Abstract
Glioblastoma (GB) remains a devastating disease for which novel therapies are urgently needed. Here we report that the Aurora-A kinase inhibitor alisertib exhibits potent efficacy against GB neurosphere tumor stem-like cells in vitro and in vivo. Many GB neurosphere cells treated with alisertib for short periods undergo apoptosis, although, some regain proliferative activity upon drug removal. Extended treatment however results in complete and irreversible loss of tumor cell proliferation. Moreover, alisertib caused GB neurosphere cells to partially differentiate and enter senescence. These effects were also observed in glioma cells treated with the Aurora-A inhibitor TC-A2317 or anti-Aurora-A siRNA. Furthermore, alisertib extended median survival of mice bearing intracranial human GB neurosphere tumor xenografts. Alisertib exerted similar effects on GB neurosphere cells in vivo based on the presence of activated phosphoThr288Aurora-A, abnormal mitoses and increased cellular ploidy, consistent with on-target activity. Our results offer preclinical proof-of-concept for alisertib as a new therapeutic for glioma treatment.
Keywords: Aurora-A, alisertib, glioblastoma, neurospheres, orthotopic xenograft model
Introduction
Aurora-A (AURKA) is a serine/threonine kinase critical for centrosome duplication, spindle assembly and mitotic exit (1–3). Aurora-A also drives cell cycle progression by promoting cyclin B1, Wnt, myc and other pro-proliferative pathways (4–6). Aurora-A knock-down blocks cellular proliferation and may induce tetraploidy, apoptosis and senescence in vitro (7–9). Aurora-A kinase inhibitors have thus emerged as unique antiproliferative agents inhibiting mitosis and important signaling pathways.
Alisertib (MLN8237) is a highly-selective Aurora-A kinase inhibitor in clinical trials for a variety of malignancies (10). Because novel approaches are urgently needed for the treatment of glioblastoma (GB), we tested alisertib against GB neurosphere tumor stem-like cells in vitro and in vivo.
Materials and Methods
Neurosphere Culture and Proliferation Assays
GB9, GB30 and GB169 neurosphere cells were derived from patient surgical samples at the Ohio State University Cancer Center under an IRB-approved protocol. Minced tissue was passed through 18- and 21-gauge needles to achieve single cell suspensions. Cells were cultured in DMEM/F12 containing N2 supplement (Invitrogen) and 20 ng/ml each of epidermal growth factor and basic fibroblast growth factor (R&D). Neurospheres were passaged by trituration with a 21-gauge needle. U87 cells obtained from the American Type Culture Collection were grown in DMEM/10% FCS. No additional authentication was performed on these cells.
The investigational Aurora-A inhibitor alisertib was provided by Takeda Pharmaceuticals. TC-A2317 (11) was from Tocris Bioscience. For growth curves, cells were plated at 104 cells/well in 24-well plates in the presence of drug or DMSO vehicle (0.0032 to 0.02%). Every 2 days 3 wells per condition were triturated and cells counted (Coulter model Z2). Metabolic activity was measured by 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide conversion to formazan (MTT assay). Cells were plated in 96-well plates at 104 cells/well in 100 µl of medium containing alisertib or DMSO. After 4 days at 37°C, 10 µl of MTT reagent (Roche) was added/well and plates processed per the manufacturer’s instructions.
Apoptosis
Neurosphere cells were treated with 200 nM alisertib or DMSO and trypsinized at indicated intervals. One-half of the cells per 60 dish were pelleted and resuspended in 100 µl Annexin-V Binding Buffer (Invitrogen). Anti-Annexin-V-FITC (BD Pharmingen, 5 µl) was added, incubated at 25°C for 15 min, diluted to 500 µl with Binding Buffer and filtered through mesh. Binding was measured by flow cytometry (488 nm excitation).
β-galactosidase assay
Neurospheres were treated for 12 days with 200 nM alisertib or DMSO alone. Cytospins were prepared by centrifuging GB neurospheres onto charged glass slides (3 min at 800 rpm). Slides were fixed in 2% formaldehyde, 0.2% glutaraldehyde and stained for β-galactosidase activity using the Senescence β-Galactosidase Staining kit (Cell Signaling).
Western blotting
Cells were lysed in RIPA containing 1 mM PMSF, 10 µg/ml aprotinin, 10 µg/ml leupeptin, 20 mM β-glycerosphosphate and 1 mM sodium orthovanadate. SDS-PAGE and western blotting of PVDF membranes was performed using TBST and 4% non-fat dry milk. Primary antibodies against phosphoThr288Aurora-A, Aurora-A (Abcam ab58494, 1:500; & ab13824, 1:500), CD44, p21, p27, cleaved PARP, BimEL, neurofilament-L (Cell Signaling 3570, 1:2000; 2947,1:1000; 3686, 1:1000; 9541, 1:1000; 2933, 1:1000; & 2837, 1:1000), β-catenin (Life Technologies 18-0226, 1:1000), Olig2 (IBL 18953, 1:200), Aldh1L1 (NeuroMab 75-140, 1:500), Mushashi-1 (R&D Systems MAB2628, 1:500), Nestin (Millipore MAB5326, 1:1000), GFAP or β-actin (Santa Cruz sc-9065, 1:500; sc-47778, 1:10,000) were incubated overnight. HRP-conjugated secondary antibodies (Santa Cruz, 1:2000) were incubated for 1 h at 25°C. Membranes were developed with Pierce Super Signal West Pico and exposed to X-ray film.
RNA interference
U87 glioblastoma cells were transfected with 300 pmol Dharmacon ON-TARGET plus SMART pool anti-human AURKA siRNA or Qiagen Allstars negative control siRNA, using Oligofectamine and Opti-MEM-I (Life Technologies).
Mouse orthotopic xenograft model
All animal experiments were IACUC approved (2013A0051). Female athymic mice (nu/nu genotype, 6–8 weeks, 20–25 g weight, NCI strain 01B74) were anesthetized with intraperitoneal ketamine (100 mg/kg)/xylazine (20 mg/kg) and fitted into stereotactic frames (David Kopf Instruments). A small incision was made just lateral to the midline and a 1.0 mm burr hole was drilled at AP= +1 mm, ML= −2.5 mm from the bregma. Two microliters of a single neurosphere cell suspension (50,000 cells/µl) was deposited at 1 µl/min into the right caudate nucleus at a depth of −3 mm from the dura. The burr hole was filled with bone wax and the incision closed (4–0 vicryl suture).
Beginning 5 days after implantation, mice were orally gavaged with 20 mg/kg alisertib dissolved in 10% (2–hydroxypropyl)-β-cylcodextrin, 1% sodium bicarbonate or vehicle alone twice daily 5 days/week. Mice were monitored daily and sacrificed according to IACUC-approved methods and criteria for neurologic symptoms.
Histology and Immunohistochemistry
FFPE sections (4 µm) of mouse brain tissue were stained with hematoxylin and eosin (H&E). For immunohistochemistry, sections were deparaffinized, rehydrated, blocked with 3% H2O2 for 5 min at 25°C and treated with Target Retrieval Solution (Dako) for 25 min at 96°C. For anti-phosphoThr288Aurora-A, slides were further blocked with Protein Block Serum-Free (Dako) for 15 min at 25°C. Slides were incubated with anti-phosphoThr288Aurora-A (Abcam ab58494, 1:200) or anti-phosphohistone-H3 (Abcam ab5176, 1:1,400) for 1 hour at 25°C. Antibodies were detected using MACH 3 Rabbit HRP-Polymer (Biocare) and DAB (Dako). Slides were counterstained with hematoxylin.
Control and alisertib-treated neurosphere cytospins were stained with H&E or immunostained with anti-β-catenin (BD Pharmingen # 610153, 1:2,500). For Feulgen staining, GB9 cells were treated for 5 days with 200 nM alisertib or DMSO and cytospins prepared. Slides were stained using the Blue Feulgen DNA Ploidy Analysis Staining Kit (ScyTek) and scanned with an Aperio XT ScanScope. Image analysis was performed with Definiens Tissue Studio 3.6 (51,480 control neurosphere nuclei and 15,864 alisertib-treated neurosphere nuclei were examined).
Results and Discussion
Effect of Aurora-A inhibition on growth and survival of glioma neurosphere cells
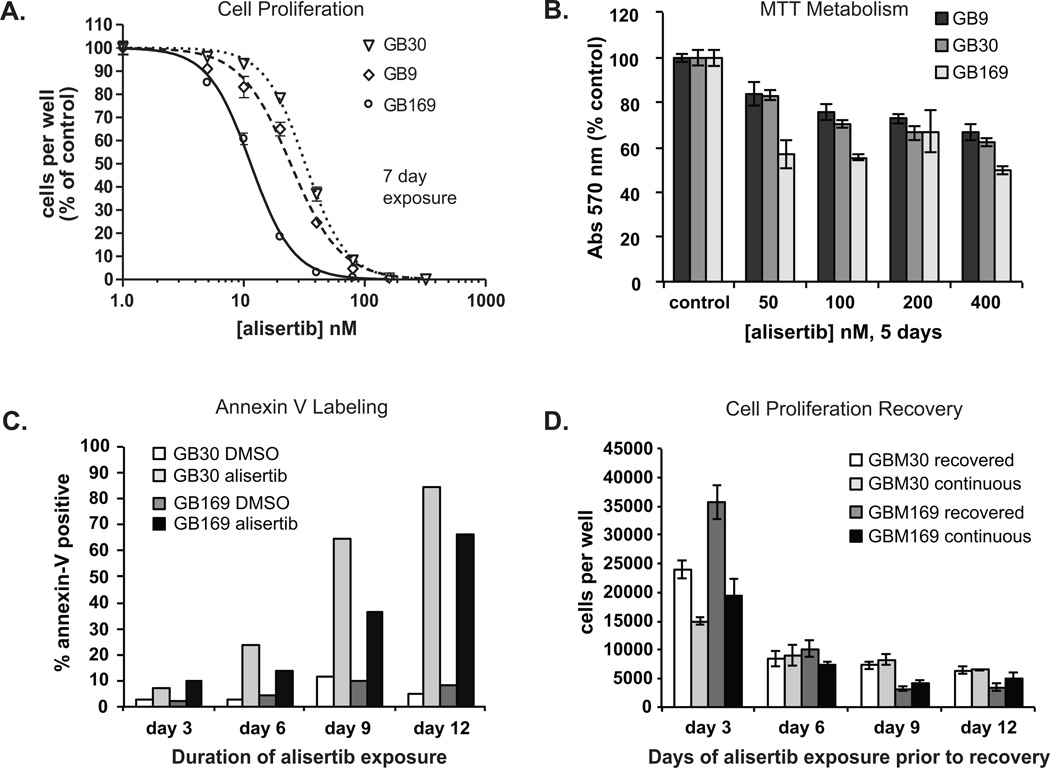
Glioblastoma neurosphere cells demonstrate tumor stem cell-like properties and are considered to closely mimic the biologic behavior of naturally occurring gliomas (12, 13). We performed growth curve analysis of GB neurosphere cells with and without small molecule Aurora-A inhibitors. Alisertib potently blocked proliferation of all 3 neurosphere lines tested (Fig. 1A). IC50 concentrations were 11, 24 and 32 nM for GB169, GB9 and GB30 cells, respectively. Another Aurora-A inhibitor, TC-A2317 (11) was less potent, demonstrating IC50 concentrations of 170, 179 and 168 nM for these cells, respectively (Fig. S1A). Observed alisertib IC50 concentrations are similar to slightly higher than those required to inhibit colony formation in 2 other GB neurosphere lines (14) and approximately 5 to 10-fold less than those effective against 10 conventional monolayer GB cell lines (15) suggesting greater efficacy against GB neurosphere cells.
Figure 1. Effect of alisertib on proliferation and viability of glioblastoma neurosphere cells.
A. GB neurospheres were cultured 7 days with various concentrations of alisertib or DMSO and counted. Means ± SD of triplicate wells are depicted. B. Neurospheres were treated for 5 days with alisertib or DMSO and MTT assays performed. Data are means ± SD of triplicate wells. Three experiments provided similar results. All alisertib treatments were significantly different from controls, p > 0.05. C. GB neurospheres were treated with 200 nM alisertib for 3 to 12 days followed by Annexin V immunostaining. D. GB neurosphere cells were plated in 60 mm dishes (5 × 105/dish) and treated with 200 nM alisertib or DMSO (neurospheres in DMSO treatments were dispersed every 3 days to prevent overgrowth). Every 3 days one-half of the cells per time point dish were pelleted, washed with PBS and recultured in 24 well plates with 200 nM alisertib (continuous) or DMSO (recovered) for an additional 3 days and counted.
We also examined the impact of alisertib on GB neurospheres using MTT assays. Although alisertib reduced the metabolic activity of these cells, the magnitude of response was less than that seen by cell counts (Fig. 1B). Short exposure to highly antiproliferative concentrations of alisertib, e.g., 200 nM, reduced total cellular metabolism by approximately 28 to 33% (Fig. 1B), similar to the fraction of cells in which an equivalent alisertib exposure induces apoptosis (Fig.s 1B & 1C). This suggests that despite attenuated proliferation, some tumor cells remained metabolically active following treatment. Therefore, the antiproliferative effect of Aurora-A kinase inhibition cannot be due solely to immediate tumor stem-like cell killing.
To test if alisertib-induced toxicity was due to increased apoptosis, we measured annexin-V staining in alisertib-treated normal human astrocytes and GB cells. Alisertib did not induce significant annexin-V staining in human astrocytes (Fig. S1B). In contrast, alisertib-treated GB neurosphere cells showed increased annexin-V staining indicative of apoptosis, reaching approximately 85% after 12 days of drug exposure (Fig. 1C & Fig. S1C).
Since alisertib caused only a limited amount of apoptosis after 6 days of exposure and did not completely prevent metabolic activity in GB neurospheres, we investigated if neurosphere cells could resume proliferation following treatment. GB neurospheres were exposed to alisertib continuously for 3 to 12 days, the drug washed out, and cells cultured with or without alisertib for an additional 3 days. Cell counts were then obtained to measure GB cell proliferative potential following drug treatment (Fig. 1D & Supp Fig. 1C). In a similar experiment, recovery of metabolic activity following extended alisertib exposure was examined by MTT assay (Fig. S1D).
If alisertib-treated cells retained proliferative capacity, total cell counts should increase when further cultured in drug-free media. As shown in Figures 1D and S1C, after 3 days of alisertib treatment GB neurosphere cells allowed to recover in drug-free media showed increased numbers compared to continually treated cells. The difference in tumor cell numbers between cultures continually treated for 6 days and those treated for 6 days and allowed to recover in drug-free media was negligible however, indicating nearly complete loss of tumor cell proliferative potential. A similar reduction of recoverable MTT activity took longer, suggesting total senescence and/or death of a small population of persisting cells (15 to 20%) required extended drug exposure, but eventually occurred (Fig. S1D).
Effect of Aurora-A inhibition on morphology, differentiation and senescence of GB neurosphere cells
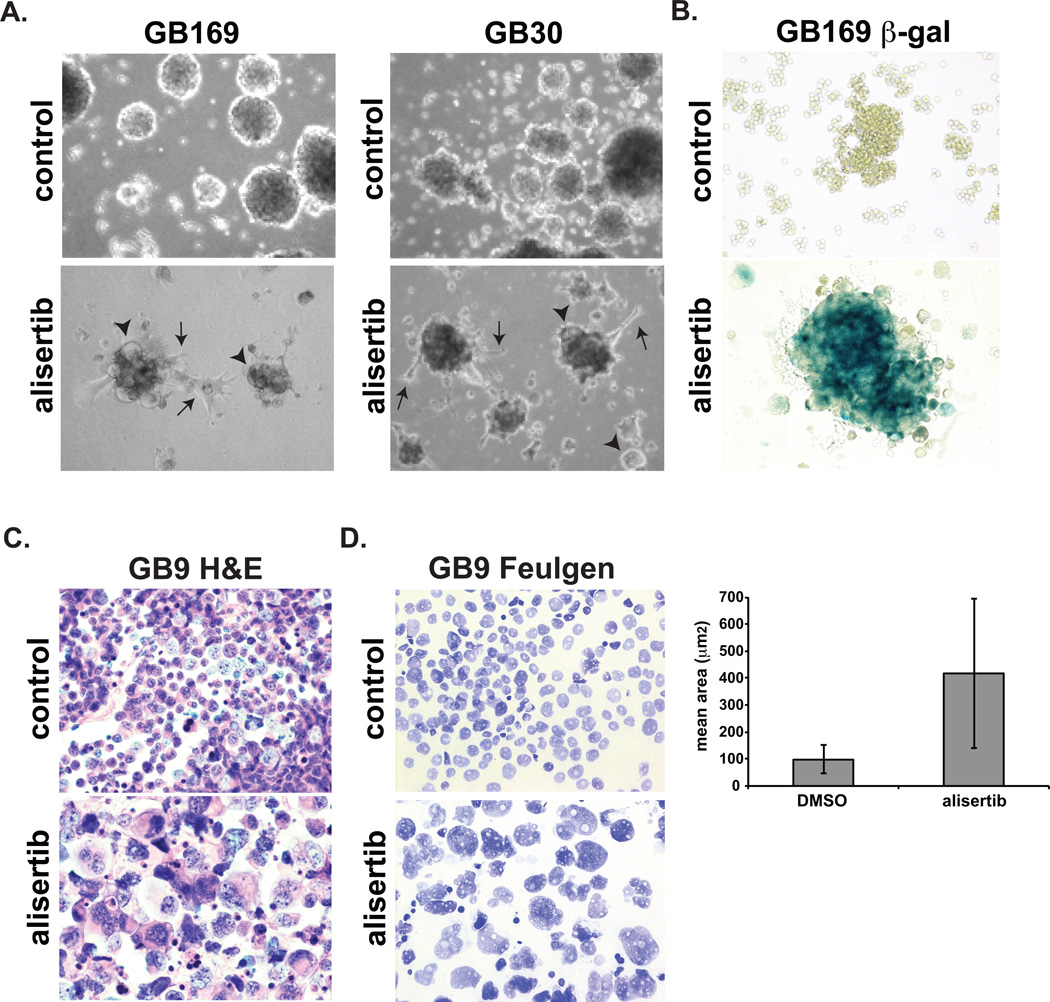
Aurora-A inhibitors had a dramatic effect on GB cell morphology after 2 weeks of exposure. Neurospheres contained fewer, but larger cells (Fig. 2A & S2). More cells were attached to the culture dish and many exhibited prominent astrocyte-like processes. Furthermore, alisertib caused a strong increase in β-galactosidase staining (Fig. 2B).
Figure 2. Morphology of GB neurosphere cells treated with alisertib.
A. Glioblastoma neurospheres were treated with DMSO or 200 nM alisertib for 2 weeks and photographed. Many cells in alisertib-treated cultures exhibited cytomegaly (arrowheads) or astrocyte-like process (arrows). B. Alisertib-treated and control GB neurosphere cytospins were stained for β-galactosidase activity, C. H&E or D. Feulgen for DNA content. The histogram depicts mean area ± SD of Feulgen stained nuclei determined by image analysis. Magnifications: C–D, 400×.
H&E-stained cytospins confirmed that individual alisertib-treated GB neurosphere cells were greatly enlarged (Fig. 2C). Quantitation of nuclear DNA by image analysis of Feulgen-stained (16) cytospins revealed that tumor nuclei mean area increased from 98.7 µm2 in control cells to 416 µm2 in alisertib-treated cells indicating failed cytokinesis and tetraploidy (Fig. 2D).
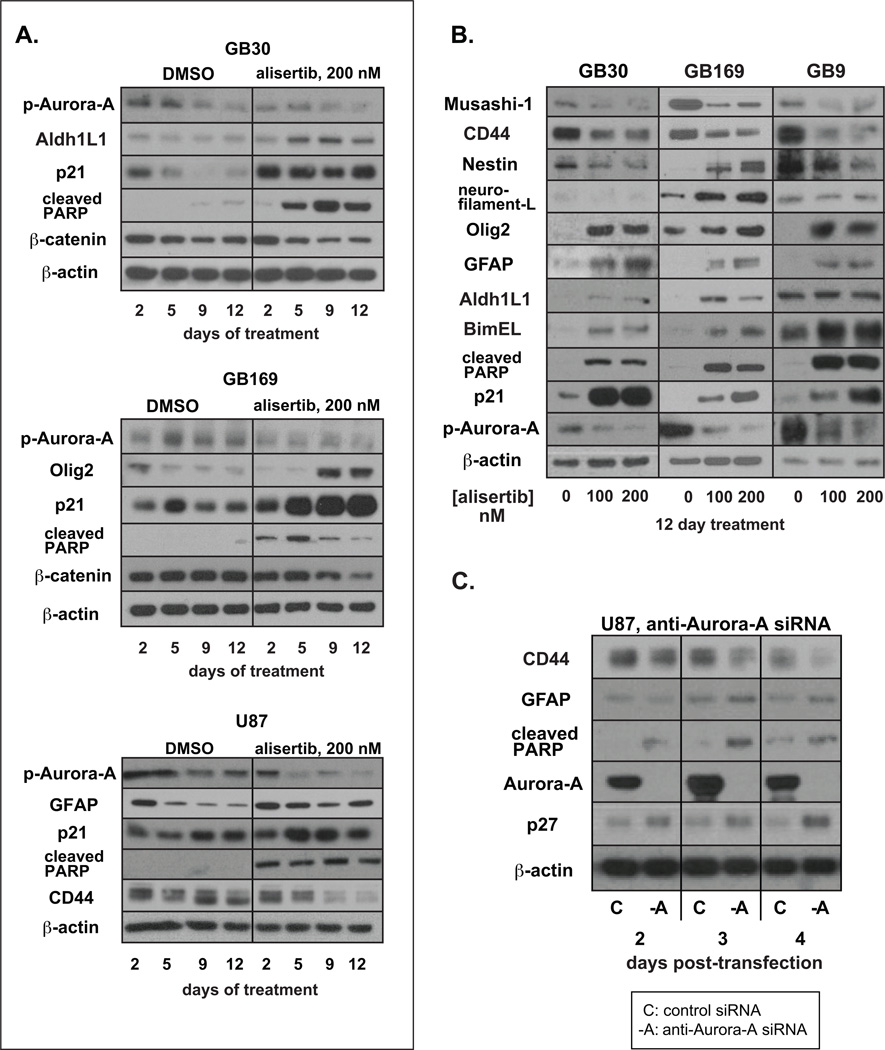
Aurora-A kinase activity depends on activation by autophosphorylation at threonine 288 (17). Western blots for phosphoThr288Aurora-A show that phosphoThr288Aurora-A is markedly decreased in alisertib- and TC-A2317-treated GB neurospheres verifying drug target-hit (Fig.s 3A, 3B & S3A).
Figure 3. Effect of alisertib on senescence, apoptosis and stem markers in GB neurospheres.
A. Glioblastoma cells were treated with 200 nM alisertib or DMSO for the indicated times and lysates immunoblotted. B. Neurospheres were treated with DMSO or 100 or 200 nM alisertib for 12 days and lysates immunoblotted. C. U87 cells were transfected with control or anti-Aurora-A siRNAs. Lysates were prepared 2, 3 and 4 days post-transfection.
To look for biochemical evidence of apoptosis, differentiation and senescence of GB neurosphere cells in response to Aurora-A inhibitors, we immunoblotted for proteins indicative of these processes. Consistent with Annexin-V staining (Fig. 1C), BimEL, a proapoptotic BH3-only protein negatively regulated by Aurora-A (18) and/or cleaved PARP were consistently elevated in alisertib- or TC-A2317-treated cells (Fig.s 3A, 3B & S3A).
Olig2, a transcription factor expressed in normal and neoplastic astrocytes and oligodendrocytes, was increased in all GB neurosphere lines treated with alisertib or TC-A2317 (Fig.s 3A, 3B & S3A). GFAP and Aldh1L1, markers of astrocytic differentiation (19), were also increased in response to alisertib (Fig.s 3A and 3B). Unlike neurosphere tumor stem-like cells, U87 glioma cells expressed high basal levels of GFAP however alisertib treatment prevented the decreased GFAP expression seen in U87 cells approaching confluence (Fig 3A). In agreement with observed elevated β-galactosidase activity (Fig. 2B), expression of the cellular senescence associated cyclin-dependent kinase inhibitor p21 (20) was strongly increased by alisertib (Fig. 3A & 3B) and TC-A2317 (Fig. S3A).
Western analysis for markers of stemness revealed that Musashi-1, an RNA-binding protein involved in stem cell self-renewal (21), the cancer stem cell marker CD44 (22) and β-catenin were all markedly decreased in alisertib-treated GB neurosphere cells (Fig.s 3A, 3B & S3B). β-catenin was also decreased by TC-A2317 (Fig. S3A). As β-catenin is required for CNS progenitor self-renewal (23), these results may correlate with a decreased capacity for GB neurosphere cell self-renewal following Aurora-A inhibition.
The neural stem cell marker nestin was also decreased by alisertib in GB9 and GB30 cells (Fig. 3B). In contrast, nestin was increased after alisertib treatment in GB169 cells. Not all GB stem-like cells express specific stem and differentiation markers equally (12). Moreover, nestin expression is not exclusive to neural stem cells but is also found in regenerating neurons and glia (24), as well as many CNS neoplasms. Unlike in GB9 and GB30 cells, alisertib also increased neurofilament protein expression in GB169 cells (Fig. 3B) possibly correlating with increased nestin and induction of an early neuronal differentiation program in these cells. Thus apoptosis, differentiation, senescence and/or loss of neurosphere tumor cell stemness are likely factors contributing to the antiproliferative effect of Aurora-A inhibition in GB cells in vitro.
To investigate whether the above observed biochemical effects of alisertib on GB cells were Aurora-A specific, we transfected U87 glioma cells with anti-Aurora-A siRNAs. Aurora-A knockdown resulted in biochemical changes similar to those caused by alisertib including reduced CD44 and increased expression of cleaved PARP, GFAP and the senescence-associated cyclin-dependent kinase inhibitor p27 (Fig. 3C). Thus, the response of GB neurosphere cells to Aurora-A kinase inhibition or mRNA knockdown includes apoptosis, differentiation and senescence. Additionally, Aurora-A itself is involved in maintaining embryonic stem cells in the undifferentiated state (25). Thus, another mechanism in which Aurora-A inhibition may be effective against glioma cells could be by inducing a loss of stemness. This may be an important mechanism possibly leading to sensitization of gliomas to other therapies or inhibiting the emergence of therapy-resistant tumor cell clones.
Effect of alisertib on GB neurosphere orthotopic xenograft growth in vivo
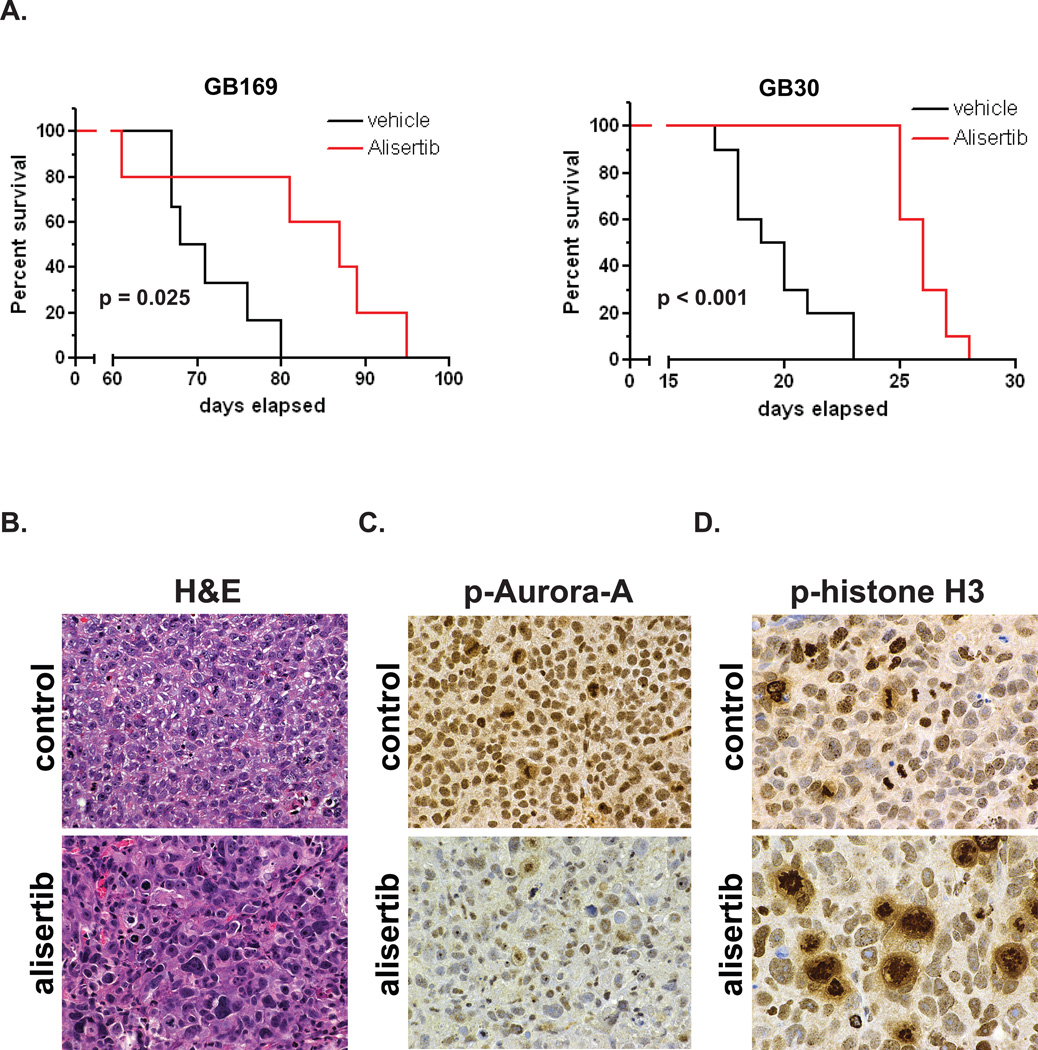
To determine whether alisertib was effective against glioblastoma in vivo we treated nude mice containing GB30 and GB169 intracranial xenografts with the drug. As shown in Figure 4A, alisertib significantly extended the survival of mice with intracranial xenografts of both GB neurosphere lines. Mice with GB169 xenografts showed a 25.2% increase in median survival (87 days for alisertib-treated compared to 69.5 days for vehicle-treated, p = 0.025), while survival was increased by 33.3% in mice with GB30 xenografts (26 days for alisertib- treated compared to 19.5 days for vehicle-treated, p < 0.0001).
Figure 4.
A. Alisertib extends survival of mice with GB neurosphere intracranial xenografts. Kaplan-Meier curves for mice with GB169 or GB30 xenografts treated with 20 mg/kg alisertib twice daily or vehicle alone are shown. B.–D. Effects of alisertib on GB neurosphere cells in vivo. Mice with GB169 xenografts were given vehicle or alisertib (20 mg/kg twice daily) for 2 weeks and sacrificed. FFPE tumor sections were stained for H&E (B) phosphoThr288Aurora-A (C) or phosphohistone-H3 (D). Magnifications: B–C, 400×; D, 600×.
Examination of GB169 xenograft tumors from alisertib-treated mice revealed similar effects to those observed in vitro. As shown in Figure 4B, glioblastomas from alisertib-treated mice demonstrated enlarged nuclei and greatly decreased phosphoThr288Aurora-A compared to control mice (Fig. 4C). Furthermore, phosphohistone-H3 immunostaining revealed large numbers of abnormal arrested mitoses as previously described for Aurora-A inhibition (26), whereas mitoses in tumors from control mice more often resembled normal metaphase or telophase (Fig. 4D). These observations confirm that alisertib exhibited target-specific effects on GB cells in vivo.
In summary, the Aurora-A inhibitor alisertib potently inhibits proliferation of GB tumor neurosphere stem-like cells in vitro and demonstrates Aurora-A-specific target hit effects in vitro and in vivo. It also significantly extends the survival of mice with intracranial GB neurosphere xenografts. Alisertib is therefore a promising new potential agent for the treatment of glioblastoma alone or in combination with other therapies.
Supplementary Material
Acknowledgments
Supported in part by Takeda Pharmaceuticals (NLL) and NCI F31CA171733 (JW).
Footnotes
No conflicts of Interest
References
- 1.Marumoto T, Honda S, Hara T, Nitta M, Hirota T, Kohmura E, et al. Aurora-A kinase maintains the fidelity of early and late mitotic events in HeLa cells. J Biol Chem. 2003;278:51786–51795. doi: 10.1074/jbc.M306275200. [DOI] [PubMed] [Google Scholar]
- 2.Seki A, Coppinger JA, Jang CY, Yates JR, Fang G. Bora and the kinase Aurora A cooperatively activate the kinase Plk1 and control mitotic entry. Science. 2008;320:1655–1658. doi: 10.1126/science.1157425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou H, Kuang J, Zhong L, Kuo WL, Gray JW, Sahin A, et al. Tumour amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and transformation. Nat Genet. 1998;20:189–193. doi: 10.1038/2496. [DOI] [PubMed] [Google Scholar]
- 4.Qin L, Tong T, Song Y, Xue L, Fan F, Zhan Q. Aurora-A interacts with Cyclin B1 and enhances its stability. Cancer Lett. 2009;275:77–85. doi: 10.1016/j.canlet.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 5.Sasayama T, Marumoto T, Kunitoku N, Zhang D, Tamaki N, Kohmura E, et al. Over-expression of Aurora-A targets cytoplasmic polyadenylation element binding protein and promotes mRNA polyadenylation of Cdk1 and cyclin B1. Genes Cells. 2005;10:627–638. doi: 10.1111/j.1365-2443.2005.00870.x. [DOI] [PubMed] [Google Scholar]
- 6.Yang S, He S, Zhou X, Liu M, Zhu H, Wang Y, et al. Suppression of Aurora-A oncogenic potential by c-Myc downregulation. Exp Mol Med. 2010;42:759–767. doi: 10.3858/emm.2010.42.11.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carol H, Boehm I, Reynolds CP, Kang MH, Maris JM, Morton CL, et al. Efficacy and pharmacokinetic/pharmacodynamic evaluation of the Aurora kinase A inhibitor MLN8237 against preclinical models of pediatric cancer. Cancer Chemother Pharmacol. 2011;68:1291–1304. doi: 10.1007/s00280-011-1618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Görgün G, Calabrese E, Hideshima T, Ecsedy J, Perrone G, Mani M, et al. A novel Aurora-A kinase inhibitor MLN8237 induces cytotoxicity and cell-cycle arrest in multiple myeloma. Blood. 2010;115:5202–5213. doi: 10.1182/blood-2009-12-259523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huck JJ, Zhang M, McDonald A, Bowman D, Hoar KM, Stringer B, et al. MLN8054, an inhibitor of Aurora A kinase, induces senescence in human tumor cells both in vitro and in vivo. Mol Cancer Res. 2010;8:373–384. doi: 10.1158/1541-7786.MCR-09-0300. [DOI] [PubMed] [Google Scholar]
- 10.ClinicalTrials.gov, a service of the U.S., National Institutes of Health
- 11.Ando R, Ikegami H, Sakiyama M, Ooike S, Hayashi M, Fujino Y, et al. 3-Cyano-6-(5-methyl-3-pyrazoloamino)pyridines: selective Aurora A kinase inhibitors. Bioorg Med Chem Lett. 2010;20:4709–4711. doi: 10.1016/j.bmcl.2010.04.119. [DOI] [PubMed] [Google Scholar]
- 12.Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 13.Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 14.Hong X, O’Donnell JP, Salazar CR, Van Brocklyn JR, Barnett KD, Pearl DK, et al. The selective Aurora-A kinase inhibitor MLN8237 (alisertib) potently inhibits proliferation of glioblastoma neurosphere tumor stem-like cells and potentiates the effects of temozolomide and ionizing radiation. Cancer Chemother Pharmacol. 2014 doi: 10.1007/s00280-014-2430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lehman NL, O'Donnell JP, Whiteley LJ, Stapp RT, Lehman TD, Roszka KM, et al. Aurora A is differentially expressed in gliomas, is associated with patient survival in glioblastoma and is a potential chemotherapeutic target in gliomas. Cell Cycle. 2012;11:489–502. doi: 10.4161/cc.11.3.18996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biesterfeld S, Beckers S, Del Carmen Villa Cadenas M, Schramm M. Feulgen staining remains the gold standard for precise DNA image cytometry. Anticancer Res. 2011;31:53–58. [PubMed] [Google Scholar]
- 17.Walter AO, Seghezzi W, Korver W, Sheung J, Lees E. The mitotic serine/threonine kinase Aurora2/AIK is regulated by phosphorylation and degradation. Oncogene. 2000;19:4906–4916. doi: 10.1038/sj.onc.1203847. [DOI] [PubMed] [Google Scholar]
- 18.Moustafa-Kamal M, Gamache I, Lu Y, Li S, Teodoro JG. BimEL is phosphorylated at mitosis by Aurora A and targeted for degradation by bTrCP1. Cell Death Differ. 2013;20:1393–1403. doi: 10.1038/cdd.2013.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romanov VS, Pospelov VA, Pospelova TV. Cyclin-dependent kinase inhibitor p21(Waf1): contemporary view on its role in senescence and oncogenesis. Biochemistry (Mosc) 2012;77:575–584. doi: 10.1134/S000629791206003X. [DOI] [PubMed] [Google Scholar]
- 21.Sakakibara S, Nakamura Y, Yoshida T, Shibata S, Koike M, Takano H, et al. RNA-binding protein Musashi family: roles for CNS stem cells and a subpopulation of ependymal cells revealed by targeted disruption and antisense ablation. Proc Natl Acad Sci U S A. 2002;99:15194–15199. doi: 10.1073/pnas.232087499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anido J, Sáez-Borderías A, Gonzàlez-Juncà A, Rodón L, Folch G, Carmona MA, et al. TGF-β receptor inhibitors target the CD44high/Id1high glioma-initiating cell population in human glioblastoma. Cancer Cell. 2010;18:655–668. doi: 10.1016/j.ccr.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 23.Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- 24.Gilyarov AV. Nestin in central nervous system cells. Neurosci Behav Physiol. 2008;38:165–169. doi: 10.1007/s11055-008-0025-z. [DOI] [PubMed] [Google Scholar]
- 25.Lee DF, Su J, Ang YS, Carvajal-Vergara X, Mulero-Navarro S, Pereira CF, et al. Regulation of embryonic and induced pluripotency by aurora kinase-p53 signaling. Cell Stem Cell. 2012;11:179–194. doi: 10.1016/j.stem.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoar K, Chakravarty A, Rabino C, Wysong D, Bowman D, Roy N, et al. MLN8054, a small-molecule inhibitor of Aurora A, causes spindle pole and chromosome congression defects leading to aneuploidy. Mol Cell Biol. 2007;27:4513–4525. doi: 10.1128/MCB.02364-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.