroll FI, Kuhar MJ. Rate of binding of various inhibitors at the dopamine transporter in vivo. Psychopharmacology (Berl) 1995;119:376–84. doi: 10.1007/BF02245852. [DOI] [PubMed] [Google Scholar]
- Suto N, Ecke LE, You ZB, Wise RA. Extracellular fluctuations of dopamine and glutamate in the nucleus accumbens core and shell associated with lever-pressing during cocaine self-administration, extinction, and yoked cocaine administration. Psychopharmacology (Berl) 2010;211:267–75. doi: 10.1007/s00213-010-1890-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsibulsky VL, Norman AB. Satiety threshold: a quantitative model of maintained cocaine self-administration. Brain Res. 1999;839:85–93. doi: 10.1016/s0006-8993(99)01717-5. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–87. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- van der Zeyden M, Oldenziel WH, Rea K, Cremers TI, Westerink BH. Microdialysis of GABA and glutamate: analysis, interpretation and comparison with microsensors. Pharmacol. Biochem. Behav. 2008;90:135–47. doi: 10.1016/j.pbb.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Venton BJ, Robinson TE, Kennedy RT. Transient changes in nucleus accumbens amino acid concentrations correlate with individual responsivity to the predator fox odor 2,5-dihydro-2,4,5-trimethylthiazoline. J. Neurochem. 2006;96:236–46. doi: 10.1111/j.1471-4159.2005.03549.x. [DOI] [PubMed] [Google Scholar]
- Vizi ES, Fekete A, Karoly R, Mike A. Non-synaptic receptors and transporters involved in brain functions and targets of drug treatment. Br. J. Pharmacol. 2010;160:785–809. doi: 10.1111/j.1476-5381.2009.00624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi KT, Kiyatkin EA. Rapid changes in extracellular glutamate induced by natural arousing stimuli and intravenous cocaine in the nucleus accumbens shell and core. J. Neurophysiol. 2012;108:285–99. doi: 10.1152/jn.01167.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Ordway GA, Woolverton W. Effects of cocaine on monoamine uptake as measured ex vivo. Neurosci. Lett. 2007;413:191–5. doi: 10.1016/j.neulet.2006.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol. Rev. 1987;94:469–92. [PubMed] [Google Scholar]
- You ZB, Chen YQ, Wise RA. Dopamine and glutamate release in the nucleus accumbens and ventral tegmental area of rat following lateral hypothalamic self-stimulation. Neuroscience. 2001;107:629–39. doi: 10.1016/s0306-4522(01)00379-7. [DOI] [PubMed] [Google Scholar]
- You ZB, Wang B, Zitzman D, Azari S, Wise RA. A role for conditioned ventral tegmental glutamate release in cocaine seeking. J. Neurosci. 2007;27:10546–55. doi: 10.1523/JNEUROSCI.2967-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zito K, Scheuss V. NMDA receptor function and physiological modulation. In: Squire LR, editor. Encyclopedia of Neuroscience. Vol. 6. Academic Press; Oxford: 2009. pp. 1157–64. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.