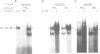Abstract
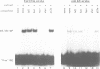
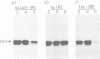
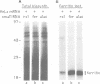
Iron-responsive elements (IREs) are regulatory RNA elements which are characterized by a phylogenetically defined sequence-structure motif. Their biological function is to provide a specific binding site for the IRE-binding protein (IRE-BP). Iron starvation of cells induces high affinity binding of the cytoplasmic IRE-BP to an IRE which has at least two different known biological consequences, repression of ferritin mRNA translation and stabilization of the transferrin receptor transcript. We report the identification of a novel, evolutionarily conserved IRE motif in the 5' UTR of murine and human erythroid-specific delta-aminolevulinic acid synthase (eALAS) mRNA which encodes the first, and possibly rate limiting, enzyme of the heme biosynthetic pathway. We demonstrate the function of the eALAS IRE as a specific binding site for the IRE-BP by gel retardation analyses and by in vitro translation experiments. In addition, we show that the 5' UTR of eALAS mRNA is sufficient to mediate iron-dependent translational regulation in vivo. These findings strongly suggest involvement of the IRE-IRE-BP system in the control of heme biosynthesis during erythroid differentiation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham N. G., Nelson J. C., Ahmed T., Konwalinka G., Levere R. D. Erythropoietin controls heme metabolic enzymes in normal human bone marrow culture. Exp Hematol. 1989 Sep;17(8):908–913. [PubMed] [Google Scholar]
- Aziz N., Munro H. N. Iron regulates ferritin mRNA translation through a segment of its 5' untranslated region. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8478–8482. doi: 10.1073/pnas.84.23.8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bawden M. J., Borthwick I. A., Healy H. M., Morris C. P., May B. K., Elliott W. H. Sequence of human 5-aminolevulinate synthase cDNA. Nucleic Acids Res. 1987 Oct 26;15(20):8563–8563. doi: 10.1093/nar/15.20.8563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. H., Daniels-McQueen S., Walden W. E., Patino M. M., Gaffield L., Bielser D., Thach R. E. Requirements for the translational repression of ferritin transcripts in wheat germ extracts by a 90-kDa protein from rabbit liver. J Biol Chem. 1989 Aug 15;264(23):13383–13386. [PubMed] [Google Scholar]
- Casey J. L., Hentze M. W., Koeller D. M., Caughman S. W., Rouault T. A., Klausner R. D., Harford J. B. Iron-responsive elements: regulatory RNA sequences that control mRNA levels and translation. Science. 1988 May 13;240(4854):924–928. doi: 10.1126/science.2452485. [DOI] [PubMed] [Google Scholar]
- Casey J. L., Koeller D. M., Ramin V. C., Klausner R. D., Harford J. B. Iron regulation of transferrin receptor mRNA levels requires iron-responsive elements and a rapid turnover determinant in the 3' untranslated region of the mRNA. EMBO J. 1989 Dec 1;8(12):3693–3699. doi: 10.1002/j.1460-2075.1989.tb08544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughman S. W., Hentze M. W., Rouault T. A., Harford J. B., Klausner R. D. The iron-responsive element is the single element responsible for iron-dependent translational regulation of ferritin biosynthesis. Evidence for function as the binding site for a translational repressor. J Biol Chem. 1988 Dec 15;263(35):19048–19052. [PubMed] [Google Scholar]
- Costanzo F., Colombo M., Staempfli S., Santoro C., Marone M., Frank R., Delius H., Cortese R. Structure of gene and pseudogenes of human apoferritin H. Nucleic Acids Res. 1986 Jan 24;14(2):721–736. doi: 10.1093/nar/14.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elferink C. J., Sassa S., May B. K. Regulation of 5-aminolevulinate synthase in mouse erythroleukemic cells is different from that in liver. J Biol Chem. 1988 Sep 15;263(26):13012–13016. [PubMed] [Google Scholar]
- Friend C., Scher W., Holland J. G., Sato T. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro: stimulation of erythroid differentiation by dimethyl sulfoxide. Proc Natl Acad Sci U S A. 1971 Feb;68(2):378–382. doi: 10.1073/pnas.68.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossen B., Caughman S. W., Harford J. B., Klausner R. D., Hentze M. W. Translational repression by a complex between the iron-responsive element of ferritin mRNA and its specific cytoplasmic binding protein is position-dependent in vivo. EMBO J. 1990 Dec;9(12):4127–4133. doi: 10.1002/j.1460-2075.1990.tb07635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile D. J., Hentze M. W., Rouault T. A., Harford J. B., Klausner R. D. Regulation of interaction of the iron-responsive element binding protein with iron-responsive RNA elements. Mol Cell Biol. 1989 Nov;9(11):5055–5061. doi: 10.1128/mcb.9.11.5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto C., Hudson K. L., Anderson K. V. The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell. 1988 Jan 29;52(2):269–279. doi: 10.1016/0092-8674(88)90516-8. [DOI] [PubMed] [Google Scholar]
- Hentze M. W., Argos P. Homology between IRE-BP, a regulatory RNA-binding protein, aconitase, and isopropylmalate isomerase. Nucleic Acids Res. 1991 Apr 25;19(8):1739–1740. doi: 10.1093/nar/19.8.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentze M. W., Caughman S. W., Casey J. L., Koeller D. M., Rouault T. A., Harford J. B., Klausner R. D. A model for the structure and functions of iron-responsive elements. Gene. 1988 Dec 10;72(1-2):201–208. doi: 10.1016/0378-1119(88)90145-x. [DOI] [PubMed] [Google Scholar]
- Hentze M. W., Caughman S. W., Rouault T. A., Barriocanal J. G., Dancis A., Harford J. B., Klausner R. D. Identification of the iron-responsive element for the translational regulation of human ferritin mRNA. Science. 1987 Dec 11;238(4833):1570–1573. doi: 10.1126/science.3685996. [DOI] [PubMed] [Google Scholar]
- Hentze M. W., Keim S., Papadopoulos P., O'Brien S., Modi W., Drysdale J., Leonard W. J., Harford J. B., Klausner R. D. Cloning, characterization, expression, and chromosomal localization of a human ferritin heavy-chain gene. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7226–7230. doi: 10.1073/pnas.83.19.7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentze M. W., Rouault T. A., Caughman S. W., Dancis A., Harford J. B., Klausner R. D. A cis-acting element is necessary and sufficient for translational regulation of human ferritin expression in response to iron. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6730–6734. doi: 10.1073/pnas.84.19.6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentze M. W., Rouault T. A., Harford J. B., Klausner R. D. Oxidation-reduction and the molecular mechanism of a regulatory RNA-protein interaction. Science. 1989 Apr 21;244(4902):357–359. doi: 10.1126/science.2711187. [DOI] [PubMed] [Google Scholar]
- Hentze M. W., Seuanez H. N., O'Brien S. J., Harford J. B., Klausner R. D. Chromosomal localization of nucleic acid-binding proteins by affinity mapping: assignment of the IRE-binding protein gene to human chromosome 9. Nucleic Acids Res. 1989 Aug 11;17(15):6103–6108. doi: 10.1093/nar/17.15.6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibraham N. G., Friedland M. L., Levere R. D. Heme metabolism in erythroid and hepatic cells. Prog Hematol. 1983;13:75–130. [PubMed] [Google Scholar]
- Johansson H. E., De Groot N., Hochberg A. A., Hentze M. W. Effect of heparin contained in preparations of small cytoplasmic RNAs on cell-free translation. J Biol Chem. 1991 Jan 25;266(3):1921–1925. [PubMed] [Google Scholar]
- Kahn P., Cameron G. EMBL Data Library. Methods Enzymol. 1990;183:23–31. doi: 10.1016/0076-6879(90)83004-s. [DOI] [PubMed] [Google Scholar]
- Kennedy M. C., Werst M., Telser J., Emptage M. H., Beinert H., Hoffman B. M. Mode of substrate carboxyl binding to the [4Fe-4S]+ cluster of reduced aconitase as studied by 17O and 13C electron-nuclear double resonance spectroscopy. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8854–8858. doi: 10.1073/pnas.84.24.8854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D., Harford J. B. cis-trans models for post-transcriptional gene regulation. Science. 1989 Nov 17;246(4932):870–872. doi: 10.1126/science.2683086. [DOI] [PubMed] [Google Scholar]
- Koeller D. M., Casey J. L., Hentze M. W., Gerhardt E. M., Chan L. N., Klausner R. D., Harford J. B. A cytosolic protein binds to structural elements within the iron regulatory region of the transferrin receptor mRNA. Proc Natl Acad Sci U S A. 1989 May;86(10):3574–3578. doi: 10.1073/pnas.86.10.3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LONDON I. M., BRUNS G. P., KARIBIAN D. THE REGULATION OF HEMOGLOBIN SYNTHESIS AND THE PATHOGENESIS OF SOME HYPOCHROMIC ANEMIAS. Medicine (Baltimore) 1964 Nov;43:789–802. doi: 10.1097/00005792-196411000-00024. [DOI] [PubMed] [Google Scholar]
- Laskey J. D., Ponka P., Schulman H. M. Control of heme synthesis during Friend cell differentiation: role of iron and transferrin. J Cell Physiol. 1986 Nov;129(2):185–192. doi: 10.1002/jcp.1041290209. [DOI] [PubMed] [Google Scholar]
- Leibold E. A., Laudano A., Yu Y. Structural requirements of iron-responsive elements for binding of the protein involved in both transferrin receptor and ferritin mRNA post-transcriptional regulation. Nucleic Acids Res. 1990 Apr 11;18(7):1819–1824. doi: 10.1093/nar/18.7.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibold E. A., Munro H. N. Cytoplasmic protein binds in vitro to a highly conserved sequence in the 5' untranslated region of ferritin heavy- and light-subunit mRNAs. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2171–2175. doi: 10.1073/pnas.85.7.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levere R. D., Granick S. Control of hemoglobin synthesis in the cultured chick blastoderm by delta-aminolevulinic acid synthetase: increase in the rate of hemoglobin formation with delta-aminolevulinic acid. Proc Natl Acad Sci U S A. 1965 Jul;54(1):134–137. doi: 10.1073/pnas.54.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May B. K., Bhasker C. R., Bawden M. J., Cox T. C. Molecular regulation of 5-aminolevulinate synthase. Diseases related to heme biosynthesis. Mol Biol Med. 1990 Oct;7(5):405–421. [PubMed] [Google Scholar]
- Milligan J. F., Groebe D. R., Witherell G. W., Uhlenbeck O. C. Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic Acids Res. 1987 Nov 11;15(21):8783–8798. doi: 10.1093/nar/15.21.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müllner E. W., Neupert B., Kühn L. C. A specific mRNA binding factor regulates the iron-dependent stability of cytoplasmic transferrin receptor mRNA. Cell. 1989 Jul 28;58(2):373–382. doi: 10.1016/0092-8674(89)90851-9. [DOI] [PubMed] [Google Scholar]
- Neupert B., Thompson N. A., Meyer C., Kühn L. C. A high yield affinity purification method for specific RNA-binding proteins: isolation of the iron regulatory factor from human placenta. Nucleic Acids Res. 1990 Jan 11;18(1):51–55. doi: 10.1093/nar/18.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponka P., Schulman H. M. Regulation of heme synthesis in erythroid cells: hemin inhibits transferrin iron utilization but not protoporphyrin synthesis. Blood. 1985 Apr;65(4):850–857. [PubMed] [Google Scholar]
- Rouault T. A., Hentze M. W., Caughman S. W., Harford J. B., Klausner R. D. Binding of a cytosolic protein to the iron-responsive element of human ferritin messenger RNA. Science. 1988 Sep 2;241(4870):1207–1210. doi: 10.1126/science.3413484. [DOI] [PubMed] [Google Scholar]
- Rouault T. A., Hentze M. W., Dancis A., Caughman W., Harford J. B., Klausner R. D. Influence of altered transcription on the translational control of human ferritin expression. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6335–6339. doi: 10.1073/pnas.84.18.6335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenhaut D. S., Curtis P. J. Nucleotide sequence of mouse 5-aminolevulinic acid synthase cDNA and expression of its gene in hepatic and erythroid tissues. Gene. 1986;48(1):55–63. doi: 10.1016/0378-1119(86)90351-3. [DOI] [PubMed] [Google Scholar]
- Schoenhaut D. S., Curtis P. J. Structure of a mouse erythroid 5-aminolevulinate synthase gene and mapping of erythroid-specific DNAse I hypersensitive sites. Nucleic Acids Res. 1989 Sep 12;17(17):7013–7028. doi: 10.1093/nar/17.17.7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theil E. C. Regulation of ferritin and transferrin receptor mRNAs. J Biol Chem. 1990 Mar 25;265(9):4771–4774. [PubMed] [Google Scholar]
- Walden W. E., Daniels-McQueen S., Brown P. H., Gaffield L., Russell D. A., Bielser D., Bailey L. C., Thach R. E. Translational repression in eukaryotes: partial purification and characterization of a repressor of ferritin mRNA translation. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9503–9507. doi: 10.1073/pnas.85.24.9503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yachou A. K., Renaudie F., Grandchamp B., Beaumont C. Nucleotide sequence of the mouse ferritin H chain gene. Nucleic Acids Res. 1989 Oct 11;17(19):8005–8005. doi: 10.1093/nar/17.19.8005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Kure S., Engel J. D., Hiraga K. Structure, turnover, and heme-mediated suppression of the level of mRNA encoding rat liver delta-aminolevulinate synthase. J Biol Chem. 1988 Nov 5;263(31):15973–15979. [PubMed] [Google Scholar]
- Zheng L., Andrews P. C., Hermodson M. A., Dixon J. E., Zalkin H. Cloning and structural characterization of porcine heart aconitase. J Biol Chem. 1990 Feb 15;265(5):2814–2821. [PubMed] [Google Scholar]