Abstract
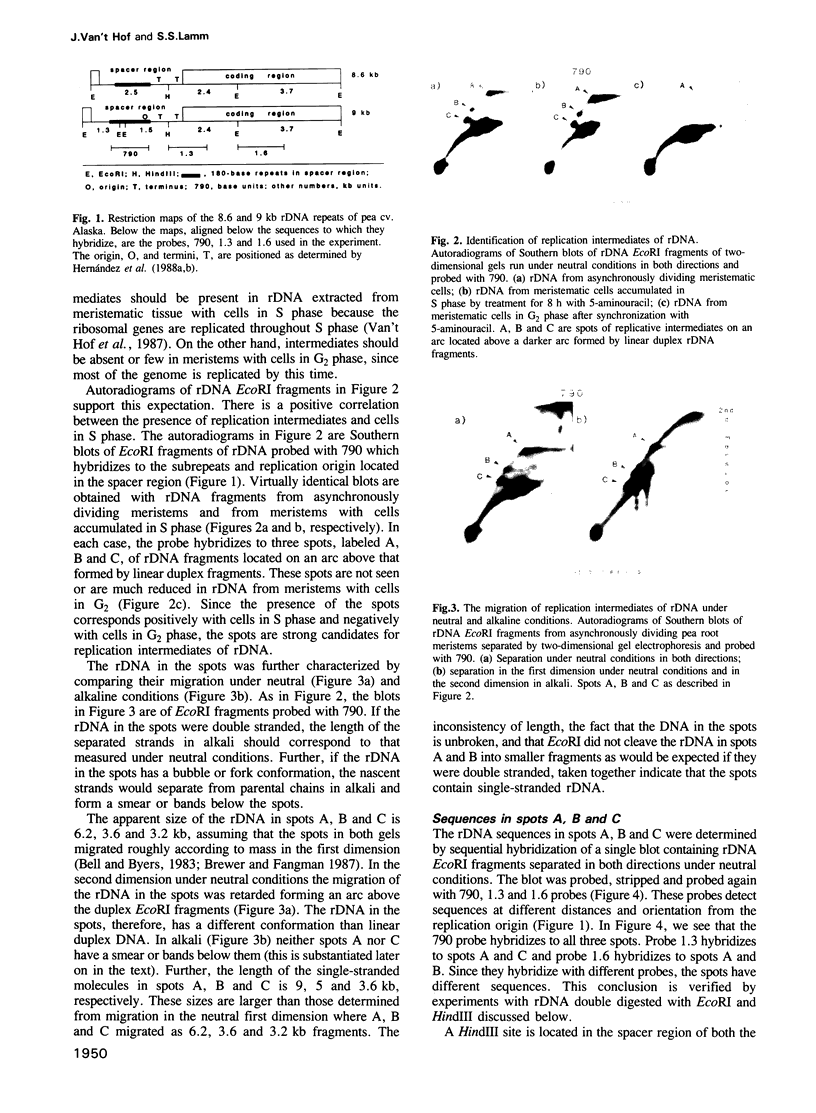
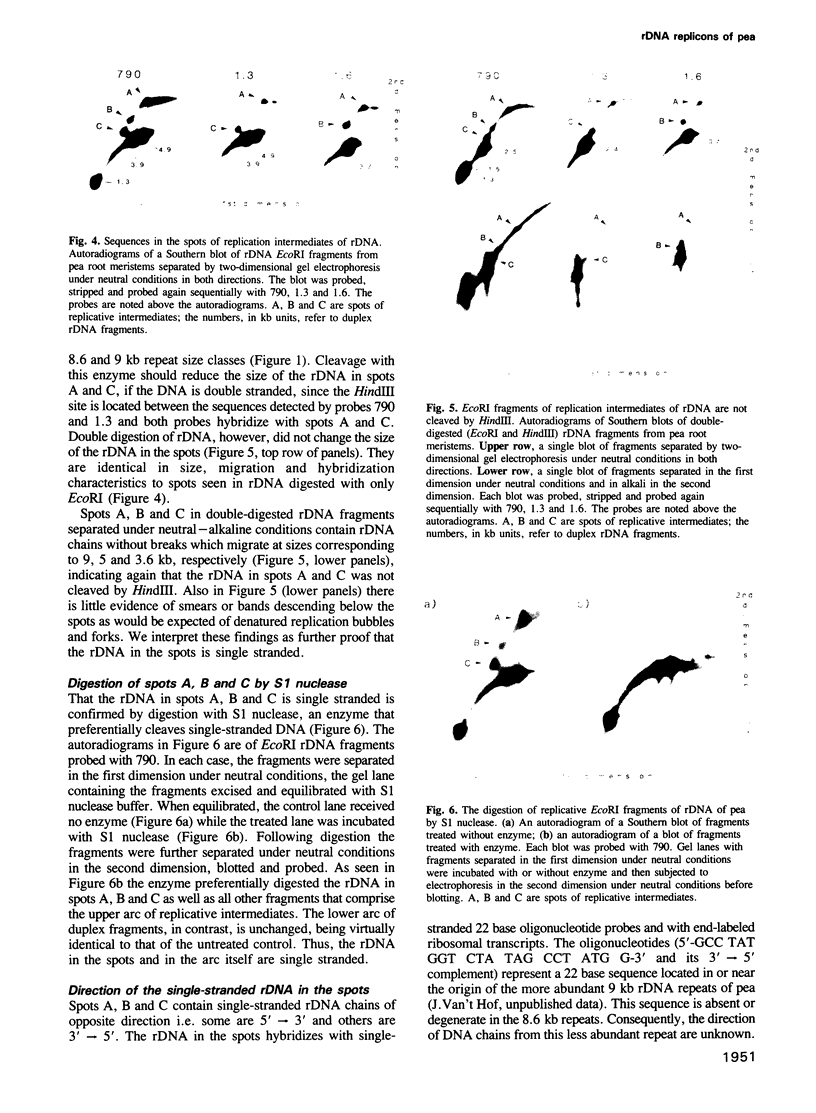
Replication of ribosomal DNA replicons in cells of Pisum sativum (cv. Alaska) occurs bidirectionally by displacement loops. Replication is initiated on opposite parental strands and nascent chains are elongated moving 5'----3' along each parental template. Replicative intermediates were analyzed by 2-dimensional agarose gel electrophoresis under neutral--neutral and neutral--alkaline conditions. Southern blots of ribosomal DNA fragments separated in the second dimension under neutral conditions show slowly migrating replicative fragments that hybridize with specific probes in a manner consistent with bidirectional replication. The replicative fragments are present in root meristems with cells in S phase; they are absent or few in number in meristems with cells in G2 phase. The following observations indicate that the replicative fragments are single stranded. The apparent length of the replicative fragments is not the same when separated under neutral and alkaline conditions. They contain rDNA without breaks and they do not exhibit the smaller nascent chains expected from replication bubbles and forks. They are not cleaved by restriction enzymes that require duplex DNA as substrate and they are digestible by S1 nuclease.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell L., Byers B. Separation of branched from linear DNA by two-dimensional gel electrophoresis. Anal Biochem. 1983 Apr 15;130(2):527–535. doi: 10.1016/0003-2697(83)90628-0. [DOI] [PubMed] [Google Scholar]
- Bjursell G., Gussander E., Lindahl T. Long regions of single-stranded DNA in human cells. Nature. 1979 Aug 2;280(5721):420–423. doi: 10.1038/280420a0. [DOI] [PubMed] [Google Scholar]
- Brewer B. J., Fangman W. L. A replication fork barrier at the 3' end of yeast ribosomal RNA genes. Cell. 1988 Nov 18;55(4):637–643. doi: 10.1016/0092-8674(88)90222-x. [DOI] [PubMed] [Google Scholar]
- Brewer B. J., Fangman W. L. The localization of replication origins on ARS plasmids in S. cerevisiae. Cell. 1987 Nov 6;51(3):463–471. doi: 10.1016/0092-8674(87)90642-8. [DOI] [PubMed] [Google Scholar]
- Carnevali F., Filetici P. Single-stranded molecules in DNA preparations from cultured mammalian cells at different moments of cell cycle. Chromosoma. 1981;82(3):377–384. doi: 10.1007/BF00285763. [DOI] [PubMed] [Google Scholar]
- Cullis C. A., Davies D. R. Ribosomal DNA Amounts in PISUM SATIVUM. Genetics. 1975 Nov;81(3):485–492. doi: 10.1093/genetics/81.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudette M. F., Benbow R. M. Replication forks are underrepresented in chromosomal DNA of Xenopus laevis embryos. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5953–5957. doi: 10.1073/pnas.83.16.5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández P., Lamm S. S., Bjerknes C. A., Hof J. V. Replication termini in the rDNA of synchronized pea root cells (Pisum sativum). EMBO J. 1988 Feb;7(2):303–308. doi: 10.1002/j.1460-2075.1988.tb02813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingle J., Sinclair J. Ribosomal RNA genes and plant development. Nature. 1972 Jan 7;235(5332):30–32. doi: 10.1038/235030a0. [DOI] [PubMed] [Google Scholar]
- Krimer D. B., Hof J. V. Extrachromosomal DNA of pea (Pisum sativum) root-tip cells replicates by strand displacement. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1933–1937. doi: 10.1073/pnas.80.7.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linskens M. H., Huberman J. A. Organization of replication of ribosomal DNA in Saccharomyces cerevisiae. Mol Cell Biol. 1988 Nov;8(11):4927–4935. doi: 10.1128/mcb.8.11.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuermann W., Klaffke-Lobsien G. On the infleunce of 5-amino uracil on the cell cycle of root tip meristems. Exp Cell Res. 1973 Feb;76(2):428–436. doi: 10.1016/0014-4827(73)90395-9. [DOI] [PubMed] [Google Scholar]
- Schimke R. T., Sherwood S. W., Hill A. B., Johnston R. N. Overreplication and recombination of DNA in higher eukaryotes: potential consequences and biological implications. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2157–2161. doi: 10.1073/pnas.83.7.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark G. R., Debatisse M., Giulotto E., Wahl G. M. Recent progress in understanding mechanisms of mammalian DNA amplification. Cell. 1989 Jun 16;57(6):901–908. doi: 10.1016/0092-8674(89)90328-0. [DOI] [PubMed] [Google Scholar]
- Wanka F., Brouns R. M., Aelen J. M., Eygensteyn A., Eygensteyn J. The origin of nascent single-stranded DNA extracted from mammalian cells. Nucleic Acids Res. 1977 Jun;4(6):2083–2097. doi: 10.1093/nar/4.6.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannis-Hadjopoulos M., Persico M., Martin R. G. The remarkable instability of replication loops provides a general method for the isolation of origins of DNA replication. Cell. 1981 Nov;27(1 Pt 2):155–163. doi: 10.1016/0092-8674(81)90369-x. [DOI] [PubMed] [Google Scholar]








