Abstract
The total syntheses of dihydrolysergic acid and dihydrolysergol are detailed based on a Pd(0)-catalyzed intramolecular Larock indole cyclization for the preparation of the embedded tricyclic indole (ABC ring system) and a subsequent powerful inverse electron demand Diels–Alder reaction of 5-carbomethoxy-1,2,3-triazine with a ketone-derived enamine for the introduction of a functionalized pyridine, serving as the precursor for a remarkably diastereoselective reduction to the N-methylpiperidine D-ring. By design, the use of the same ketone-derived enamine and a set of related complementary heterocyclic azadiene [4 + 2] cycloaddition reactions permitted the late stage divergent preparation of a series of alternative heterocyclic derivatives not readily accessible by more conventional approaches.
Keywords: 1,2,3-triazine cycloaddition; ergot alkaloids; palladium catalyzed indole synthesis; Diels-Alder cycloaddition
1. Introduction
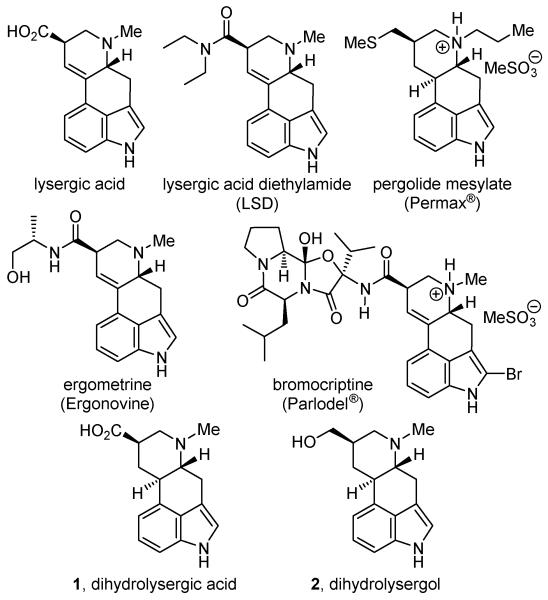
The ergot alkaloids isolated from the fungus Claviceps purpurea comprise a large group of pharmacologically potent indole alkaloids (Figure 1).1 Among this family of natural products, lysergic acid2 is the most widely recognized member and several semisynthetic derivatives are clinically used in the treatment of a range of neurological diseases. The dopamine agonists, pergolide3 and bromocriptine,4 are used for the treatment of Parkinson’s disease and type 2 diabetes, respectively, and ergometrine5 is used as a uterotonic agent in combination with oxytocin. Most notorious among the semisynthetic derivatives is lysergic acid diethylamide (LSD), a powerful psychoactive and psychedelic drug. Embedded in the structures of the ergot alkaloids are conformationally-restricted variants of the phenethylamine pharmacophores of both dopamine and related biogenic amines as well as that of serotonin. Consequently, extensive studies have been conducted to explore not only the biological properties of the ergot alkaloids, but to also define the structural features responsible for the activity and the underlying origin of the pharmacological effects.
Figure 1.
Natural products and key semisynthetic analogues.
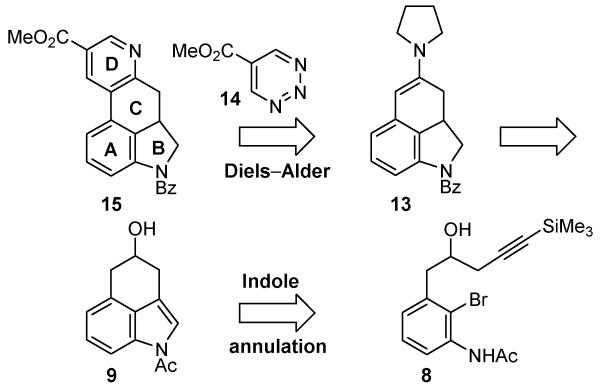
As a result, the total synthesis of the ergot alkaloids has been the subject of extensive study over the last half century.1a,6 Herein, we report the total synthesis of dihydrolysergic acid (1)7,8 and dihydrolysergol (2)9 based on two key reactions we recently introduced. Notably, the latter natural product has only been prepared by semisynthesis from lysergic acid and the work herein discloses the first total synthesis of this natural product by an alternate route. The first of these reactions is a powerful intramolecular Larock indole cyclization10,11 for formation of an embedded tricyclic indole isomeric with Uhle’s12 and Kornfeld’s6a ketones widely used in accessing the ergot alkaloids (Figure 2). The second of the reactions is a powerful inverse electron demand Diels–Alder reaction of 5-carbomethoxy-1,2,3-triazine with a conjugated enamine for the regiospecific introduction of a functionalized pyridine,13 serving as the precursor for a subsequent and remarkably diastereoselective reduction to the N-methylpiperidine D-ring. An additional and deliberate key feature of the approach is that use of the same advanced ketone-derived enamine and a series of inverse electron demand [4 + 2] cycloaddition reactions of heterocyclic azadienes14 permitted the divergent15 preparation of isomeric and alternative heterocyclic derivatives bearing deep-seated structural changes to the ergot skeleton not as readily accessible by conventional approaches.
Figure 2.
Strategic reactions employed.
2. Results and Discussion
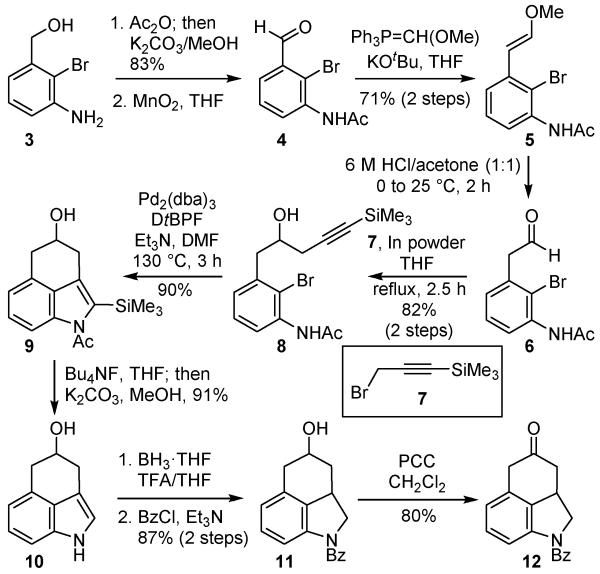
The synthesis began with the preparation of the tricyclic ketone 12 as illustrated in Scheme 1. Although both the synthesis of 8 (in Supporting Information10a) and its participation in the key indole cyclization were detailed in our original studies disclosing the Pd(0)-catalyzed cyclization,10a the synthesis of 8 reported herein is significantly improved and its cyclization has been successfully increased to 0.5–1 g scales. Bis-acetylation of the known 2-bromoaniline 316 and subsequent methanolysis of the O-acetate furnished the free alcohol (83%). This was followed by oxidation (MnO2) of the benzylic alcohol to provide the aldehyde 4 (92%). Wittig olefination of the resulting aldehyde with (methoxymethylene)-triphenylphosphorane17 afforded the enol ether 5 (86%) as a mixture of olefin isomers (71% yield over two steps without intermediate purification of 4). Hydrolysis effected by treatment of 5 with aqueous 6 N HCl/acetone (1:1) furnished aldehyde 6. The substrate for the key intramolecular indole annulation was accessed from 6 using an indium variant of a Grignard reagent, which was prepared from 1-(trimethylsilyl)propargyl bromide (7).18 The addition reaction of the in situ generated indium reagent (3 equiv indium powder, THF, 25 °C to reflux, 2.5 h) proceeded smoothly to afford the homopropargylic alcohol 8 in excellent yield (82% over two steps from enol ether 5) without detection of the isomeric allene or competitive reactions derived from enolization of the reactive starting aldehyde that were observed with the Grignard reagent itself. The key transformation for the preparation of the tricyclic ketone skeleton was realized with use of the intramolecular Pd(0)-catalyzed Larock indole annulation of 8, possessing the pendant alkyne and forming the desired tricyclic indole 9 in excellent yield (90%).
Scheme 1.
Preparation of tricyclic ketone 12.
Our introduction and subsequent investigation10 of the catalytic indole cyclization indicated that substrates including 8 were inert towards many candidate palladium catalysts including PdCl2, PdCl2(PhCN)2 or PdCl2(PPh3)2, whereas other readily accessible catalysts including PdCl2(PPh3)2, Pd(PPh3)4 and Pd2(dba)3 supported the cyclization reaction. However, significantly improved results were observed with a Pd2(dba)3 catalyst system that was first found to be remarkably successful in a challenging Suzuki coupling reaction in our vancomycin synthetic studies, arising from its unusually rapid oxidative addition to a hindered, electron-rich aryl bromide.19 Optimization of this catalyst system revealed that DtBPF was the best added ligand of those examined, that DMF was the most effective solvent examined although others were effective, and that soluble organic bases such as Et3N were found to be superior to insoluble bases like K2CO310. With the use of 15 mol % of the catalyst and with the DtBPF ligand in DMF at 130 °C for 3 h, the reaction provided the indole 9 in yields as high as 90% now on scales ranging from 0.5–1 g. Despite the expectedly slow oxidative addition with substrates like 8 bearing a hindered, electron-rich ortho disubstituted aryl bromide, they are effective participants in the cyclization reaction under these conditions. Presumably, this may be attributed to a combination of the unique Pd2(dba)3 derived catalyst reactivity19 and potentially its coordination to the proximal polar amide of the substrate.
At this stage, oxidation of the secondary alcohol to the corresponding ketone was examined. However, all efforts were ineffective under a variety of oxidation conditions. Although clean oxidation product could be detected (TLC and LCMS), all efforts resulted in competitive benzylic oxidation, naphthalene aromatization, or potential decomposition upon SiO2 purification. Thus, the reduced indoline precursor was prepared, which provided two advantages over the candidate indole precursor. In addition to preventing the competitive over-oxidation, the subsequent requisite enamine now could be generated regioselectively by virtue of the single stabilizing aryl conjugation. Thus, removal of the trimethylsilyl group in 9 (Bu4NF, THF) followed by methanolysis of the resulting crude N-acetate (K2CO3, MeOH) provided 10 (91%). After exploration of several reduction conditions, treatment of 10 with bis(trifluoroacetoxy)borane20 generated in situ from BH3·THF in trifluoroacetic acid proved to be highly effective and afforded the corresponding indoline, which was protected as the benzoyl amide 11 (87% over two steps). Other reduction methods were also successful (Et3SiH, TFA/CH2Cl2, 69%; H2, PtO2, TFA/H2O; BH3–Py, TFA) but initially afforded 11 in lower conversions. Subsequent oxidation of the alcohol by PCC21 now uneventfully provided the requisite tricyclic ketone 12 in 80% yield.
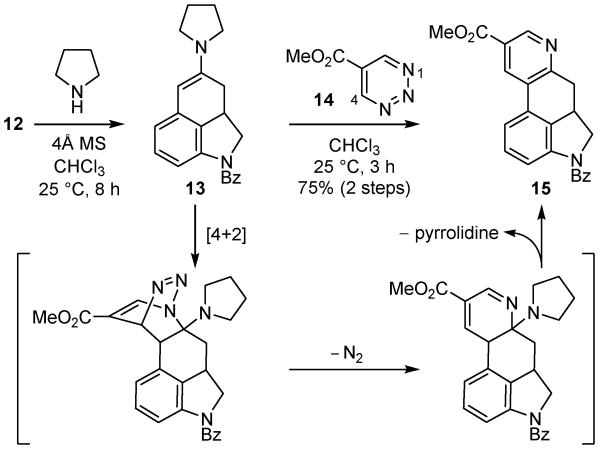
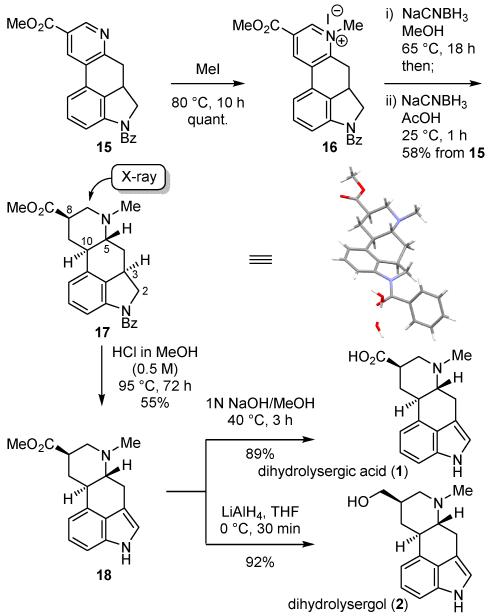
With the requisite tricyclic ketone 12 in hand, its use in a key inverse electron demand Diels–Alder reaction with 5-carbomethoxy-1,2,3-triazine13a (14, ALD00106)22 was explored (Scheme 2). The enamine 13 was prepared by treatment of ketone 12 with pyrrolidine (4Å MS, CHCl3, 25 °C, 8 h) and its Diels–Alder reaction with the 1,2,3-triazine 14 (4 equiv, 0.1 M CHCl3, 25 °C, 3 h)23 proceeded rapidly to provide the pyridine cycloaddition product 15 (75% from ketone 12) as a single regioisomer without detection of the intermediates even when conducted at room temperature. The inclusion of 4Å MS in the reaction mixture or use of higher reaction concentrations (0.3 M) or temperatures (65 °C) even with prolonged reaction times (15 h) did not further improve the already excellent cycloaddition yield. The reaction of the 1,2,3-triazine 14 exhibited the now characteristic exclusive N1/C4 cycloaddition regioselectivity13 with the nucleophilic carbon of the electron-rich enamine attached to C4, benefiting from the complementary azadiene substitution (C5-CO2Me). Importantly, the remarkable reactivity of 14 imparted by the C5 electron-withdrawing substituent provides substantially improved cycloaddition reactivity toward enamine dienophiles such that the reaction with 13 occurred at 25 °C, representing a synthetic scope not observed with 1,2,3-triazine itself.
Scheme 2.
Inverse electron demand Diels–Alder reaction.
The dihydro analogues of lysergic acid comprise an important class of central nervous system drugs that includes pergolide mesylate, which is produced commercially through the hydrogenation of lysergic acid. Although the potential reduction of pyridine precursors to the ergot alkaloid D-ring has always been an attractive strategy, only one successful implementation has been reported6b and was conducted with a precursor lacking the intact ABCD-ring system. In a large measure, this may be attributed to a problematic pyridine reduction with substrates bearing the core indole, which suffers from a facile tautomerization to a core naphthalene. Our approach enlists a core indoline incapable of this competitive reaction and we first examined the use of 15 targeting the fully reduced pyridine ring to afford saturated derivatives of lysergic acid (Scheme 3).
Scheme 3.
Completion of the total synthesis of dihydrolysergic acid (1) and dihydrolysergol (2).
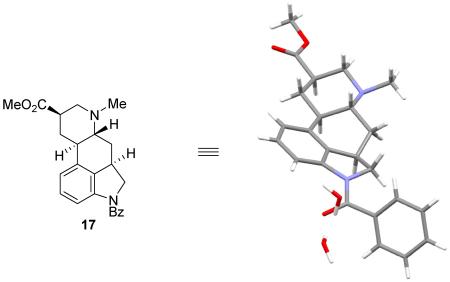
To this end, pyridine 15 was N-methylated by treatment with MeI (80 °C, 10 h), providing the pyridinium salt 16 quantitatively. In turn, this crude salt was exposed to a variety of reduction conditions. After exploration of several methods, a stepwise reduction (NaCNBH3, MeOH, 65 °C, 18 h; then NaCNBH3, HOAc, 25 °C, 1 h) proved to be highly effective and directly produced near exclusively a single diastereomer 17 (58%, >10:1), whose structure and relative stereochemistry were established by a single-crystal X-ray structure determination.24 The trans CD ring fusion (C5/C10), the thermodynamically more stable equatorial disposition of the C8 methyl ester, and the relative C3/C5 trans stereochemistry that places all substituents on the central C-ring in an equatorial disposition, collectively represent the thermodynamically most stable relative stereochemistry among all stereocenters. It is remarkable that essentially a single diastereomer emerged from the stepwise reduction and only a trace of a minor diastereomer(s) was detected (ca. 5%) that likely represents predominately the C8 diastereomer. These remarkable results sharply contrast prior efforts where reduction of such pyridyl precursors with the imbedded indole intact proved unmanageable25 or were erroneously reported.26 Removal of the N-benzoyl amide was found to occur under conditions that promote concomitant oxidation of the indoline to the corresponding indole. This was optimized such that treatment of 17 with HCl in MeOH (0.5 M, 95 °C, 72 h) provided 18 (55%) directly in a single step and subsequent hydrolysis of the methyl ester (1 N NaOH/MeOH, 40 °C, 3 h) afforded dihydrolysergic acid (1), whose spectroscopic properties perfectly matched those of authentic synthetic material.27 The same intermediate 18 was also used to access dihydrolysergol (2) where ester reduction (LiAlH4, THF, 0 °C, 30 min, 92%) afforded dihydrolysergol (2), whose properties proved identical in all respects with those of an authentic sample of the natural product.28,29
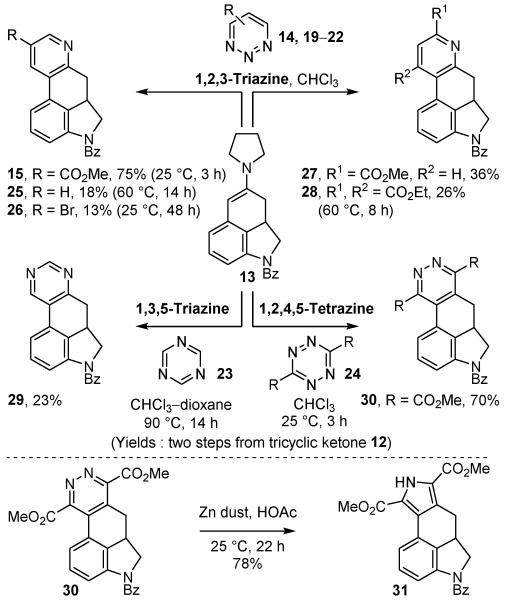
A key element of the approach was the use of the enamine 13 derived from ketone 12 in a cycloaddition reaction with a heterocyclic azadiene for installation of a D-ring pyridine. This strategy deliberately permits additional cycloaddition reactions of the advanced electron-rich dienophile 13 with other reactive heterocyclic azadienes (19–24) for the late-stage, divergent preparation of a series of complementary heterocyclic derivatives (Scheme 4). Representative of this and without individual optimization, this included the reaction of 1,2,3-triazine13d (19, ALD00112),22 5-bromo-1,2,3-triazine (20),13a 4-carbomethoxy-1,2,3-triazine13b (21, ALD00104),22 and 4,6-dicarboethoxy-1,2,3-triazine13b (22, ALD00110),22 each of which displayed the 1,2,3-triazine C4/N1 mode of cycloaddition and provided the alternatively substituted pyridines 25–28, 1,3,5-triazine30 (23)22 that provided the pyrimidine 29, and 3,6-dicarbomethoxy-1,2,4,5-tetrazine31 (24, ALD00098)22 that provided the substituted pyridazine 30 and served further as a precursor to the pyrrole 31 via a unique zinc-mediated reductive ring contraction.32 It is of note that it was the exploration of the ergot alkaloids at Lilly and the preparation of their simplified ring systems that provided the first synthetic use of such heterocyclic azadiene Diels–Alder reactions (use of 30) and the first report of the effective zinc-mediated pyridazine to pyrrole reductive ring contraction reaction.33
Scheme 4.
Divergent synthesis of heterocyclic derivatives.
3. Conclusions
Herein, effective total syntheses of dihydrolysergic acid (1) and dihydrolysergol (2) are disclosed. The approach employed an inverse electron demand Diels–Alder reaction of the tricyclic ketone 12 derived enamine 13 with 5-carbomethoxy-1,2,3-triazine (14) for the late-stage preparation of the tetracyclic framework of the ergot alkaloid core structure. Key to the preparation of the tricyclic ketone 12 was our recently disclosed intramolecular Pd(0)-catalyzed Larock indole annulation of a N-acetyl 2-bromoaniline derivative substituted with a pendant alkyne to assemble the tricyclic fused indole found in ergot alkaloids. This strategy deliberately permits the late-stage cycloaddition reaction of the electron-rich dienophile 13 with additional reactive heterocyclic azadienes for the divergent preparation of a series of complementary heterocyclic D-ring derivatives not as readily accessible by more conventional approaches, including alternatively substituted pyridines, pyrimidines, pyridazines and pyrroles.
4. Experimental34
4.1. (3-Acetylamino-2-bromophenyl)methanol
A stirred solution of 316 (1.81 g, 9.00 mmol) in THF (90 mL) was treated with pyridine (4.4 mL, 54.0 mmol) and acetic anhydride (3.4 mL, 36.0 mmol) at 25 °C. After stirring for 12 h at the same temperature, the resulting mixture was concentrated under reduced pressure. The mixture was dissolved in MeOH (90 mL) and was treated with potassium carbonate (1.87 g, 13.5 mmol). After stirring for 3 h at 25 °C, the resulting mixture was quenched with the addition of H2O and diluted with EtOAc. The layers were separated, and the aqueous layer was extracted with EtOAc. The combined organic layers were washed with saturated aqueous NaCl, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The residue was purified by flash chromatography (SiO2, 70% EtOAc–hexanes) to provide the title compound (1.82 g, 83%) as a white solid: 1H NMR (600 MHz, (CD3)2CO) δ 8.50 (br s, 1H), 7.93 (d, J = 8.0 Hz, 1H), 7.39 (d, J = 8.0 Hz, 1H), 7.35 (t, J = 7.8 Hz, 1H), 4.67 (d, J = 6.0 Hz, 2H), 4.50 (t, J = 6.0 Hz, 1H), 2.18 (s, 3H); 13C NMR (150 MHz, CDCl3) δ 169.0, 142.8, 137.3, 128.1, 124.4, 123.6, 115.3, 64.8, 24.1; IR (film) υmax 3278, 1659, 1592, 1536, 1353, 1300, 1074, 1025, 722 cm−1; HRMS (ESI) m/z 243.9968 [(M+H)+, C9H10BrNO2 requires 243.9968].
4.2. N-(2-Bromo-3-formylphenyl)acetamide (4)
A solution of N-acetyl 2-bromo-3-hydroxymethylaniline (1.76 g, 7.24 mmol) in THF (70 mL) was treated with MnO2 (3.15 g, 36.2 mmol) in a portion at 25 °C. The reaction mixture was stirred for 1 h, followed by another two additions of MnO2 (3.15 g, 36.2 mmol) every 1 h. After stirring for an additional 6 h at room temperature, the resulting mixture was filtered through a pad of Celite and concentrated in vacuo to provide crude 4 as a white solid: 1H NMR (600 MHz, CDCl3) δ 10.29 (s, 1H), 8.44 (d, J = 8.2 Hz, 1H), 7.86 (br s, 1H), 7.60 (d, J = 7.6 Hz, 1H), 7.37 (t, J = 7.9 Hz, 1H), 2.25 (s, 3H); 13C NMR (150 MHz, CDCl3) δ 191.2, 168.4, 136.4, 133.6, 128.1, 127.8, 125.6, 117.9, 24.6; IR (film) υmax 3253, 2924, 1661, 1521, 1371, 1234, 1024, 792 cm−1; HRMS (ESI) m/z 241.9812 [(M+H)+, C9H8BrNO2 requires 241.9811]. In initial studies, but on smaller scales (0.94 mmol), the purification of the product provided aldehyde 4 (135 mg) in 92% yield.
4.3. (E)-N-(2-Bromo-3-(2-methoxyvinyl)phenyl)acetamide (5)
A cooled (−78 °C) solution of (methoxymethyl)triphenylphosphonium chloride (7.45 g, 21.7 mmol) in anhydrous THF (100 mL) was treated with a solution of potassium tert-butoxide (18.1 mL, 1.0 M in THF, 18.1 mmol) and the mixture was warmed to 0 °C. The bright red solution was stirred for 10 min and a solution of aldehyde 4 in THF (45 mL) was added dropwise by syringe. The reaction mixture was allowed to warm to 25 °C and stirred for 30 min before the resulting mixture was quenched with the addition of saturated aqueous NH4Cl and diluted with EtOAc and H2O. The layers were separated and the aqueous layer was extracted with EtOAc. The combined organic layers were dried over Na2SO4, filtered, and concentrated in vacuo. The residue was purified by flash chromatography (SiO2, 50% EtOAc–hexanes) and provided 5 (1.38 g, 71% over two steps) as a mixture of E/Z (1.4:1) isomers. For major E isomer: 1H NMR (600 MHz, CDCl3) δ 7.96 (t, J = 8.9 Hz, 1H), 7.79 (s, 1H), 7.11 (t, J = 7.9 Hz, 1H), 7.02 (d, J = 7.7 Hz, 1H), 6.89 (d, J = 12.8 Hz, 1H), 6.00 (d, J = 12.8 Hz, 1H), 3.66 (s, 3H), 2.15 (s, 3H). For minor Z isomer: 1H NMR (600 MHz, CDCl3) δ 7.96 (t, J = 8.9 Hz, 1H), 7.79 (s, 1H), 7.73 (d, J = 7.9 Hz, 1H), 7.16 (t, J = 8.0 Hz, 1H), 6.19 (d, J = 7.2 Hz, 1H), 5.50 (d, J = 7.2 Hz, 1H), 3.70 (s, 3H), 2.15 (s, 3H); 13C NMR (150 MHz, CDCl3, a mixture of E/Z isomers) δ 168.13, 168.08, 150.6, 149.3, 136.7, 135.8, 135.4, 135.3, 127.4, 126.9, 125.8, 121.2, 119.8, 119.6, 114.6, 114.3, 104.5, 103.7, 60.6, 56.4, 24.4; IR (film) υmax 3274, 2931, 2863, 1646, 1521, 1210, 1101, 668 cm−1; HRMS (ESI) m/z 270.0123 [(M+H)+, C11H12BrNO requires 270.0124].
4.4. N-(2-Bromo-3-(2-hydroxy-5-(trimethylsilyl)pent-4-yn-1-yl)phenyl)acetamide (8)
[Hydrolysis] A cooled (0 °C) solution of 5 (1.79 g, 6.65 mmol) in acetone (50 mL) was treated dropwise with 6 N HCl (50 mL). After stirring for 30 min at 0 °C, the reaction mixture was warmed to 25 °C and stirred for an additional 1 h. The resulting mixture was quenched with the addition of saturated aqueous NaHCO3 and diluted with EtOAc. The layers were separated, and the aqueous layer was extracted with EtOAc. The combined organic layers were dried over anhydrous Na2SO4, concentrated in vacuo, and azeotroped with toluene to afford the crude 6 as a white solid, which was employed in the next step without further purification: 1H NMR (600 MHz, CDCl3) δ 9.74 (s, 1H), 8.30 (d, J = 8.3 Hz, 1H), 7.68 (s, 1H), 7.33 (t, J = 7.9 Hz, 1H), 7.01 (d, J = 7.5 Hz, 1H), 3.89 (s, 2H), 2.26 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 198.0, 168.5, 136.5, 133.3, 128.3, 127.2, 121.8, 116.5, 51.3, 24.8; IR (film) υmax 3274, 2934, 1719, 1657, 1533, 1323, 1299, 1101, 1026, 665 cm−1; HRMS (ESI) m/z 255.9967 [(M+H)+, C10H10BrNO2 requires 255.9968]. [Indium Grignard Addition] A two neck flask charged with indium powder (2.29 g, 19.96 mmol) in THF (60 mL) was treated with (3-bromoprop-1-ynyl)trimethylsilane (2.2 mL, 13.31 mmol) at 25 °C. After stirring for 15 min, the mixture was subsequently treated dropwise with a solution of the above aldehyde 6 in THF (60 mL). The reaction mixture was allowed to stir for 2.5 h at reflux before the resulting mixture was cooled to room temperature. The resulting mixture was filtered through a pad of Celite and diluted with EtOAc and H2O. The layers were separated and the aqueous layer was extracted with EtOAc. The combined organic layers were dried over Na2SO4, filtered, and concentrated in vacuo. The residue was purified by column chromatography (SiO2, gradient elution: 30–50% EtOAc–hexanes) and provided 8 (2.00 g, 82% for 2 steps) as a white solid: 1H NMR (600 MHz, CDCl3) δ 8.19 (d, J = 8.2 Hz, 1H), 7.71 (s, 1H), 7.19–7.34 (m, 1H), 7.06 (d, J = 7.5 Hz, 1H), 4.06 (t, J = 6.6 Hz, 1H), 3.12 (dd, J = 13.8, 5.1 Hz, 1H), 2.96 (dd, J = 13.8, 7.9 Hz, 1H), 2.52 (dd, J = 16.8, 5.3 Hz, 1H), 2.45 (dd, J = 16.8, 6.1 Hz, 1H), 2.24 (s, 3H), 0.17 (s, 9H); 13C NMR (150 MHz, CDCl3) δ 168.2, 138.1, 136.0, 127.8, 127.0, 120.6, 116.2, 102.5, 88.3, 69.1, 43.3, 28.3, 24.9, 0.0; IR (film) υmax 3398, 2957, 1670, 1521, 1404, 1247, 1022, 839, 733 cm−1; HRMS (ESI) m/z 368.0677 [(M+H)+, C16H22BrNO2 Si requires 368.0676].
4.5. 1-(4-Hydroxy-2-(trimethylsilyl)-4,5-dihydrobenzo[cd]indol-1(3H)-yl)ethan-1-one (9)
A solution of 8 (778 mg, 2.12 mmol), Pd2(dba)3 (291 mg, 0.318 mmol) and DtBPF (301 mg, 0.636 mmol) in DMF (106 mL, 0.02 M) in a sealed vessel was degassed with argon for 20 min. This solution was treated with triethylamine (1.5 mL, 10.6 mmol) and the reaction mixture was allowed to stir at 130 °C for 3 h. The resulting mixture was cooled to room temperature, filtered through a short pad of silica gel and diluted with Et2O. Excess DMF was removed by washing with H2O and extracting with Et2O. The combined organic layers were dried over Na2SO4 and concentrated in vacuo. The residue was purified by column chromatography (SiO2, gradient elution: 25–40% EtOAc–hexanes) to afford 9 (548 mg, 90%) as a light tan oil: 1H NMR (600 MHz, CDCl3) δ 7.41 (d, J = 8.3 Hz, 1H), 7.27 (t, J = 7.8 Hz, 1H), 7.02 (d, J = 7.2 Hz, 1H), 4.39 (d, J = 7.6 Hz, 1H), 3.22 (dd, J = 16.0, 3.9 Hz, 1H), 3.14 (d, J = 14.9 Hz, 1H), 3.00 (d, J = 7.4 Hz, 1H), 2.97 (d, J = 7.9 Hz, 1H), 2.77 (s, 3H), 2.07 (d, J = 4.9 Hz, 1H), 0.36 (s, 9H); 13C NMR (150 MHz, CDCl3) δ 169.1, 135.7, 135.0, 130.4, 129.7, 128.4, 125.8, 121.2, 111.8, 67.8, 35.9, 33.3, 26.0, 2.3; IR (film) υmax 3366, 2897, 1689, 1609, 1376, 1326, 1243, 839, 730 cm−1; HRMS (ESI) m/z 288.1413 [(M+H)+, C16H21NO2Si requires 288.1414].
4.6. 1,3,4,5-Tetrahydrobenzo[cd]indol-4-ol (10)
[Desilylation] A cooled (0 °C) solution of 9 (435 mg, 1.52 mmol) in THF (15.2 mL, 0.1 M) was treated with Bu4NF (4.55 mL, 1.0 M in THF, 4.55 mmol). After stirring for 2 h at 25 °C, the resulting mixture was quenched with the addition of saturated aqueous NH4Cl and diluted with EtOAc (50 mL). The layers were separated and the aqueous layer was extracted with EtOAc. The combined organic layers were washed with saturated aqueous NaCl, dried over anhydrous Na2SO4, and concentrated in vacuo. The crude indole was taken to next step without further purification. [Methanolysis] The crude product was dissolved in MeOH (7.6 mL) and was treated with K2CO3 (628 mg, 4.54 mmol). After stirring for 2 h at 25 °C, the resulting mixture was quenched with the addition of H2O and diluted with EtOAc. The layers were separated, and the aqueous layer was extracted with EtOAc. The combined organic layers were washed with saturated aqueous NaCl, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The residue was purified by flash chromatography (SiO2, 70% EtOAc–hexanes) to provide 10 (241 mg, 91%) as a viscous light tan oil: 1H NMR (600 MHz, CDCl3) δ 8.02 (s, 1H), 7.10–7.22 (m, 2H), 6.90 (dd, J = 5.2, 2.4 Hz, 1H), 6.87–6.89 (m, 1H), 4.48 (tt, J = 7.1, 3.8 Hz, 1H), 3.21 (dd, J = 15.8, 3.7 Hz, 1H), 3.15 (dd, J = 15.1, 3.9 Hz, 1H), 3.07 (dd, J = 15.7, 6.9 Hz, 1H), 2.96 (dd, J = 15.1, 6.8 Hz, 1H), 2.08 (d, J = 7.9 Hz, 1H); 13C NMR (150 MHz, CDCl3) δ 133.5, 128.2, 126.3, 123.2, 118.9, 117.0, 110.3, 108.6, 68.2, 36.3, 31.0; IR (film) υmax 3388, 2923, 1605, 1442, 1338, 1034, 907, 728 cm−1; HRMS (ESI) m/z 174.0913 [(M+H)+, C11H11NO requires 174.0913].
4.7. (4-Hydroxy-2a,3,4,5-tetrahydrobenzo[cd]indol-1(2H)-yl)(phenyl)methanone (11)
[Reduction of Indole] A cooled (0 °C) solution of 10 (302 mg, 1.75 mmol) in THF and TFA (8 mL/8 mL) was treated dropwise with borane-tetrahydrofuran complex (3.5 mL, 1.0 M in THF, 3.50 mmol). The reaction mixture was allowed to stir for 10 min at 0 °C at which time an additional portion of borane-tetrahydrofuran complex (3.5 mL, 1.0 M in THF, 3.50 mmol) was added dropwise. After stirring for an additional 30 min at the same temperature, the mixture was quenched with the addition of MeOH and H2O. The resulting mixture was neutralized by addition of 1 N NaOH and diluted with EtOAc (40 mL). The layers were separated and the aqueous layer was extracted with EtOAc. The combined organic layers were washed with saturated aqueous NaCl, dried over anhydrous Na2SO4, and concentrated in vacuo. The residue was purified by flash chromatography (SiO2, 70% EtOAc–hexanes) to provide indoline as a colorless oil. [Benzoylation] A cooled (0 °C) solution of the indoline (300 mg, 1.71 mmol) in CH2Cl2 (15 mL) was treated with Et3N (716 μL, 5.14 mmol) followed by dropwise addition of benzoyl chloride (289 μL, 2.056 mmol) in CH2Cl2 (2 mL). After stirring for 30 min at 25 °C, the resulting mixture was quenched with the addition of saturated aqueous NaHCO3 and diluted with CH2Cl2. The layers were separated, and the aqueous layer was extracted with CH2Cl2. The combined organic layers were washed with saturated aqueous NaCl, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The residue was purified by flash chromatography (SiO2, 70% EtOAc–hexanes) to provide a diastereomeric mixture of 11 (423 mg, 87% for 2 steps). For less polar 11a: 1H NMR (500 MHz, CDCl3, 50 °C) δ 7.57 (d, J = 7.3 Hz, 2H), 7.38–7.52 (m, 3H), 6.99 (br s, 1H), 6.78 (d, J = 7.5 Hz, 1H), 4.47 (s, 1H), 4.36 (br s, 1H), 3.52–3.70 (m, 2H), 2.97 (dd, J = 18.0, 4.8 Hz, 1H), 2.85 (d, J = 18.0 Hz, 1H), 2.21–2.31 (m, 1H), 2.15 (br s, 1H), 1.48 (t, J = 11.6 Hz, 1H); 13C NMR (150 MHz, CDCl3, 50 °C) δ 168.7, 141.3, 136.6, 132.9, 131.8, 130.5, 128.5, 127.7, 127.4, 123.4, 113.7, 65.8, 58.4, 34.9, 33.5, 31.0; IR (film) υmax 3374, 2919, 1625, 1455, 1391, 1228, 770 cm−1; HRMS (ESI) m/z 280.1333 [(M+H)+, C18H17NO2 requires 280.1332]. For polar 11b: 1H NMR (500 MHz, CDCl3, 50 °C) δ 7.58 (d, J = 7.4 Hz, 2H), 7.40–7.53 (m, 3H), 7.02 (br s, 1H), 6.80 (d, J = 7.5 Hz, 1H), 4.34 (br s, 1H), 4.23 (dq, J = 10.1, 5.7, 4.3 Hz, 1H), 3.68 (t, J = 11.1 Hz, 1H), 3.36 (dq, J = 18.0, 11.5, 8.2 Hz, 1H), 3.24 (dd, J = 16.7, 6.5 Hz, 1H), 2.63 (dd, J = 16.7, 9.6 Hz, 1H), 2.29–2.42 (m, 1H), 1.85 (br s, 1H), 1.52 (q, J = 11.3 Hz, 1H); 13C NMR (150 MHz, CDCl3, 50 °C) δ 168.7, 141.4, 136.6, 132.7, 132.3, 130.6, 128.6, 128.1, 127.5, 123.1, 114.0, 68.6, 58.1, 36.7, 36.3, 36.2; IR (film) υmax 3305, 2922, 1626, 1455, 1395, 770, 668 cm−1; HRMS (ESI) m/z 280.1333 [(M+H)+, C18H17NO2 requires 280.1332]. The 1H and 13C NMR spectra of 11 in CDCl3 at 25 °C exhibit broadened peaks due to Bz rotamers.34
4.8. 1-Benzoyl-1,2,2a,5-tetrahydrobenzo[cd]indol-4(3H)-one (12)
A cooled (0 °C) solution of 11 (650 mg, 2.33 mmol) in CH2Cl2 (20 mL, 0.12 M) was treated with PCC (2.51 g, 11.64 mmol, 5 equiv) and the mixture was warmed to 25 °C. After stirring for 2 h at the same temperature, the resulting mixture was diluted with Et2O (100 mL) and stirred for 30 min. The solid residue was filtered through a pad of Celite and washed with Et2O. The crude residue was concentrated under reduced pressure and the residue was purified by flash chromatography (SiO2, gradient elution: 30–50% EtOAc–hexanes) to provide 12 (517 mg, 80%) as a yellow oil: 1H NMR (500 MHz, CDCl3, 50 °C) δ 7.58 (d, J = 7.5 Hz, 2H), 7.43–7.53 (m, 3H), 7.10 (s, 1H), 6.84 (d, J = 7.7 Hz, 1H), 4.49 (s, 1H), 3.78 (q, J = 11.0 Hz, 2H), 3.54 (s, 3H), 2.94 (d, J = 15.9, 1H), 2.22–2.40 (m, 1H); 13C NMR (125 MHz, CDCl3, 50 °C) δ 207.5, 168.8, 141.3, 136.4, 131.8, 130.9, 130.7, 129.0, 128.7, 127.4, 122.6, 114.8, 58.1, 43.8, 42.0, 34.9; IR (film) υmax 3365, 2922, 1717, 1600, 1459, 1241, 743, 670 cm−1; HRMS (ESI) m/z 278.1174 [(M+H)+, C18H15NO2 requires 278.1175]. The 1H and 13C NMR spectra of 12 in CDCl3 at 25 °C exhibit broadened peaks due to Bz rotamers.34
4.9. Methyl 4-Benzoyl-4,5,5a,6-tetrahydroindolo[4,3-fg]quinoline-9-carboxylate (15)
[Enamine Formation] A solution of 12 (90.2 mg, 0.325 mmol) and 4Å molecular sieves (~450 mg) in CHCl3 (3.3 mL, 0.1 M, passed through basic alumina) was treated dropwise with pyrrolidine (152 μL, 1.63 mmol) at 25 °C. After stirring for 8 h at room temperature, the mixture was filtered through a pad of Celite (10% MeOH-CH2Cl2 + 1% Et3N) and the solvent was removed under reduced pressure. The crude enamine was dried under high vacuum for 5 h. [Inverse Electron Demand Diels–Alder Reaction] A solution of the enamine 13 in CHCl3 (2.5 mL, passed through basic alumina) was treated dropwise with 5-carbomethoxy-1,2,3-triazine (14, 90.5 mg, 0.651 mmol) in CHCl3 (0.8 mL) during which smooth evolution of gas took place. The reaction mixture was stirred for 1 h, followed by another addition of 5-carbomethoxy-1,2,3-triazine (14, 90.5 mg, 0.651 mmol) in CHCl3 (0.8 mL). After stirring for additional 2 h at 25 °C, the solvent was removed under reduced pressure. The resulting residue was purified by column chromatography (SiO2, 5% MeOH–CH2Cl2) to provide 15 (90.2 mg, 75%) as a dark red solid: 1H NMR (500 MHz, CDCl3, 50 °C) δ 9.03 (d, J = 2.0 Hz, 1H), 8.58 (d, J = 2.0 Hz, 1H), 7.61 (d, J = 7.1 Hz, 2H), 7.45–7.54 (m, 3H), 7.41 (d, J = 7.7 Hz, 1H), 7.24 (s, 1H), 4.53 (s, 1H), 3.99 (s, 3H), 3.92 (t, J = 10.7 Hz, 1H), 3.72–3.86 (m, 1H), 3.46 (dd, J = 15.7, 6.3 Hz, 1H), 3.01 (t, J = 15.1 Hz, 1H); 13C NMR (125 MHz, CDCl3, 50 °C) δ 168.7, 165.5, 160.8, 149.0, 141.7, 136.3, 132.2, 131.0, 130.6, 129.4, 129.2, 128.6, 128.5, 127.2, 125.1, 117.9, 58.5, 52.2, 37.0, 34.7; IR (film) υmax 3309, 1646, 1428, 1257, 668 cm−1; HRMS (ESI) m/z 371.1391 [(M+H)+, C23H18N2O3 requires 371.1390]. The 1H and 13C NMR spectra of 15 in CDCl at 25 °C exhibit broadened peaks due to Bz rotamers.34
4.10. Methyl (5aR,6aR,9R,10aR)-4-Benzoyl-7-methyl-4,5,5a,6,6a,7,8,9,10,10a-decahydroindolo[4,3-fg]quinoline-9-carboxylate (17)
[Formation of Pyridinium Salt] A sealed tube charged with 15 (90.0 mg, 0.243 mmol) in MeI (2.4 mL, 0.1 M) was warmed to 80 °C and stirred for 10 h, at which point complete conversion of the starting material to the pyridinium salt had been achieved as monitored by LCMS. The brown mixture was cooled to room temperature and concentrated in vacuo to provide crude 16. [Reduction of Pyridinium Salt] A solution of 16 in anhydrous MeOH (8.1 mL, 0.03 M) was treated with NaCNBH3 (153 mg, 2.43 mmol) at 25 °C under Ar. The reaction mixture was warmed to 65 °C and stirred for 18 h before it was cooled to 0 °C and quenched with the addition of saturated aqueous NaHCO3. The organic layers were dried over anhydrous Na2SO4, and concentrated in vacuo. The residue was purified by passage through a short pad of silica gel to provide a mixture of partial and full reduction products and concentrated under reduced pressure. A solution of the mixture of reduction products in acetic acid (8.1 mL, 0.03 M) was treated with NaCNBH3 (153 mg, 2.43 mmol) at 25 °C under Ar. The reaction mixture was allowed to stir for 1 h at 25 °C before being cooled to 0 °C. The resulting mixture was quenched with the addition of saturated aqueous NaHCO3 and diluted with EtOAc (10 mL). The layers were separated, and the aqueous layer was extracted with EtOAc. The combined organic layers were further washed with 1 N NaOH, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The residue was purified by preparative thin-layer chromatography (Et3N pre-treated SiO2, 3% MeOH–CH2Cl2 eluted 3×) to provide 17 (55.3 mg, 58%) and an inseparable mixture of minor reduction products (4.6 mg, 5%): For 17: 1H NMR (500 MHz, CDCl3, 50 °C) δ 7.55 (d, J = 7.4 Hz, 2H), 7.41–7.50 (m, 3H), 7.09 (br s, 1H), 6.88 (d, J = 7.5 Hz, 1H), 4.48 (s, 1H), 3.73 (s, 3H), 3.68 (t, J = 10.8 Hz, 1H), 3.48 (qd, J = 10.2, 6.1 Hz, 1H), 3.17 (dd, J = 11.6, 3.6 Hz, 1H), 2.85 (tt, J = 12.0, 3.7 Hz, 1H), 2.74 (d, J = 13.0 Hz, 1H), 2.45–2.56 (m, 1H), 2.30 (s, 3H), 2.18 (t, J = 11.6 Hz, 1H), 2.05–2.15 (m, 1H), 1.86 (dt, J = 13.8, 7.1 Hz, 1H), 1.74 (q, J = 8.8 Hz, 1H), 1.59 (q, J = 12.5 Hz, 1H); 13C NMR (150 MHz, CDCl3, 50 °C) δ 174.0, 168.8, 140.5, 137.6, 136.8, 132.5, 130.4, 128.6, 127.8, 127.3, 118.6, 114.4, 65.5, 60.3, 58.5, 51.7, 43.2, 41.3, 38.6, 32.32, 32.31, 29.9; IR (film) υmax 2924, 1731, 1635, 1456, 1388, 1254, 732 cm−1; HRMS (ESI) m/z 391.2017 [(M+H)+, C24H26N2O3 requires 391.2016]. The 1H and 13C NMR spectra of 17 in CDCl3 at 25 °C exhibit broadened peaks due to Bz rotamers.34
The structure and relative stereochemistry of 17 were confirmed upon X-ray (CCDC 1047936) analysis enlisting a colorless monoclinic plate crystal obtained from MeOH.
4.11. Methyl (6aR,9R,10aR)-7-Methyl-4,6,6a,7,8,9,10,10a-octahydroindolo[4,3-fg]quinoline-9-carboxylate (18)
A microwave sealed vessel charged with 17 (4.7 mg, 0.012 mmol) was treated with a solution of HCl (0.5 M in MeOH, 1 mL). The reaction mixture was warmed to 95 °C and stirred for 72 h. The resulting mixture was cooled to room temperature and basified with the addition of 1 N aqueous NaOH and diluted with CH2Cl2. The layers were separated, and the aqueous layer was extracted with CH2Cl2. The combined organic layers were dried over anhydrous Na2SO4, and concentrated in vacuo. The residue was purified by preparative thin-layer chromatography (Et3N pre-treated SiO2, 5% MeOH–CH2Cl2 eluted 2×) to afford 18 (1.9 mg, 55%) as an off-white solid: 1H NMR (600 MHz, CDCl3) δ 7.96 (s, 1H), 7.15–7.21 (m, 2H), 6.96 (d, J = 6.6 Hz, 1H), 6.89 (s, 1H), 3.41 (dd, J = 14.6, 4.3 Hz, 1H), 3.26 (dt, J = 11.5, 2.9 Hz, 1H), 2.92–3.01 (m, 3H), 2.69 (dd, J = 14.6, 11.2 Hz, 1H), 2.51 (s, 3H), 2.36 (t, J = 11.7 Hz, 1H), 2.16–2.24 (m, 1H), 1.60 (q, J = 13.2 Hz, 1H); 13C NMR (150 MHz, CDCl3) δ 174.3, 133.3, 132.5, 126.1, 123.1, 117.8, 113.3, 111.6, 108.7, 66.7, 58.5, 51.8, 42.9, 41.3, 40.0, 30.5, 26.8, 8.9; IR (film) υmax 3360, 2922, 2851, 1731, 1456, 749 cm−1; HRMS (ESI) m/z 285.1597 [(M+H)+, C17H20N2O2 requires 285.1597].
4.12. Dihydrolysergic Acid (1)
A solution of 18 (2.6 mg, 9.1 μmol) in MeOH (600 μL) was treated with 1 N NaOH (600 μL). After stirring for 3 h at 40 °C, 1 N HCl was added to the mixture to carefully adjust the pH to 6. The resulting mixture was concentrated in vacuo. The residue was washed with ice cold water three times and the resulting solid was dried in vacuo to afford dihydrolysergic acid (1, 2.2 mg, 89%) as an off-white solid identical in all respects with authentic material previously reported27: 1H NMR (600 MHz, DMSO-d6) δ 10.63 (s, 1H), 7.12 (d, J = 8.0 Hz, 1H), 7.01 (t, J = 7.6 Hz, 1H), 6.97 (br s, 1H), 6.79 (d, J = 7.0 Hz, 1H), 3.29 (d, J = 14.7 Hz, 1H), 3.11 (d, J = 11.3 Hz, 1H), 2.70–2.85 (m, 3H), 2.50–2.56 (m, 1H), 2.37 (s, 3H), 2.16 (t, J = 11.5 Hz, 1H), 2.00 (t, J = 11.1 Hz, 1H), 1.34 (q, J = 13.0 Hz, 1H); 13C NMR (150 MHz, DMSO-d6) δ 175.0, 133.1, 132.3, 125.9, 122.1, 118.5, 112.0, 110.0, 108.7, 66.6, 58.6,. 42.6, 40.9, 30.5, 26.5; IR (film) υmax 3192, 2919, 2851, 1611, 1573, 1259, 1094,. 779 cm−1; HRMS (ESI) m/z 271.1440 [(M+H)+, C16H18N2O2 requires 271.1441].
4.13. Dihydrolysergol (2)
A cooled (0 °C) solution of 18 (3.2 mg, 11.3 μmol) in anhydrous THF (0.2 mL, 0.056 M) was treated dropwise with a solution of LiAlH4 (56 μL, 1.0 M in THF, 56.3 μmol). After stirring for 30 min at the same temperature, the resulting mixture was quenched with the addition of MeOH followed by saturated aqueous Rochelle’s salt solution and diluted with CH2Cl2. The resulting biphasic mixture was stirred for 3 h. The layers were separated, and the aqueous layer was extracted with CH2Cl2. The combined organic layers were dried over anhydrous Na2SO4, and concentrated in vacuo. The residue was purified by preparative thin-layer chromatography (Et3N pre-treated SiO2, 5% MeOH–CH2Cl2) to provide dihydrolysergol (2, 2.7 mg, 92%) as a white solid identical in all respects with data reported for authentic material and with a sample of authentic material 28,29: 1H NMR (600 MHz, CD3OD) δ 7.12 (d, J = 8.1 Hz, 1H), 7.04 (t, J = 7.6 Hz, 1H), 6.90 (s, 1H), 6.83 (d, J = 7.1 Hz, 1H), 3.58 (dd, J = 10.9, 5.6 Hz, 1H), 3.48 (dd, J = 10.9, 7.0 Hz, 1H), 3.43 (dd, J = 14.5, 4.4 Hz, 1H), 3.14 (dt, J = 11.4, 2.8 Hz, 1H), 2.91 (ddd, J = 12.8, 9.9, 3.9 Hz, 1H), 2.48 (s, 3H), 2.07–2.15 (m, 2H), 2.00 (t, J = 11.5 Hz, 1H), 1.10 (q, J = 12.4 Hz, 1H); 13C NMR (150 MHz, CD3OD) δ 135.1, 133.6, 127.4, 123.5, 119.2, 113.4, 111.4, 109.7, 69.1, 66.4, 61.8, 43.6, 41.4, 39.5, 32.0, 27.7; IR (film) υmax 3228, 2920, 2849, 1443, 1348, 1226, 1028, 745, 668 cm−1; HRMS (ESI) m/z 257.1649 [(M+H)+, C16H20N2O requires 257.1648].
4.14. Methyl 4-Benzoyl-4,5,5a,6-tetrahydroindolo[4,3-fg]quinoline-9-carboxylate (25)
A solution of 12 (7.0 mg, 0.025 mmol) and 4Å molecular sieves (35 mg) in CHCl3 (0.51 mL, 0.05 M, passed through basic alumina) was treated dropwise with pyrrolidine (10 μL, 0.13 mmol) at 25 °C. After stirring for 10 h at room temperature, the mixture was filtered through a pad of Celite (10% MeOH–CH2Cl2 + 1% Et3N) and the solvent was removed under reduced pressure. The crude enamine was dried under high vacuum for 5 h. A solution of the enamine 13 in CHCl3 (0.1 mL, passed through basic alumina) was treated dropwise with 1,2,3-triazine (19, 4.1 mg, 0.12 mmol) in CHCl3 (0.15 mL). The reaction mixture was stirred for 14 h at 60 °C before the solvent was removed under reduced pressure. The residue was purified by preparative thin-layer chromatography (SiO2, 5% MeOH–CH2Cl2) to provide 25 (1.4 mg, 18%) as a white solid: 1H NMR (500 MHz, CDCl3, 50 °C) δ 8.45 (d, J = 4.9 Hz, 1H), 8.01 (d, J = 7.8 Hz, 1H), 7.61 (d, J = 7.3 Hz, 2H), 7.44–7.55 (m, 3H), 7.33 (d, J = 7.6 Hz, 1H), 7.13–7.30 (m, 2H), 4.52 (br s, 1H), 3.91 (t, J = 10.7 Hz, 1H), 3.73–3.85 (m, 1H), 3.38 (dd, J = 15.2, 6.2 Hz, 1H), 2.98 (t, J = 14.8 Hz, 1H); 13C NMR (150 MHz, CDCl3, 50 °C) δ 168.9, 156.6, 148.3, 141.7, 136.6, 132.4, 130.7, 130.5, 129.1, 128.9, 128.7, 127.4, 122.5, 117.8, 116.4, 58.7, 37.1, 35.1; IR (film) υmax 3360, 2921, 2851, 1638, 1470, 1413, 1384, 1085, 779, 698 cm−1; HRMS (ESI) m/z 313.1336 [(M+H)+, C21H16N2O requires 313.1335]. The 1H and 13C NMR spectra of 25 in CDCl3 at 25 °C exhibit broadened peaks due to Bz rotamers.34
4.15. (9-Bromo-5a,6-dihydroindolo[4,3-fg]quinolin-4(5H)-yl)(phenyl)methanone (26)
A solution of 12 (16.9 mg, 0.0609 mmol) and 4Å molecular sieves (85 mg) in CHCl3 (1.2 mL, 0.05 M, passed through basic alumina) was treated dropwise with pyrrolidine (22 μL, 0.30 mmol) at 25 °C. After stirring for 10 h at room temperature, the mixture was filtered through a pad of Celite (10% MeOH–CH2Cl2 + 1% Et3N) and the solvent was removed under reduced pressure. The crude enamine was dried under high vacuum for 5 h. A solution of the enamine 13 in CHCl3 (0.31 mL, passed through basic alumina) was treated dropwise with 5-bromo-1,2,3-triazine (20, 19.4 mg, 0.122 mmol) in CHCl3 (0.3 mL). The reaction mixture was stirred for 48 h at 25 °C before the solvent was removed under reduced pressure. The residue was purified by preparative thin-layer chromatography (SiO2, 5% MeOH–CH2Cl2) to provide 26 (3.1 mg, 13%) as a light tan solid: 1H NMR (500 MHz, CDCl3, 50 °C) δ 8.50 (d, J = 2.2 Hz, 1H), 8.12 (d, J = 2.2 Hz, 1H), 7.61 (d, J = 7.1 Hz, 2H), 7.44–7.55 (m, 3H), 7.10–7.36 (m, 2H), 4.51 (br s, 1H), 3.90 (t, J = 10.7 Hz, 1H), 3.70–3.84 (m, 1H), 3.35 (dd, J = 15.4, 6.3 Hz, 1H), 2.90 (t, J = 14.9 Hz, 1H); 13C NMR (150 MHz, CDCl3, 50 °C) δ 168.9, 155.0, 149.0, 141.9, 136.4, 133.0, 132.6, 130.7, 130.6, 129.3, 128.7, 127.4, 119.5, 117.9, 117.2, 58.6, 36.6, 35.0; IR (film) υmax 3345, 2923, 1642, 1419, 1384, 1077, 787, 667 cm−1; HRMS (ESI) m/z 391.0439 [(M+H)+, C21H15BrN2O requires 391.0440]. The 1H and 13C NMR spectra of 26 in CDCl3 at 25 °C exhibit broadened peaks due to Bz rotamers.34
4.16. Methyl 4-Benzoyl-4,5,5a,6-tetrahydroindolo[4,3-fg]quinoline-8-carboxylate (27)
A solution of 12 (19.3 mg, 0.0696 mmol) and 4Å molecular sieves (~100 mg) in CHCl3 (1.4 mL, 0.05 M, passed through basic alumina) was treated dropwise with pyrrolidine (29 μL, 0.35 mmol) at 25 °C. After stirring for 10 h at room temperature, the mixture was filtered through a pad of Celite (10% MeOH–CH2Cl2 + 1% Et3N) and the solvent was removed under reduced pressure. The crude enamine was dried under high vacuum for 5 h. A solution of the enamine 13 in CHCl3 (0.4 mL, passed through basic alumina) was treated dropwise with 4-carbomethoxy-1,2,3-triazine (21, 19.4 mg, 0.139 mmol) in CHCl3 (0.3 mL). The reaction mixture was warmed to 60 °C and stirred for 8 h before the solvent was removed under reduced pressure. The residue was purified by preparative thin-layer chromatography (SiO2, 5% MeOH–CH2Cl2) to provide 27 (9.3 mg, 36%) as a white solid: 1H NMR (500 MHz, CDCl3, 50 °C) δ 9.03 (d, J = 2.0 Hz, 1H), 8.58 (d, J = 2.0 Hz, 1H), 7.61 (d, J = 7.1 Hz, 2H), 7.45–7.54 (m, 3H), 7.41 (d, J = 7.7 Hz, 1H), 7.24 (s, 1H), 4.53 (s, 1H), 3.99 (s, 3H), 3.92 (t, J = 10.7 Hz, 1H), 3.72–3.86 (m, 1H), 3.46 (dd, J = 15.7, 6.3 Hz, 1H), 3.01 (t, J = 15.1 Hz, 1H); 13C NMR (125 MHz, CDCl3, 50 °C) δ 168.7, 165.5, 160.8, 149.0, 141.7, 136.3, 132.2, 131.0, 130.6, 129.4, 129.2, 128.6, 128.5, 127.2, 125.1, 117.9, 58.5, 52.2, 37.0, 34.7; IR (film) υmax 3309, 1646, 1428, 1257, 668 cm−1; HRMS (ESI) m/z 371.1391 [(M+H)+, C23H18N2O3 requires 371.1390]. The 1H and 13C NMR spectra of 27 in CDCl3 at 25 °C exhibit broadened peaks due to Bz rotamers.34
4.17. Diethyl 4-Benzoyl-4,5,5a,6-tetrahydroindolo[4,3-fg]quinoline-8,10-dicarboxylate (28)
A solution of 12 (18.1 mg, 0.0653 mmol) and 4Å molecular sieves (~100 mg) in CHCl3 (1.3 mL, 0.05 M, passed through basic alumina) was treated dropwise with pyrrolidine (27 μL, 0.33 mmol) at 25 °C. After stirring for 10 h at room temperature, the mixture was filtered through a pad of Celite (10% MeOH–CH2Cl2 + 1% Et3N) and solvent was removed under reduced pressure. The crude enamine was dried under high vacuum for 5 h. A solution of the enamine 13 in CHCl3 (0.4 mL, passed through basic alumina) was treated dropwise with 4,6-dicarboethoxy-1,2,3-triazine (22, 29.4 mg, 0.139 mmol) in CHCl3 (0.25 mL). The reaction mixture was warmed to 60 °C and stirred for 8 h before the solvent was removed under reduced pressure. The residue was purified by preparative thin-layer chromatography (SiO2, 5% MeOH–CH2Cl2) to provide 28 (7.7 mg, 26%) as a light yellow solid: 1H NMR (500 MHz, CDCl3, 50 °C) δ 8.12 (s, 1H), 7.61 (d, J = 7.3 Hz, 2H), 7.45–7.55 (m, 3H), 7.10–7.23 (m, 2H), 4.37–4.57 (m, 5H), 3.91 (t, J = 10.4 Hz, 1H), 3.73–3.85 (m, 1H), 3.58 (dd, J = 15.5, 5.9 Hz, 1H), 2.98 (t, J = 14.8 Hz, 1H), 1.45 (t, J = 7.1 Hz, 3H), 1.36 (t, J = 7.2 Hz, 3H); 13C NMR (150 MHz, CDCl3, 50 °C) δ 168.9, 168.0, 164.4, 159.3, 146.4, 141.7, 138.0, 136.3, 134.8, 130.8, 129.5, 128.7, 128.4, 127.4, 123.2, 121.8, 117.8, 62.5, 62.1, 58.0, 38.2, 34.6, 14.3, 13.9; IR (film) υmax 2927, 1723, 1644, 1387, 1244, 1019, 732 cm−1; HRMS (ESI) m/z 457.1759 [(M+H)+, C27H24N2O5 requires 457.1758]. The 1H and 13C NMR spectra of 28 in CDCl3 at 25 °C exhibit broadened peaks due to Bz rotamers.34
4.18. (5a,6-Dihydroindolo[4,3-fg]quinazolin-4(5H)-yl)(phenyl)methanone (29)
A solution of 12 (24 mg, 0.087 mmol) and 4Å molecular sieves (120 mg) in CHCl3 (1.7 mL, 0.05 M, passed through basic alumina) was treated dropwise with pyrrolidine (36 μL, 0.43 mmol) at 25 °C. After stirring for 10 h at room temperature, the mixture was filtered through a pad of Celite (10% MeOH–CH2Cl2 + 1% Et3N) and the solvent was removed under reduced pressure. The crude enamine was dried under high vacuum for 5 h. A solution of the enamine 13 in CHCl3 (430 μL, passed through basic alumina) was treated dropwise with 1,3,5-triazine (23, 42 mg, 0.519 mmol) in dioxane (430 μL). The reaction mixture was stirred for 14 h at 90 °C before the solvent was removed under reduced pressure. The residue was purified by preparative thin-layer chromatography (SiO2, 5% MeOH–CH2Cl2) to provide 29 (6.2 mg, 23%) as a off white solid: 1H NMR (500 MHz, CDCl3, 50 °C) δ 9.04 (s, 1H), 9.02 (s, 1H), 7.61 (d, J = 7.3 Hz, 2H), 7.44–7.55 (m, 3H), 7.39 (d, J = 7.5 Hz, 1H), 7.15–7.30 (m, 1H), 4.53 (br s, 1H), 3.91 (t, J = 10.7 Hz, 1H), 3.74–3.84 (m, 1H), 3.33 (dd, J = 15.9, 6.4 Hz, 1H), 2.96 (t, J = 15.1 Hz, 1H); 13C NMR (150 MHz, CDCl3, 50 °C) δ 168.9, 164.8, 157.6, 150.8, 142.0, 136.3, 132.1, 130.8, 129.6, 128.7, 127.5, 127.4, 126.8, 117.4, 58.6, 36.2, 34.5; IR (film) υmax 2925, 1723, 1643, 1383, 1247, 787, 667 cm−1; HRMS (ESI) m/z 314.1287 [(M+H)+, C20H15N3O requires 314.1288]. The 1H and 13C NMR spectra of 29 in CDCl3 at 25 °C exhibit broadened peaks due to Bz rotamers.34
4.19. Dimethyl 4-Benzoyl-4,5,5a,6-tetrahydroindolo[4,3-fg]phthalazine-7,10-dicarboxylate (30)
A solution of 12 (11.4 mg, 0.0411 mmol) and 4Å molecular sieves (55 mg) in CHCl3 (0.8 mL, 0.05 M, passed through basic alumina) was treated dropwise with pyrrolidine (17 μL, 0.21 mmol) at 25 °C. After stirring for 10 h at room temperature, the mixture was filtered through a pad of Celite (10% MeOH–CH2Cl2 + 1% Et3N) and the solvent was removed under reduced pressure. The crude enamine was dried under high vacuum for 5 h. A solution of the enamine in CHCl3 (0.4 mL, passed through basic alumina) was treated dropwise with 3,6-dicarbomethoxy-1,2,4,5-tetrazine (24, 16.3 mg, 0.0822 mmol) in CHCl3 (0.41 mL). The reaction mixture was stirred for 3 h at 25 °C before the solvent was removed under reduced pressure. The residue was purified by preparative thin-layer chromatography (SiO2, 5% MeOH–CH2Cl2) to provide 30 (12.4 mg, 70%) as a white solid: 1H NMR (600 MHz, CDCl3, 25 °C) δ 7.60 (d, J = 7.2 Hz, 2H), 7.53 (t, J = 7.4 Hz, 1H), 7.47 (t, J = 7.4 Hz, 2H), 7.03–7.21 (m, 2H), 4.53 (s, 1H), 4.06 (s, 3H), 4.05 (s, 3H), 3.93 (t, J = 10.6 Hz, 1H), 3.67–3.77 (m, 1H), 3.58–3.67 (m, 1H), 2.73 (dd, J = 16.4, 14.4 Hz, 1H); 13C NMR (150 MHz, CDCl3, 50 °C) δ 168.8, 166.4, 165.1, 152.9, 150.9, 142.1, 135.9, 135.0, 131.1, 131.0, 129.5, 128.8, 127.4, 125.2, 120.3, 118.9, 58.0, 53.4, 53.3, 33.4, 29.0; IR (film) υmax 3446, 2953, 1732, 1644, 1455, 1387, 1237, 1155, 730 cm−1; HRMS (ESI) m/z 430.1397 [(M+H)+, C24H19N3O5 requires 430.1397]. The 1H and 13C NMR spectra of 30 in CDCl3 at 25 °C exhibit broadened peaks due to Bz rotamers.34
4.20. Dimethyl 4-Benzoyl-5,5a,6,8-tetrahydro-4H-isoindolo[6,5,4-cd]indole-7,9-dicarboxylate (31)
A solution of 12 (4.5 mg, 0.011 mmol) in acetic acid (0.2 mL, 0.05 M) was treated with zinc dust (34.3 mg, 0.525 mmol) in a portion at 25 °C. After stirring for 22 h at the same temperature, the resulting mixture was filtered through a short pad of Celite and the solvent was removed under reduced pressure. The residue was purified by preparative thin-layer chromatography (SiO2, 5% MeOH–CH2Cl2) to provide 31 (3.4 mg, 78%) as a white solid: 1H NMR (500 MHz, CDCl3, 50 °C) δ 9.53 (s, 1H), 8.01 (d, J = 7.8 Hz, 1H), 7.60 (d, J = 7.2 Hz, 2H), 7.48 (dq, J = 14.5, 7.3 Hz, 3H), 7.16 (br s, 1H), 4.48 (br s, 1H), 3.97 (s, 3H), 3.93 (s, 3H), 3.85 (t, J = 10.7 Hz, 1H), 3.71 (dd, J = 15.3, 6.7 Hz, 1H), 3.52–3.65 (m, 1H), 2.46 (t, J = 14.5 Hz, 1H); 13C NMR (150 MHz, CDCl3, 50 °C) δ 168.7, 160.8, 160.4, 141.1, 136.7, 133.0, 130.5, 128.6, 128.4, 128.2, 127.7, 127.4, 125.8, 121.6, 120.8, 120.2, 115.7, 57.8, 52.0, 51.8, 36.3, 26.1; IR (film) υmax 3325, 1699, 1635, 1472, 1395, 1256, 1013, 668 cm−1; HRMS (ESI) m/z 417.1446 [(M+H)+, C24H20N2O5 requires 417.1445]. The 1H and 13C NMR spectra of 31 in CDCl3 at 25 °C exhibit broadened peaks due to Bz rotamers.34
Supplementary Material
Table 1.
Comparison of 1H NMR data for 1 (Solvent: DMSO-d6; δ in ppm, J in Hz)
| chemical shifts (δ) | |
|---|---|
| Semi synthetica (500 MHz) |
Synthetic 1 (600 MHz) |
| 10.65 (s, 1H) | 10.63 (s, 1H) |
| 7.13 (d, J = 8.1 Hz, 1H) | 7.12 (d, J = 8.0 Hz, 1H) |
| 7.01 (t, J = 7.6 Hz, 1H) | 7.01 (t, J = 7.6 Hz, 1H) |
| 6.98 (bs, 1H) | 6.97 (br s, 1H) |
| 6.79 (d, J = 7.1 Hz, 1H) | 6.79 (d, J = 7.0 Hz, 1H) |
| 3.29 (dd, J = 14.6, 4.1 Hz, 1H) | 3.29 (d, J = 14.7 Hz, 1H) |
| 3.12 (dd, J = 11.0, 2.1 Hz, 1H) | 3.11 (d, J = 11.3 Hz, 1H) |
| 2.70–2.85 (m, 3H) | 2.70–2.85 (m, 3H) |
| 2.48–2.55 (m, 1H) | 2.50–2.56 (m, 1H) |
| 2.37 (s, 3H) | 2.37 (s, 3H) |
| 2.17 (t, J = 11.5 Hz, 1H) | 2.16 (t, J = 11.5 Hz, 1H) |
| 2.00 (dt, J = 10.9, 4.1 Hz, 1H) | 2.00 (t, J = 11.1 Hz, 1H) |
| 1.34 (q, J = 12.9 Hz, 1H) | 1.34 (q, J = 13.0 Hz, 1H) |
Wagger, J.; Požes, A.; Požgan, F. RSC Adv. 2013, 3, 23146.
Table 2.
Comparison of 13C NMR data for 1 (Solvent: DMSO-d6; δ in ppm)
| chemical shifts (δ) | |
|---|---|
| Semi synthetica (125 MHz) |
Synthetic 1 (150 MHz) |
| 175.1 | 175.0 |
| 133.2 | 133.1 |
| 132.3 | 132.3 |
| 125.9 | 125.9 |
| 122.1 | 122.1 |
| 118.6 | 118.5 |
| 112.0 | 112.0 |
| 110.0 | 110.0 |
| 108.8 | 108.7 |
| 66.6 | 66.6 |
| 58.6 | 58.6 |
| 42.6 | 42.6 |
| 40.9 | 40.9 |
| 30.5 | 30.5 |
| 26.5 | 26.5 |
Wagger, J.; Požes, A.; Požgan, F. RSC Adv. 2013, 3, 23146.
Acknowledgements
We gratefully acknowledge the financial support of the National Institutes of Health (CA042056 and CA041101) and wish to thank Curtis Moore (UCSD) for the X-ray structure of 17.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Copies of the 1H and 13C NMR spectra of all synthetic intermediates and final products are provided.
References and notes
- 1.(a) Ninomiya I, Kiguchi T. In: The Alkaloids. Brossi A, editor. Vol. 38. Academic; San Diego: 1990. pp. 1–156. [Google Scholar]; (b) The Merck Index. 12th ed. Merck; Whitehouse Station, NY: 1996. p. 231. [Google Scholar]; (c) Somei M, Yokoyama Y, Murakami Y, Ninomiya I, Kiguchi T, Naito T. In: The Alkaloids. Cordell G, editor. Vol. 54. Academic; San Diego: 2000. pp. 191–257. [Google Scholar]
- 2.(a) Jacobs WA, Craig LC. J. Biol. Chem. 1934;104:547. [Google Scholar]; (b) Stoll A, Hofmann A, Troxler F. Helv. Chim. Acta. 1949;32:506. doi: 10.1002/hlca.19490320219. [DOI] [PubMed] [Google Scholar]
- 3.(a) Yen TT, Stamm NB, Clemens JA. Life Sci. 1979;25:209. doi: 10.1016/0024-3205(79)90287-x. [DOI] [PubMed] [Google Scholar]; (b) Pezzoli G, Martignoni E, Pacchetti C, Angeleri V, Lamberti P, Muratorio A, Bonuccelli U, De Mari M, Foschi N, Cossutta E. Neurology. 1995;45:22. doi: 10.1212/wnl.45.3_suppl_3.s22. [DOI] [PubMed] [Google Scholar]
- 4.(a) Flückiger E, Wagner H. Experientia. 1968;24:1130. doi: 10.1007/BF02147804. [DOI] [PubMed] [Google Scholar]; (b) Schneider HR, Stadler PA, Stütz P, Troxler F, Seres J. Experientia. 1977;33:1412. doi: 10.1007/BF01918774. [DOI] [PubMed] [Google Scholar]; (c) Kebabian JW, Calne DB. Nature. 1979;277:93. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- 5.(a) Dudley HW, Moir JC. Br. Med. J. 1935;1:520. doi: 10.1136/bmj.1.3871.520. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Moir JC. Br. Med. J. 1936;2:799. doi: 10.1136/bmj.2.3955.799. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) De Costa C. Lancet. 2002;359:1768. doi: 10.1016/S0140-6736(02)08658-0. [DOI] [PubMed] [Google Scholar]
- 6.Kornfeld EC, Fornefeld EJ, Kline GB, Mann MJ, Morrison DE, Jones RG, Woodward RB. J. Am. Chem. Soc. 1956;78:3087. Julia M, Legoffic F, Igolen J, Baillarg M. Tetrahedron Lett. 1969:1569. Oppolzer W, Francotte E, Bättig K. Helv. Chim. Acta. 1981;64:478. Ramage R, Armstrong VW, Coulton S. Tetrahedron. 1981;37:157. Rebek J, Tai DF, Shue Y-K. J. Am. Chem. Soc. 1984;106:1813. Ninomyia I, Hashimoto C, Kiguchi T, Naito T. J. Chem. Soc., Perkin Trans. 1. 1985:941. doi: 10.1039/p19760001868. Kurihara T, Terada T, Harusawa S, Yoneda R. Chem. Pharm. Bull. 1987;35:4793. Cacchi S, Ciattini PG, Morera E, Ortar G. Tetrahedron Lett. 1988;29:3117. Saá C, Crotts DD, Hsu G, Vollhardt KPC. Synlett. 1994:487. Moldvai I, Temesvári-Major E, Incze M, Szentirmay É, Gács-Baitz E, Szántay C. J. Org. Chem. 2004;69:5993. doi: 10.1021/jo049209b. Inuki S, Oishi S, Fujii N, Ohno H. Org. Lett. 2008;10:5239. doi: 10.1021/ol8022648. Kurokawa T, Isomura M, Tokuyama H, Fukuyama T. Synlett. 2009:775. Inuki S, Iwata A, Oishi S, Fujii N, Ohno H. J. Org. Chem. 2011;76:2072. doi: 10.1021/jo102388e. Iwata A, Inuki S, Oishi S, Fujii N, Ohno H. J. Org. Chem. 2011;76:5506. doi: 10.1021/jo2008324. Liu Q, Jia Y. Org. Lett. 2011;13:4810. doi: 10.1021/ol2018467. Umezaki S, Yokoshima S, Fukuyama T. Org. Lett. 2013;15:4230. doi: 10.1021/ol4019562. Liu Q, Zhang Y-A, Xu P, Jia Y. J. Org. Chem. 2013;78:10885. doi: 10.1021/jo4018777. Lysergol and isolysergol: Kiguchi T, Hashimoto C, Ninomiya I. Heterocycles. 1985;23:1377. Ninomiya I, Hashimoto C, Kiguchi T. Heterocycles. 1984;22:1035. Ninomiya I, Hashimoto C, Kiguchi T, Naito T, Barton DHR, Lusinchi X, Milliet P. J. Chem. Soc., Perkin Trans. 1. 1990:707. Deck JA, Martin SF. Org. Lett. 2010;12:2610. doi: 10.1021/ol100819f.
- 7.Stoll A, Petrzilka Th., Rutschmann J, Hofmann A, Günthard Hs. H. Helv. Chim. Acta. 1954;37:2039. [Google Scholar]
- 8.Total synthesis of dihydrolysergic acid: Uhle FC, Jacobs WA. J. Org. Chem. 1945;10:76. Stoll A, Rutschmann J, Schlientz W. Helv. Chim. Acta. 1950;33:375. Haefliger WE. Helv. Chim. Acta. 1984;67:1942.
- 9.(a) Der Marderosian AH, Cho E, Chao JM. Planta Med. 1974;25:6. doi: 10.1055/s-0028-1097906. [DOI] [PubMed] [Google Scholar]; (b) Lee TM, Chao JM, Der Marderosian AH. Planta Med. 1979;35:247. doi: 10.1055/s-0028-1097212. [DOI] [PubMed] [Google Scholar]
- 10.(a) Breazzano SP, Poudel YB, Boger DL. J. Am. Chem. Soc. 2013;135:1600. doi: 10.1021/ja3121394. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Shimamura H, Breazzano SP, Garfunkle J, Kimball FS, Trzupek JD, Boger DL. J. Am. Chem. Soc. 2010;132:7776. doi: 10.1021/ja102304p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Garfunkle J, Kimball FS, Trzupek JD, Takazawa S, Shimamura H, Tomishima M, Boger DL. J. Am. Chem. Soc. 2009;131:16036. doi: 10.1021/ja907193b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larock RC, Yum E, Refvik M. J. Org. Chem. 1998;63:7652. [Google Scholar]
- 12.Uhle FC. J. Am. Chem. Soc. 1949;71:761. doi: 10.1021/ja01171a001. [DOI] [PubMed] [Google Scholar]
- 13.(a) Anderson ED, Boger DL. J. Am. Chem. Soc. 2011;133:12285. doi: 10.1021/ja204856a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Anderson ED, Duerfeldt AS, Zhu K, Glinkerman CM, Boger DL. Org. Lett. 2014;16:5084. doi: 10.1021/ol502436n. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Duerfeldt AS, Boger DL. J. Am. Chem. Soc. 2014;136:2119. doi: 10.1021/ja412298c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Anderson ED, Boger DL. Org. Lett. 2011;13:2492. doi: 10.1021/ol2007428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.(a) Boger DL. Tetrahedron. 1983;39:2869. [Google Scholar]; (b) Boger DL. Chem. Rev. 1986;86:781. [Google Scholar]; (c) Boger DL, Weinreb SM. Hetero Diels–Alder Methodology in Organic Synthesis. Academic; San Diego: 1987. [Google Scholar]
- 15.(a) Boger DL, Brotherton CE. J. Org. Chem. 1984;49:4050. [Google Scholar]; (b) Mullican MD, Boger DL. J. Org. Chem. 1984;49:4033. [Google Scholar]; (c) Mullican MD, Boger DL. J. Org. Chem. 1984;49:4045. [Google Scholar]
- 16.Ozlu Y, Cladingboel D, Parsons P. Tetrahedron. 1994;50:2183. [Google Scholar]
- 17.Levine SG. J. Am. Chem. Soc. 1958;80:6150. [Google Scholar]
- 18.Lin M-J, Loh T-P. J. Am. Chem. Soc. 2003;125:13042. doi: 10.1021/ja037410i. [DOI] [PubMed] [Google Scholar]
- 19.(a) Boger DL, Miyazaki S, Kim SH, Wu JH, Loiseleur O, Castle SL. J. Am. Chem. Soc. 1999;121:3226. [Google Scholar]; (b) Boger DL, Miyazaki S, Kim SH, Wu JH, Castle SL, Loiseleur O, Jin Q. J. Am. Chem. Soc. 1999;121:10004. [Google Scholar]
- 20.Maryanoff BE, McComsey DF. J. Org. Chem. 1978;43:2733. doi: 10.1021/jo000363h. [DOI] [PubMed] [Google Scholar]
- 21.(a) Corey EJ, Suggs JW. Tetrahedron Lett. 1975:2647. [Google Scholar]; (b) Corey EJ, Boger DL. Tetrahedron Lett. 1978:2461. [Google Scholar]
- 22.Commercially available from Aldrich.
- 23.CHCl3 was slurried with neutral Al2O3 and decanted prior to use.
- 24.The structure and stereochemistry of 17 were established with a single crystal X-ray structure determination conducted on colorless monoclinic plate obtained from MeOH (CCDC 1047936).
- 25.Burley SD, Lam VV, Lakner FJ, Bergdahl BM, Parker MA. Org. Lett. 2013;15:2598. doi: 10.1021/ol400620a. [DOI] [PubMed] [Google Scholar]
- 26.(a) Hendrickson JB, Wang J. Org. Lett. 2004;6:3. doi: 10.1021/ol0354369. [DOI] [PubMed] [Google Scholar]; (b) Bekkam M, Mo H, Nichols DE. Org. Lett. 2012;14:296. doi: 10.1021/ol203048q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.(a) Wagger J, Požes A, Požgan F. RSC Adv. 2013;3:23146. [Google Scholar]; (b) Dosa PI, Ward T, Walters MA, Kim SW. ACS Med. Chem. Lett. 2013;4:254. doi: 10.1021/ml3003814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.(a) Ohno S, Adachi Y, Koumori M, Mizukoshi K, Nagasaka M, Ichihara K, Kato E. Chem. Pharm. Bull. 1994;42:1463. doi: 10.1248/cpb.42.1463. [DOI] [PubMed] [Google Scholar]; (b) Chirivi C, Fontana G, Monti D, Ottolina G, Riva S, Danieli B. Chem.–Eur. J. 2012;18:10355. doi: 10.1002/chem.201201076. [DOI] [PubMed] [Google Scholar]
- 29.Although not exhaustive, initial efforts to generate a tetrahydropyridine through partial reduction of 16 have not yet been successful.
- 30.(a) Boger DL, Schumacher J, Panek JS, Mullican MD, Patel M. J. Org. Chem. 1982;47:2673. [Google Scholar]; (b) Boger DL, Mullican MD, Patel M. Tetrahedron Lett. 1982;23:4559. [Google Scholar]; (c) Boger DL, Dang Q. Tetrahedron. 1988;44:3379. [Google Scholar]; (d) Boger DL, Honda T, Dang Q. J. Am. Chem. Soc. 1994;116:5619. [Google Scholar]; (e) Boger DL, Kochanny MJ. J. Org. Chem. 1994;59:4950. [Google Scholar]
- 31.(a) Boger DL, Panek JS, Coleman RS, Sauer J, Huber FX. J. Org. Chem. 1985;50:5377. [Google Scholar]; (b) Boger DL, Panek JS. J. Am. Chem. Soc. 1985;107:5745. [Google Scholar]; (c) Boger DL, Panek JS, Duff SR, Yasuda M. J. Org. Chem. 1985;50:5790. [Google Scholar]; (d) Boger DL, Coleman RS. J. Am. Chem. Soc. 1987;109:2717. [Google Scholar]; (e) Boger DL, Zhang M. J. Am. Chem. Soc. 1991;113:4230. [Google Scholar]
- 32.(a) Boger DL, Coleman RS, Panek JS, Yohannes D. J. Org. Chem. 1984;49:4405. [Google Scholar]; (b) Boger DL, Patel M. J. Org. Chem. 1988;53:1405. [Google Scholar]; (c) Boger DL, Baldino CM. J. Am. Chem. Soc. 1993;115:11418. [Google Scholar]; (d) Boger DL, Boyce CW, Labroli MA, Sehon CA, Jin Q. J. Am. Chem. Soc. 1999;121:54. [Google Scholar]; (e) Boger DL, Soenen DR, Boyce CW, Hedrick MP, Jin Q. J. Org. Chem. 2000;65:2479. doi: 10.1021/jo9916535. [DOI] [PubMed] [Google Scholar]; (f) Boger DL, Hong J. J. Am. Chem. Soc. 2001;123:8515. doi: 10.1021/ja011271s. [DOI] [PubMed] [Google Scholar]; (g) Hamasaki A, Zimpleman JM, Hwang I, Boger DL. J. Am. Chem. Soc. 2005;127:10767. doi: 10.1021/ja0526416. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Oakdale JS, Boger DL. Org. Lett. 2010;12:1132. doi: 10.1021/ol100146b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bach NJ, Kornfeld EC, Jones ND, Chaney MO, Dorman DE, Paschal JW, Clemens JA, Smalstig EB. J. Med. Chem. 1980;23:481. doi: 10.1021/jm00179a003. [DOI] [PubMed] [Google Scholar]
- 34.Intermediates bearing the N1-benzoyl group display broadened signals in the NMR. At room temperature, the three aromatic protons of the indoline in 11-13, 15-17, and 25-31 are so broad in the 1H NMR that they are not observed. At 50 °C, two of the three aromatic protons are observed as broadened signals and the third is still so broad that it is not observable.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.