Abstract
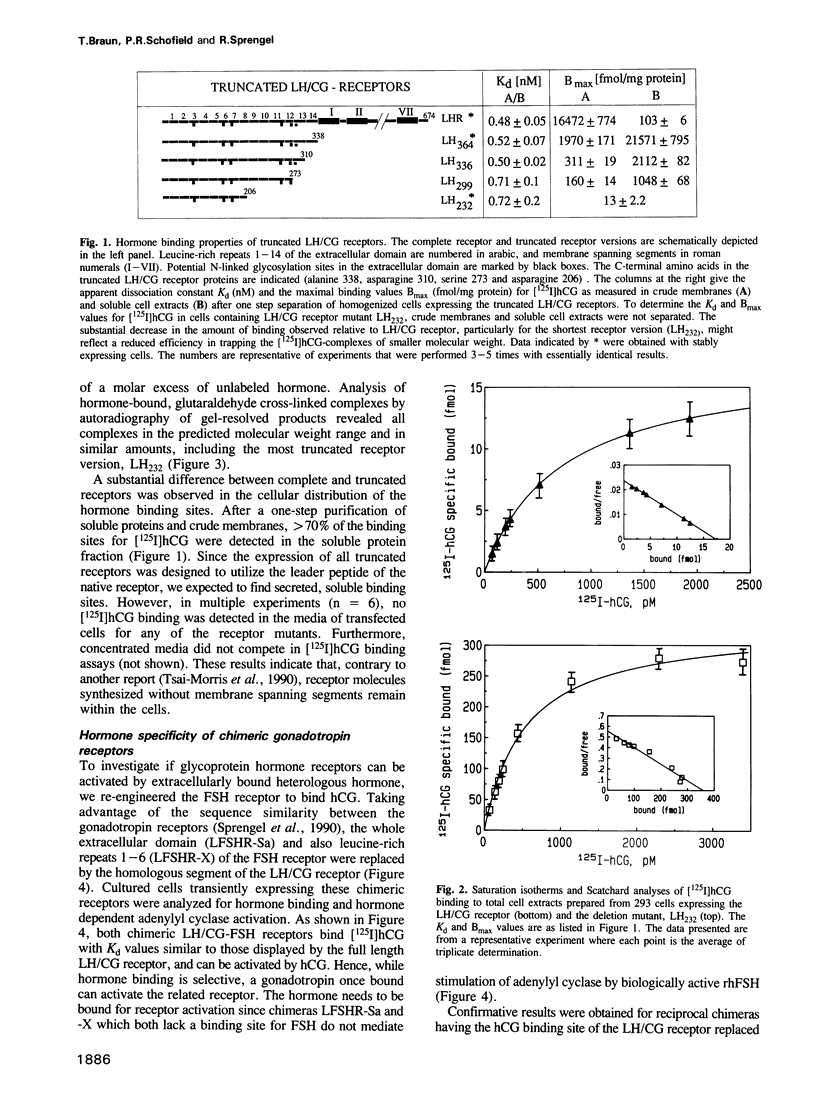
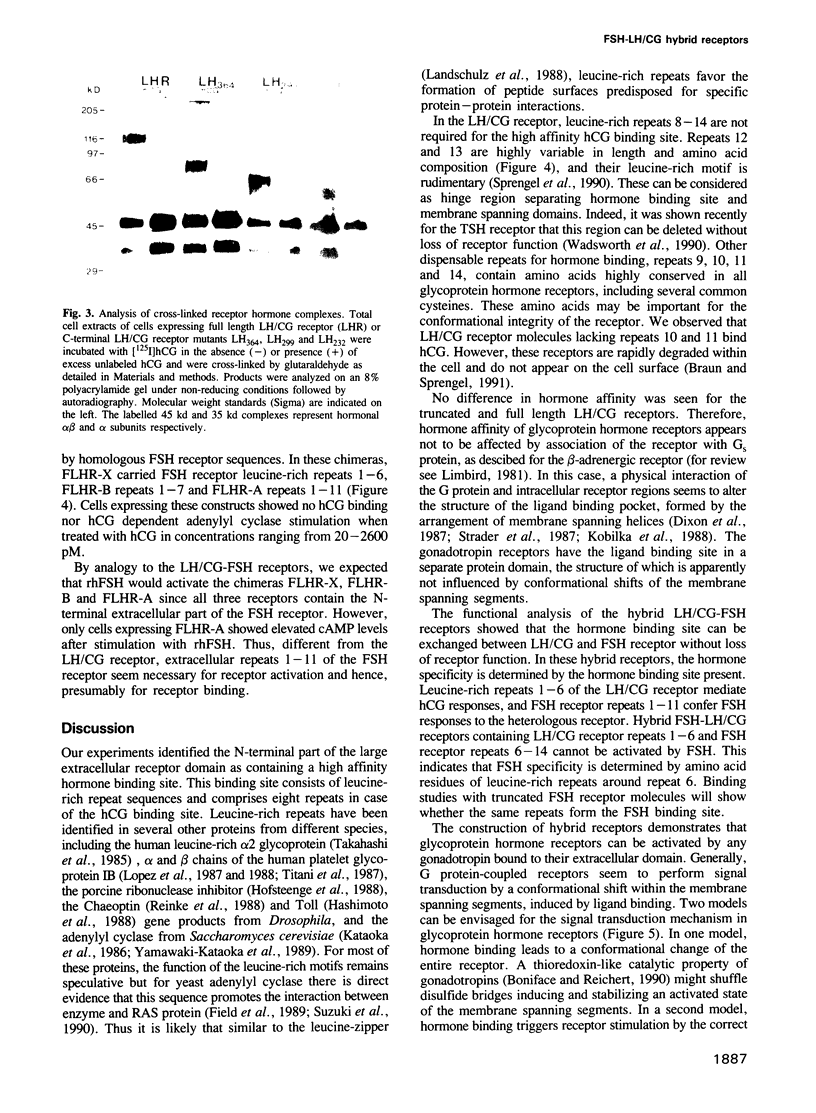
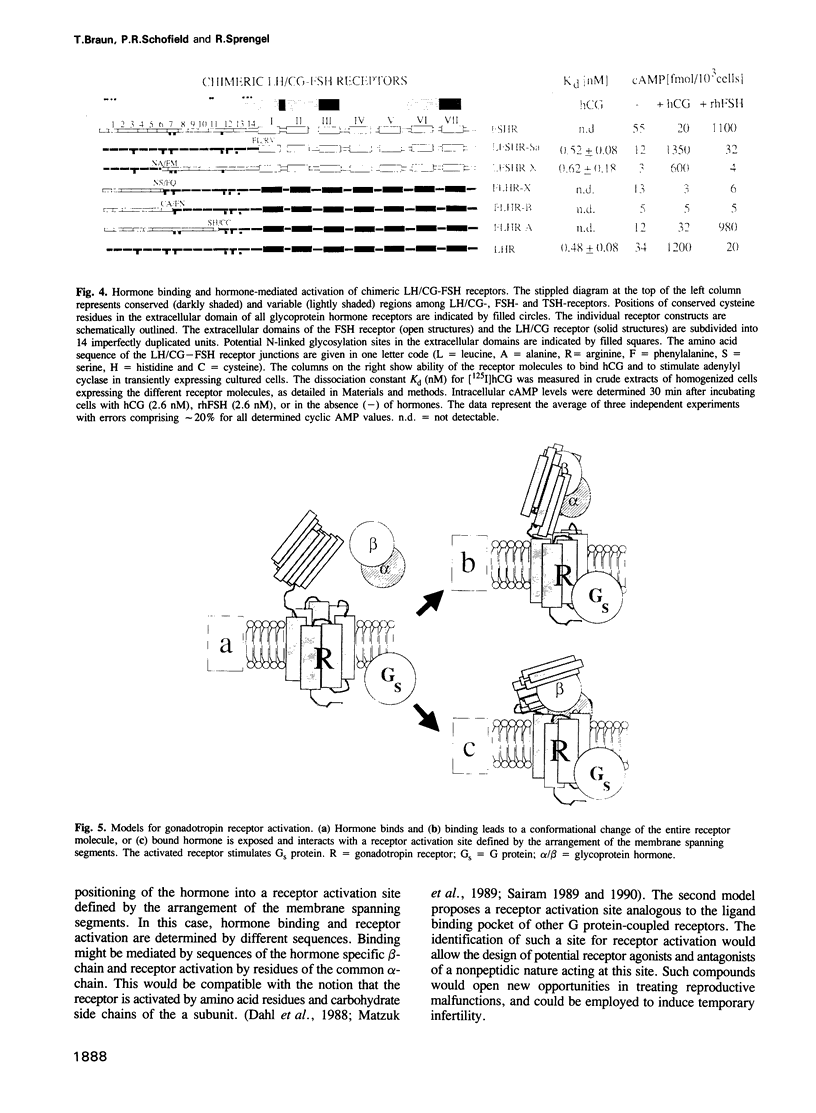
Recombinant expression of truncated receptors for luteinizing hormone/chorionic gonadotropin (LH/CG) revealed that the amino-terminal leucine-rich repeats 1-8 of the extracellular receptor domain bind human chorionic gonadotropin (hCG) with an affinity (Kd = 0.72 +/- 0.2 nM) similar to that of the native LH/CG receptor (Kd = 0.48 +/- 0.05 nM). LH/CG receptor leucine-rich repeats 1-8 were used to replace homologous sequences in the closely related receptor for follicle stimulating hormone (FSH). Cells expressing such chimeric LH/CG-FSH receptors bind hCG and show elevated cylic AMP levels when stimulated by hCG but not by recombinant human FSH (rhFSH). Similarly, a chimeric LH/CG receptor in which leucine-rich repeats 1-11 originated from the FSH receptor is activated by rhFSH but not by hCG. For this chimera, no residual [125I] hCG binding was observed in a range of 2 pM to 10 nM. Our results demonstrate that specificity of gonadotropin receptors is determined by a high affinity hormone binding site formed by the amino-terminal leucine-rich receptor repeats.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boniface J. J., Reichert L. E., Jr Evidence for a novel thioredoxin-like catalytic property of gonadotropic hormones. Science. 1990 Jan 5;247(4938):61–64. doi: 10.1126/science.2104678. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl K. D., Bicsak T. A., Hsueh A. J. Naturally occurring antihormones: secretion of FSH antagonists by women treated with a GnRH analog. Science. 1988 Jan 1;239(4835):72–74. doi: 10.1126/science.3122320. [DOI] [PubMed] [Google Scholar]
- Dixon R. A., Sigal I. S., Rands E., Register R. B., Candelore M. R., Blake A. D., Strader C. D. Ligand binding to the beta-adrenergic receptor involves its rhodopsin-like core. Nature. 1987 Mar 5;326(6108):73–77. doi: 10.1038/326073a0. [DOI] [PubMed] [Google Scholar]
- Field J., Xu H. P., Michaeli T., Ballester R., Sass P., Wigler M., Colicelli J. Mutations of the adenylyl cyclase gene that block RAS function in Saccharomyces cerevisiae. Science. 1990 Jan 26;247(4941):464–467. doi: 10.1126/science.2405488. [DOI] [PubMed] [Google Scholar]
- Frazier A. L., Robbins L. S., Stork P. J., Sprengel R., Segaloff D. L., Cone R. D. Isolation of TSH and LH/CG receptor cDNAs from human thyroid: regulation by tissue specific splicing. Mol Endocrinol. 1990 Aug;4(8):1264–1276. doi: 10.1210/mend-4-8-1264. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Gies D., McCray G., Huang M. The human cytomegalovirus major immediate early promoter can be trans-activated by adenovirus early proteins. Virology. 1989 Aug;171(2):377–385. doi: 10.1016/0042-6822(89)90605-3. [DOI] [PubMed] [Google Scholar]
- Hashimoto C., Hudson K. L., Anderson K. V. The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell. 1988 Jan 29;52(2):269–279. doi: 10.1016/0092-8674(88)90516-8. [DOI] [PubMed] [Google Scholar]
- Hofsteenge J., Kieffer B., Matthies R., Hemmings B. A., Stone S. R. Amino acid sequence of the ribonuclease inhibitor from porcine liver reveals the presence of leucine-rich repeats. Biochemistry. 1988 Nov 15;27(23):8537–8544. doi: 10.1021/bi00423a006. [DOI] [PubMed] [Google Scholar]
- Kataoka T., Broek D., Wigler M. DNA sequence and characterization of the S. cerevisiae gene encoding adenylate cyclase. Cell. 1985 Dec;43(2 Pt 1):493–505. doi: 10.1016/0092-8674(85)90179-5. [DOI] [PubMed] [Google Scholar]
- Kobilka B. K., Kobilka T. S., Daniel K., Regan J. W., Caron M. G., Lefkowitz R. J. Chimeric alpha 2-,beta 2-adrenergic receptors: delineation of domains involved in effector coupling and ligand binding specificity. Science. 1988 Jun 3;240(4857):1310–1316. doi: 10.1126/science.2836950. [DOI] [PubMed] [Google Scholar]
- Krantz D. E., Zipursky S. L. Drosophila chaoptin, a member of the leucine-rich repeat family, is a photoreceptor cell-specific adhesion molecule. EMBO J. 1990 Jun;9(6):1969–1977. doi: 10.1002/j.1460-2075.1990.tb08325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., McKnight S. L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988 Jun 24;240(4860):1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- Libert F., Lefort A., Gerard C., Parmentier M., Perret J., Ludgate M., Dumont J. E., Vassart G. Cloning, sequencing and expression of the human thyrotropin (TSH) receptor: evidence for binding of autoantibodies. Biochem Biophys Res Commun. 1989 Dec 29;165(3):1250–1255. doi: 10.1016/0006-291x(89)92736-8. [DOI] [PubMed] [Google Scholar]
- Limbird L. E. Activation and attenuation of adenylate cyclase. The role of GTP-binding proteins as macromolecular messengers in receptor--cyclase coupling. Biochem J. 1981 Apr 1;195(1):1–13. doi: 10.1042/bj1950001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loosfelt H., Misrahi M., Atger M., Salesse R., Vu Hai-Luu Thi M. T., Jolivet A., Guiochon-Mantel A., Sar S., Jallal B., Garnier J. Cloning and sequencing of porcine LH-hCG receptor cDNA: variants lacking transmembrane domain. Science. 1989 Aug 4;245(4917):525–528. doi: 10.1126/science.2502844. [DOI] [PubMed] [Google Scholar]
- Lopez J. A., Chung D. W., Fujikawa K., Hagen F. S., Davie E. W., Roth G. J. The alpha and beta chains of human platelet glycoprotein Ib are both transmembrane proteins containing a leucine-rich amino acid sequence. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2135–2139. doi: 10.1073/pnas.85.7.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez J. A., Chung D. W., Fujikawa K., Hagen F. S., Papayannopoulou T., Roth G. J. Cloning of the alpha chain of human platelet glycoprotein Ib: a transmembrane protein with homology to leucine-rich alpha 2-glycoprotein. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5615–5619. doi: 10.1073/pnas.84.16.5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzuk M. M., Keene J. L., Boime I. Site specificity of the chorionic gonadotropin N-linked oligosaccharides in signal transduction. J Biol Chem. 1989 Feb 15;264(5):2409–2414. [PubMed] [Google Scholar]
- McFarland K. C., Sprengel R., Phillips H. S., Köhler M., Rosemblit N., Nikolics K., Segaloff D. L., Seeburg P. H. Lutropin-choriogonadotropin receptor: an unusual member of the G protein-coupled receptor family. Science. 1989 Aug 4;245(4917):494–499. doi: 10.1126/science.2502842. [DOI] [PubMed] [Google Scholar]
- Nagayama Y., Kaufman K. D., Seto P., Rapoport B. Molecular cloning, sequence and functional expression of the cDNA for the human thyrotropin receptor. Biochem Biophys Res Commun. 1989 Dec 29;165(3):1184–1190. doi: 10.1016/0006-291x(89)92727-7. [DOI] [PubMed] [Google Scholar]
- Parmentier M., Libert F., Maenhaut C., Lefort A., Gérard C., Perret J., Van Sande J., Dumont J. E., Vassart G. Molecular cloning of the thyrotropin receptor. Science. 1989 Dec 22;246(4937):1620–1622. doi: 10.1126/science.2556796. [DOI] [PubMed] [Google Scholar]
- Patthy L. Detecting homology of distantly related proteins with consensus sequences. J Mol Biol. 1987 Dec 20;198(4):567–577. doi: 10.1016/0022-2836(87)90200-2. [DOI] [PubMed] [Google Scholar]
- Pierce J. G., Parsons T. F. Glycoprotein hormones: structure and function. Annu Rev Biochem. 1981;50:465–495. doi: 10.1146/annurev.bi.50.070181.002341. [DOI] [PubMed] [Google Scholar]
- Reichert L. E., Jr, Dattatreyamurty B. The follicle-stimulating hormone (FSH) receptor in testis: interaction with FSH, mechanism of signal transduction, and properties of the purified receptor. Biol Reprod. 1989 Jan;40(1):13–26. doi: 10.1095/biolreprod40.1.13. [DOI] [PubMed] [Google Scholar]
- Reinke R., Krantz D. E., Yen D., Zipursky S. L. Chaoptin, a cell surface glycoprotein required for Drosophila photoreceptor cell morphogenesis, contains a repeat motif found in yeast and human. Cell. 1988 Jan 29;52(2):291–301. doi: 10.1016/0092-8674(88)90518-1. [DOI] [PubMed] [Google Scholar]
- Sairam M. R. Complete dissociation of gonadotropin receptor binding and signal transduction in mouse Leydig tumour cells. Obligatory role of glycosylation in hormone action. Biochem J. 1990 Feb 1;265(3):667–674. doi: 10.1042/bj2650667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sairam M. R. Role of carbohydrates in glycoprotein hormone signal transduction. FASEB J. 1989 Jun;3(8):1915–1926. doi: 10.1096/fasebj.3.8.2542111. [DOI] [PubMed] [Google Scholar]
- Sayers J. R., Schmidt W., Eckstein F. 5'-3' exonucleases in phosphorothioate-based oligonucleotide-directed mutagenesis. Nucleic Acids Res. 1988 Feb 11;16(3):791–802. doi: 10.1093/nar/16.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprengel R., Braun T., Nikolics K., Segaloff D. L., Seeburg P. H. The testicular receptor for follicle stimulating hormone: structure and functional expression of cloned cDNA. Mol Endocrinol. 1990 Apr;4(4):525–530. doi: 10.1210/mend-4-4-525. [DOI] [PubMed] [Google Scholar]
- Strader C. D., Sigal I. S., Dixon R. A. Structural basis of beta-adrenergic receptor function. FASEB J. 1989 May;3(7):1825–1832. doi: 10.1096/fasebj.3.7.2541037. [DOI] [PubMed] [Google Scholar]
- Strader C. D., Sigal I. S., Register R. B., Candelore M. R., Rands E., Dixon R. A. Identification of residues required for ligand binding to the beta-adrenergic receptor. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4384–4388. doi: 10.1073/pnas.84.13.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N., Choe H. R., Nishida Y., Yamawaki-Kataoka Y., Ohnishi S., Tamaoki T., Kataoka T. Leucine-rich repeats and carboxyl terminus are required for interaction of yeast adenylate cyclase with RAS proteins. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8711–8715. doi: 10.1073/pnas.87.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N., Takahashi Y., Putnam F. W. Periodicity of leucine and tandem repetition of a 24-amino acid segment in the primary structure of leucine-rich alpha 2-glycoprotein of human serum. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1906–1910. doi: 10.1073/pnas.82.7.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titani K., Takio K., Handa M., Ruggeri Z. M. Amino acid sequence of the von Willebrand factor-binding domain of platelet membrane glycoprotein Ib. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5610–5614. doi: 10.1073/pnas.84.16.5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai-Morris C. H., Buczko E., Wang W., Dufau M. L. Intronic nature of the rat luteinizing hormone receptor gene defines a soluble receptor subspecies with hormone binding activity. J Biol Chem. 1990 Nov 15;265(32):19385–19388. [PubMed] [Google Scholar]
- Wadsworth H. L., Chazenbalk G. D., Nagayama Y., Russo D., Rapoport B. An insertion in the human thyrotropin receptor critical for high affinity hormone binding. Science. 1990 Sep 21;249(4975):1423–1425. doi: 10.1126/science.2169649. [DOI] [PubMed] [Google Scholar]
- Xie Y. B., Wang H., Segaloff D. L. Extracellular domain of lutropin/choriogonadotropin receptor expressed in transfected cells binds choriogonadotropin with high affinity. J Biol Chem. 1990 Dec 15;265(35):21411–21414. [PubMed] [Google Scholar]
- Yamawaki-Kataoka Y., Tamaoki T., Choe H. R., Tanaka H., Kataoka T. Adenylate cyclases in yeast: a comparison of the genes from Schizosaccharomyces pombe and Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5693–5697. doi: 10.1073/pnas.86.15.5693. [DOI] [PMC free article] [PubMed] [Google Scholar]






