Abstract
5-Aminolevulinate synthase (ALAS) catalyzes the first step of the heme biosynthetic pathway. cDNA clones for the human erythroid ALAS isozyme were isolated from a fetal liver library. It can be deduced that the erythroid ALAS precursor protein has a molecular weight of 64.6 kd, and is similar in size to the previously isolated human housekeeping ALAS precursor of molecular weight 70.6 kd. The mature mitochondrial forms of the erythroid and housekeeping ALAS isozymes are predicted to have molecular weights of 59.5 kd and 64.6 kd, respectively. The two isozymes show little amino acid identity in their N-terminal signal sequences but have considerable sequence identity in the C-terminal two-thirds of their proteins. An analysis of the immediate promoter of the human erythroid ALAS gene revealed several putative erythroid-specific cis-acting elements including both a GATA-1 and an NF-E2 binding site. An iron-responsive element (IRE) motif has been identified in the 5'-untranslated region of the human erythroid ALAS mRNA, but is not present in the housekeeping ALAS mRNA. Gel retardation experiments established that this IRE motif formed a protein - RNA complex with cytosolic extracts from human K562 cells and this binding was strongly competed with IRE transcripts from ferritin or transferrin receptor mRNAs. A transcript of the ALAS IRE, mutated in the conserved loop of the IRE, did not readily form this protein - RNA complex. These results suggest that the IRE motif in the ALAS mRNA is functional and imply that translation of the mRNA is controlled by cellular iron availability during erythropoiesis.
Full text
PDF


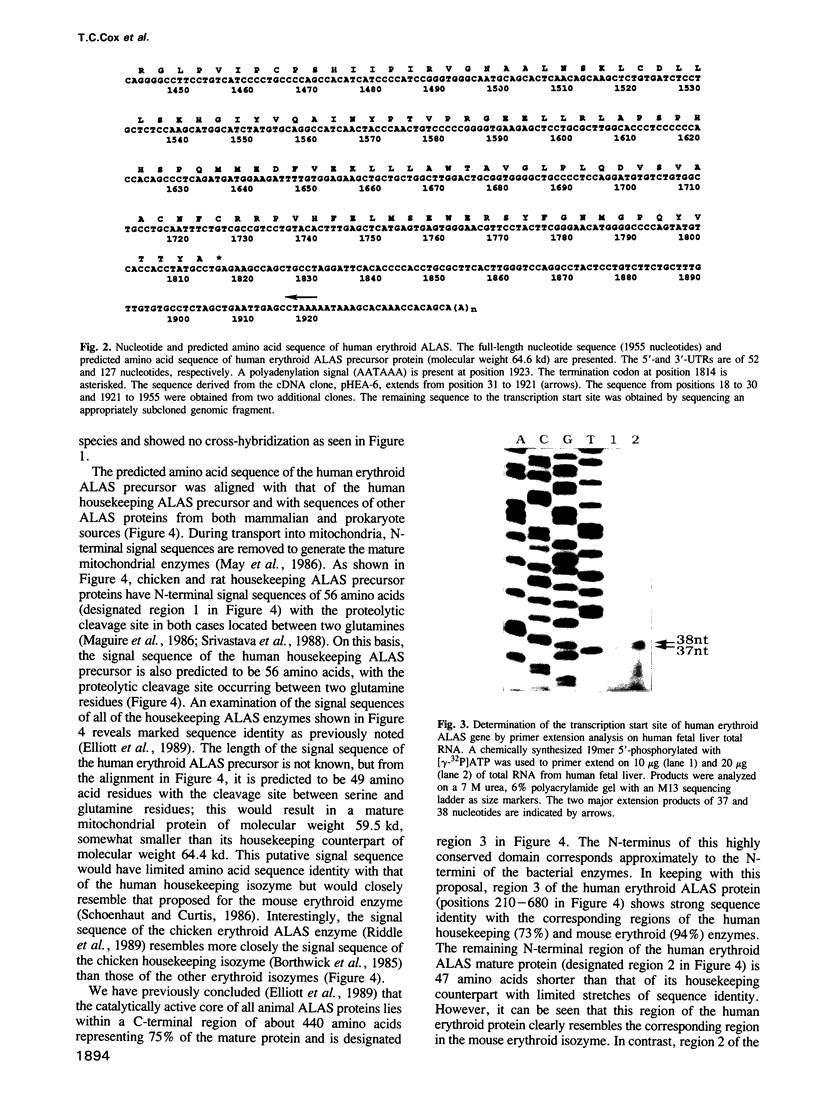
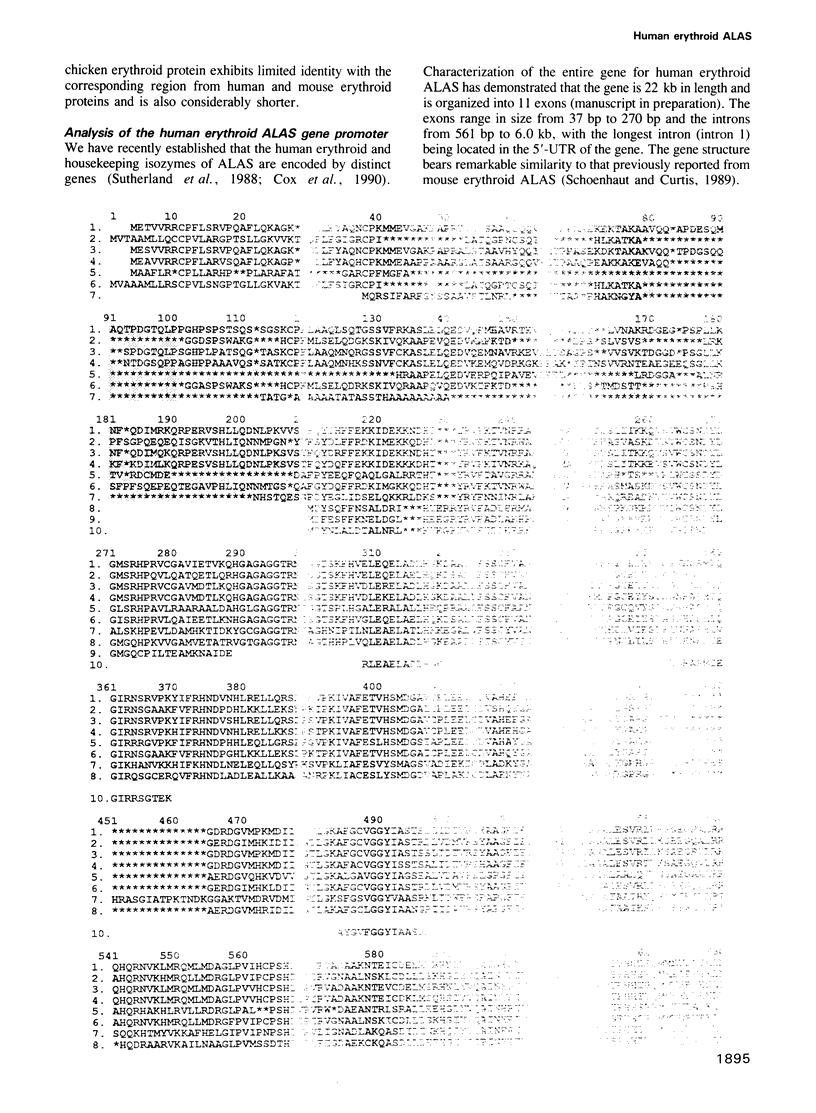

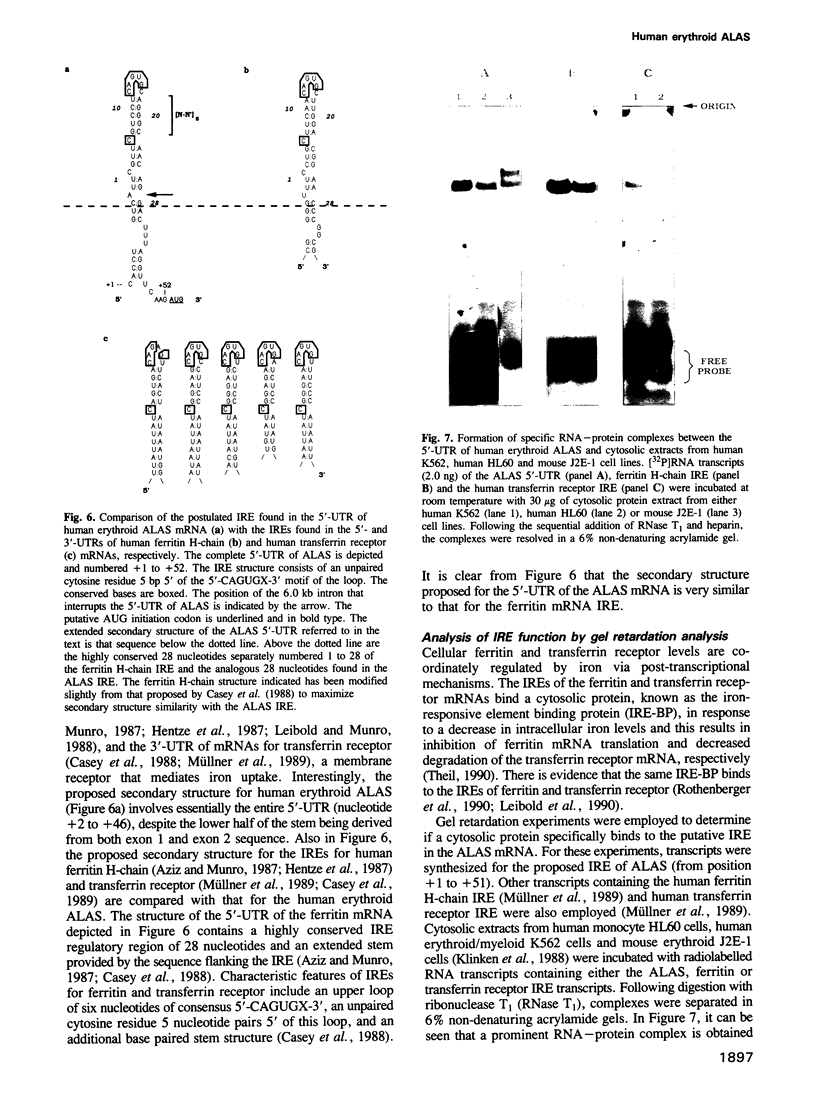





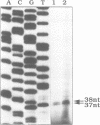
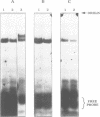
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avissar Y. J., Beale S. I. Identification of the enzymatic basis for delta-aminolevulinic acid auxotrophy in a hemA mutant of Escherichia coli. J Bacteriol. 1989 Jun;171(6):2919–2924. doi: 10.1128/jb.171.6.2919-2924.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz N., Munro H. N. Iron regulates ferritin mRNA translation through a segment of its 5' untranslated region. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8478–8482. doi: 10.1073/pnas.84.23.8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton H. A., Eisenstein R. S., Bomford A., Munro H. N. Determinants of the interaction between the iron-responsive element-binding protein and its binding site in rat L-ferritin mRNA. J Biol Chem. 1990 Apr 25;265(12):7000–7008. [PubMed] [Google Scholar]
- Bawden M. J., Borthwick I. A., Healy H. M., Morris C. P., May B. K., Elliott W. H. Sequence of human 5-aminolevulinate synthase cDNA. Nucleic Acids Res. 1987 Oct 26;15(20):8563–8563. doi: 10.1093/nar/15.20.8563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Beaumont C., Deybach J. C., Grandchamp B., da Silva V., de Verneuil H., Nordmann Y. Effects of succinylacetone on dimethylsulfoxide-mediated induction of heme pathway enzymes in mouse friend virus-transformed erythroleukemia cells. Exp Cell Res. 1984 Oct;154(2):474–484. doi: 10.1016/0014-4827(84)90171-x. [DOI] [PubMed] [Google Scholar]
- Bishop D. F., Henderson A. S., Astrin K. H. Human delta-aminolevulinate synthase: assignment of the housekeeping gene to 3p21 and the erythroid-specific gene to the X chromosome. Genomics. 1990 Jun;7(2):207–214. doi: 10.1016/0888-7543(90)90542-3. [DOI] [PubMed] [Google Scholar]
- Borthwick I. A., Srivastava G., Day A. R., Pirola B. A., Snoswell M. A., May B. K., Elliott W. H. Complete nucleotide sequence of hepatic 5-aminolaevulinate synthase precursor. Eur J Biochem. 1985 Aug 1;150(3):481–484. doi: 10.1111/j.1432-1033.1985.tb09047.x. [DOI] [PubMed] [Google Scholar]
- Bottomley S. S., Muller-Eberhard U. Pathophysiology of heme synthesis. Semin Hematol. 1988 Oct;25(4):282–302. [PubMed] [Google Scholar]
- Casey J. L., Hentze M. W., Koeller D. M., Caughman S. W., Rouault T. A., Klausner R. D., Harford J. B. Iron-responsive elements: regulatory RNA sequences that control mRNA levels and translation. Science. 1988 May 13;240(4854):924–928. doi: 10.1126/science.2452485. [DOI] [PubMed] [Google Scholar]
- Casey J. L., Koeller D. M., Ramin V. C., Klausner R. D., Harford J. B. Iron regulation of transferrin receptor mRNA levels requires iron-responsive elements and a rapid turnover determinant in the 3' untranslated region of the mRNA. EMBO J. 1989 Dec 1;8(12):3693–3699. doi: 10.1002/j.1460-2075.1989.tb08544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughman S. W., Hentze M. W., Rouault T. A., Harford J. B., Klausner R. D. The iron-responsive element is the single element responsible for iron-dependent translational regulation of ferritin biosynthesis. Evidence for function as the binding site for a translational repressor. J Biol Chem. 1988 Dec 15;263(35):19048–19052. [PubMed] [Google Scholar]
- Chretien S., Dubart A., Beaupain D., Raich N., Grandchamp B., Rosa J., Goossens M., Romeo P. H. Alternative transcription and splicing of the human porphobilinogen deaminase gene result either in tissue-specific or in housekeeping expression. Proc Natl Acad Sci U S A. 1988 Jan;85(1):6–10. doi: 10.1073/pnas.85.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox T. C., Bawden M. J., Abraham N. G., Bottomley S. S., May B. K., Baker E., Chen L. Z., Sutherland G. R. Erythroid 5-aminolevulinate synthase is located on the X chromosome. Am J Hum Genet. 1990 Jan;46(1):107–111. [PMC free article] [PubMed] [Google Scholar]
- Dierks P., van Ooyen A., Cochran M. D., Dobkin C., Reiser J., Weissmann C. Three regions upstream from the cap site are required for efficient and accurate transcription of the rabbit beta-globin gene in mouse 3T6 cells. Cell. 1983 Mar;32(3):695–706. doi: 10.1016/0092-8674(83)90055-7. [DOI] [PubMed] [Google Scholar]
- Elferink C. J., Sassa S., May B. K. Regulation of 5-aminolevulinate synthase in mouse erythroleukemic cells is different from that in liver. J Biol Chem. 1988 Sep 15;263(26):13012–13016. [PubMed] [Google Scholar]
- Gardner L. C., Cox T. M. Biosynthesis of heme in immature erythroid cells. The regulatory step for heme formation in the human erythron. J Biol Chem. 1988 May 15;263(14):6676–6682. [PubMed] [Google Scholar]
- Glass C. K., Holloway J. M., Devary O. V., Rosenfeld M. G. The thyroid hormone receptor binds with opposite transcriptional effects to a common sequence motif in thyroid hormone and estrogen response elements. Cell. 1988 Jul 29;54(3):313–323. doi: 10.1016/0092-8674(88)90194-8. [DOI] [PubMed] [Google Scholar]
- Goossen B., Caughman S. W., Harford J. B., Klausner R. D., Hentze M. W. Translational repression by a complex between the iron-responsive element of ferritin mRNA and its specific cytoplasmic binding protein is position-dependent in vivo. EMBO J. 1990 Dec;9(12):4127–4133. doi: 10.1002/j.1460-2075.1990.tb07635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentze M. W., Rouault T. A., Caughman S. W., Dancis A., Harford J. B., Klausner R. D. A cis-acting element is necessary and sufficient for translational regulation of human ferritin expression in response to iron. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6730–6734. doi: 10.1073/pnas.84.19.6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson S., Nienhuis A. W. Developmental regulation of human globin genes. Annu Rev Biochem. 1985;54:1071–1108. doi: 10.1146/annurev.bi.54.070185.005231. [DOI] [PubMed] [Google Scholar]
- Klinken S. P., Nicola N. A., Johnson G. R. In vitro-derived leukemic erythroid cell lines induced by a raf- and myc-containing retrovirus differentiate in response to erythropoietin. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8506–8510. doi: 10.1073/pnas.85.22.8506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibold E. A., Laudano A., Yu Y. Structural requirements of iron-responsive elements for binding of the protein involved in both transferrin receptor and ferritin mRNA post-transcriptional regulation. Nucleic Acids Res. 1990 Apr 11;18(7):1819–1824. doi: 10.1093/nar/18.7.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibold E. A., Munro H. N. Characterization and evolution of the expressed rat ferritin light subunit gene and its pseudogene family. Conservation of sequences within noncoding regions of ferritin genes. J Biol Chem. 1987 May 25;262(15):7335–7341. [PubMed] [Google Scholar]
- Leong S. A., Williams P. H., Ditta G. S. Analysis of the 5' regulatory region of the gene for delta-aminolevulinic acid synthetase of Rhizobium meliloti. Nucleic Acids Res. 1985 Aug 26;13(16):5965–5976. doi: 10.1093/nar/13.16.5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebhaber S. A., Goossens M. J., Kan Y. W. Cloning and complete nucleotide sequence of human 5'-alpha-globin gene. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7054–7058. doi: 10.1073/pnas.77.12.7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire D. J., Day A. R., Borthwick I. A., Srivastava G., Wigley P. L., May B. K., Elliott W. H. Nucleotide sequence of the chicken 5-aminolevulinate synthase gene. Nucleic Acids Res. 1986 Feb 11;14(3):1379–1391. doi: 10.1093/nar/14.3.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D. I., Tsai S. F., Orkin S. H. Increased gamma-globin expression in a nondeletion HPFH mediated by an erythroid-specific DNA-binding factor. Nature. 1989 Mar 30;338(6214):435–438. doi: 10.1038/338435a0. [DOI] [PubMed] [Google Scholar]
- May B. K., Bhasker C. R., Bawden M. J., Cox T. C. Molecular regulation of 5-aminolevulinate synthase. Diseases related to heme biosynthesis. Mol Biol Med. 1990 Oct;7(5):405–421. [PubMed] [Google Scholar]
- McClung C. R., Somerville J. E., Guerinot M. L., Chelm B. K. Structure of the Bradyrhizobium japonicum gene hemA encoding 5-aminolevulinic acid synthase. Gene. 1987;54(1):133–139. doi: 10.1016/0378-1119(87)90355-6. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Gavis E. R., Kingsbury R., Axel R. Analysis of transcriptional regulatory signals of the HSV thymidine kinase gene: identification of an upstream control region. Cell. 1981 Aug;25(2):385–398. doi: 10.1016/0092-8674(81)90057-x. [DOI] [PubMed] [Google Scholar]
- Mignotte V., Eleouet J. F., Raich N., Romeo P. H. Cis- and trans-acting elements involved in the regulation of the erythroid promoter of the human porphobilinogen deaminase gene. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6548–6552. doi: 10.1073/pnas.86.17.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignotte V., Wall L., deBoer E., Grosveld F., Romeo P. H. Two tissue-specific factors bind the erythroid promoter of the human porphobilinogen deaminase gene. Nucleic Acids Res. 1989 Jan 11;17(1):37–54. doi: 10.1093/nar/17.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R. M., Tilly K., Maniatis T. Fine structure genetic analysis of a beta-globin promoter. Science. 1986 May 2;232(4750):613–618. doi: 10.1126/science.3457470. [DOI] [PubMed] [Google Scholar]
- Müllner E. W., Neupert B., Kühn L. C. A specific mRNA binding factor regulates the iron-dependent stability of cytoplasmic transferrin receptor mRNA. Cell. 1989 Jul 28;58(2):373–382. doi: 10.1016/0092-8674(89)90851-9. [DOI] [PubMed] [Google Scholar]
- Ney P. A., Sorrentino B. P., Lowrey C. H., Nienhuis A. W. Inducibility of the HS II enhancer depends on binding of an erythroid specific nuclear protein. Nucleic Acids Res. 1990 Oct 25;18(20):6011–6017. doi: 10.1093/nar/18.20.6011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin S. H. Globin gene regulation and switching: circa 1990. Cell. 1990 Nov 16;63(4):665–672. doi: 10.1016/0092-8674(90)90133-y. [DOI] [PubMed] [Google Scholar]
- Philipsen S., Talbot D., Fraser P., Grosveld F. The beta-globin dominant control region: hypersensitive site 2. EMBO J. 1990 Jul;9(7):2159–2167. doi: 10.1002/j.1460-2075.1990.tb07385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponka P., Schulman H. M., Martinez-Medellin J. Haem inhibits iron uptake subsequent to endocytosis of transferrin in reticulocytes. Biochem J. 1988 Apr 1;251(1):105–109. doi: 10.1042/bj2510105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raich N., Mignotte V., Dubart A., Beaupain D., Leboulch P., Romana M., Chabret C., Charnay P., Papayannopoulou T., Goossens M. Regulated expression of the overlapping ubiquitous and erythroid transcription units of the human porphobilinogen deaminase (PBG-D) gene introduced into non-erythroid and erythroid cells. J Biol Chem. 1989 Jun 15;264(17):10186–10192. [PubMed] [Google Scholar]
- Riddle R. D., Yamamoto M., Engel J. D. Expression of delta-aminolevulinate synthase in avian cells: separate genes encode erythroid-specific and nonspecific isozymes. Proc Natl Acad Sci U S A. 1989 Feb;86(3):792–796. doi: 10.1073/pnas.86.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roméo P. H., Raich N., Dubart A., Beaupain D., Pryor M., Kushner J., Cohen-Solal M., Goossens M. Molecular cloning and nucleotide sequence of a complete human uroporphyrinogen decarboxylase cDNA. J Biol Chem. 1986 Jul 25;261(21):9825–9831. [PubMed] [Google Scholar]
- Rothenberger S., Müllner E. W., Kühn L. C. The mRNA-binding protein which controls ferritin and transferrin receptor expression is conserved during evolution. Nucleic Acids Res. 1990 Mar 11;18(5):1175–1179. doi: 10.1093/nar/18.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenhaut D. S., Curtis P. J. Nucleotide sequence of mouse 5-aminolevulinic acid synthase cDNA and expression of its gene in hepatic and erythroid tissues. Gene. 1986;48(1):55–63. doi: 10.1016/0378-1119(86)90351-3. [DOI] [PubMed] [Google Scholar]
- Schoenhaut D. S., Curtis P. J. Structure of a mouse erythroid 5-aminolevulinate synthase gene and mapping of erythroid-specific DNAse I hypersensitive sites. Nucleic Acids Res. 1989 Sep 12;17(17):7013–7028. doi: 10.1093/nar/17.17.7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava G., Borthwick I. A., Maguire D. J., Elferink C. J., Bawden M. J., Mercer J. F., May B. K. Regulation of 5-aminolevulinate synthase mRNA in different rat tissues. J Biol Chem. 1988 Apr 15;263(11):5202–5209. [PubMed] [Google Scholar]
- Sutherland G. R., Baker E., Callen D. F., Hyland V. J., May B. K., Bawden M. J., Healy H. M., Borthwick I. A. 5-Aminolevulinate synthase is at 3p21 and thus not the primary defect in X-linked sideroblastic anemia. Am J Hum Genet. 1988 Sep;43(3):331–335. [PMC free article] [PubMed] [Google Scholar]
- Theil E. C. Regulation of ferritin and transferrin receptor mRNAs. J Biol Chem. 1990 Mar 25;265(9):4771–4774. [PubMed] [Google Scholar]
- Urban-Grimal D., Volland C., Garnier T., Dehoux P., Labbe-Bois R. The nucleotide sequence of the HEM1 gene and evidence for a precursor form of the mitochondrial 5-aminolevulinate synthase in Saccharomyces cerevisiae. Eur J Biochem. 1986 May 2;156(3):511–519. doi: 10.1111/j.1432-1033.1986.tb09610.x. [DOI] [PubMed] [Google Scholar]
- Wall L., deBoer E., Grosveld F. The human beta-globin gene 3' enhancer contains multiple binding sites for an erythroid-specific protein. Genes Dev. 1988 Sep;2(9):1089–1100. doi: 10.1101/gad.2.9.1089. [DOI] [PubMed] [Google Scholar]
- Wang Y. H., Sczekan S. R., Theil E. C. Structure of the 5' untranslated regulatory region of ferritin mRNA studied in solution. Nucleic Acids Res. 1990 Aug 11;18(15):4463–4468. doi: 10.1093/nar/18.15.4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenke M., Muñoz A., Sap J., Vennström B., Beug H. v-erbA oncogene activation entails the loss of hormone-dependent regulator activity of c-erbA. Cell. 1990 Jun 15;61(6):1035–1049. doi: 10.1016/0092-8674(90)90068-p. [DOI] [PubMed] [Google Scholar]
- deBoer E., Antoniou M., Mignotte V., Wall L., Grosveld F. The human beta-globin promoter; nuclear protein factors and erythroid specific induction of transcription. EMBO J. 1988 Dec 20;7(13):4203–4212. doi: 10.1002/j.1460-2075.1988.tb03317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]