ABSTRACT
Interleukin (IL)-1β is associated with hypotension and cardiovascular collapse in mammals during heat stroke, and the mRNA expression of this pro-inflammatory cytokine increases dramatically in the blood of Atlantic cod (Gadus morhua) at high temperatures. These data suggest that release of IL-1β at high temperatures negatively impacts fish cardiovascular function and could be a primary determinant of upper thermal tolerance in this taxa. Thus, we measured the concentration-dependent response of isolated steelhead trout (Oncorhynchus mykiss) coronary microvessels (<150 μm in diameter) to recombinant (r) IL-1β at two temperatures (10 and 20°C). Recombinant IL-1β induced a concentration-dependent vasodilation with vessel diameter increasing by approximately 8 and 30% at 10−8 and 10−7 mol l−1, respectively. However, this effect was not temperature dependent. Both vessel denudation and cyclooxygenase blockade (by indomethacin), but not the nitric oxide (NO) antagonist L-NIO, inhibited the vasodilator effect of rIL-1β. In contrast, the concentration-dependent dilation caused by the endothelium-dependent calcium ionophore A23187 was completely abolished by L-NIO and indomethacin, suggesting that both NO and prostaglandin signaling mechanisms exist in the trout coronary microvasculature. These data: (1) are the first to demonstrate a functional link between the immune and cardiovascular systems in fishes; (2) suggest that IL-1β release at high temperatures may reduce systemic vascular resistance, and thus, the capacity of fish to maintain blood pressure; and (3) provide evidence that both NO and prostaglandins play a role in regulating coronary vascular tone, and thus, blood flow.
KEY WORDS: Vasomotor responses, Oncorhynchus mykiss, Heart, Cytokine, Tone, Vascular resistance
Summary: The pro-inflammatory cytokine (r)interleukin-1β dilates isolated coronary microvessels of steelhead trout in a concentration-dependent manner: an effect that is not influenced by incubation at high temperature (20°C) and is partially mediated by prostaglandins.
INTRODUCTION
Interleukin (IL)-1β is a pro-inflammatory cytokine that has a number of localized and systemic biological effects, and is essential to the initiation and regulation of immune and inflammatory responses in mammals (Dinarello, 1997). However, not all of its biological and physiological effects appear to be immune or inflammation related. Increases in plasma IL-1β levels are associated with hypotension and cardiovascular collapse in mammals during heat stroke (Chiu et al., 1995; Lin et al., 1997; Leon, 2006), a phenomenon that can be mimicked by IL-1β injection and eliminated by pre-treatment with IL-1β receptor antagonists (Chiu et al., 1995; Lin et al., 1997). IL-1β induces vasodilation of porcine cerebral arterioles (Shibata et al., 1996) and canine basilar arteries (Osuka et al., 1997). Finally, IL-1β alters the excitation–contraction coupling characteristics of cardiomyocytes (Combes et al., 2002; Radin et al., 2008), including calcium regulation and cycling (Duncan et al., 2010), and thus reduces cardiomyocyte contractility (Combes et al., 2002; Duncan et al., 2010).
While it is clear that IL-1β can significantly affect mammalian cardiac function and vascular resistance (Bataillard and Sassard, 1994; Graff and Gozal, 1999; Maher et al., 2003 and above references), the mechanism(s) that modulates the effects of IL-1β on the vasculature is not well understood. There is evidence that IL-1β induces the production and/or release of vasoactive substances such as prostaglandins (PGs) and nitric oxide (NO) from mammalian vascular cells (Rossi et al., 1985; Hirafuji et al., 2002; Proescholdt et al., 2002; Schiltz and Sawchenko, 2002; Jedrzejowska-Szypulka et al., 2009). However, the results of experiments investigating the role of these two biological agents in modulating vascular resistance have been quite variable. For example, IL-1β induces inducible nitric oxide synthase (iNOS) gene expression and de novo synthesis in rat vascular endothelial cells and cardiomyocytes (Kanno et al., 1994; Tsujino et al., 1994). However, in the canine basilar artery (McKean et al., 1994), human vascular smooth muscle cells (Beasley and McGuiggin, 1995) and isolated rabbit mesenteric arteries (Marceau et al., 1991), IL-1β induces the release of cyclooxygenase 2 (COX-2), a key enzyme in the catalytic conversion of arachidonic acid to PGs, but not NO. Furthermore, a positive-feedback mechanism has been suggested for human endothelial and vascular smooth muscle (VSM) cells (Warner et al., 1987a,b; Proescholdt et al., 2002), where IL-1β stimulates the synthesis of more IL-1β, and induces the production and release of other vasoactive agents such as endothelin-1 (Jedrzejowska-Szypulka et al., 2009) and serotonin (Chiu et al., 1995).
Interestingly, the mRNA expression of this cytokine increases dramatically (by ∼25-fold) in the blood of Atlantic cod (Gadus morhua) when water temperature is gradually increased to 19°C (Pérez-Casanova et al., 2008). A significant increase in IL-1β expression is seen in LPS (bacterial lipopolysaccharide)-infected trout exposed to an increase in water temperature (i.e. to stimulate behavioral fever) when compared with trout kept at control temperature (Gräns et al., 2012). Finally, systemic vascular resistance falls in rainbow trout at elevated temperatures (>18°C) (Gamperl et al., 2011). These findings suggest that in fish, as in mammals, this cytokine plays an important role in the loss of cardiovascular function observed at high temperatures (Farrell et al., 1996, 2009; Farrell, 2009; Gollock et al., 2006; Gamperl et al., 2011) and has direct effects on the microvasculature. Changes in tissue perfusion or vascular resistance are primarily mediated by the dilation or constriction of small arteries and arterioles (<150 µm in diameter) (Duling et al., 1981; Kuo et al., 1988; Berne and Levy, 1997). Therefore, the main goals of this study were to use recombinant trout IL-1β (rIL-1β) to: (1) determine whether this cytokine has vasoactive effects on steelhead trout (Oncorhynchus mykiss) isolated coronary microvessels, and if so, whether these effects are temperature dependent; and (2) examine the potential roles of the endothelium, NO and PGs in IL-1β-induced changes in isolated coronary microvessel tone. This rIL-1β has been shown to be strongly bioactive both in vitro (Hong et al., 2001; Peddie et al., 2001, 2002) and in vivo (Holland et al., 2002; Hong et al., 2003).
List of abbreviations
- ANOVA
analysis of variance
- CHAPS
3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate, ionic detergent
- COX
cyclooxygenase
- DTT
dithiothreitol
- EDRF
endothelium-derived relaxing factor
- eNOS
endothelial nitric oxide synthase
- ID
internal diameter
- IL
interleukin
- INDO
indomethacin, non-selective cyclooxygenase inhibitor
- iNOS
inducible nitric oxide synthase
- L-NIO
N5-(1-iminoethyl)-l-ornithine, dihydrochloride, NO synthase inhibitor
- LPS
bacterial lipopolysaccharide
- nNOS
neurally derived nitric oxide synthase
- NO
nitric oxide
- NOS
nitric oxide synthase
- PG
prostaglandin
- rIL-1β
recombinant interleukin-1β
- SNP
sodium nitroprusside
- TEM
transmission electron microscopy
- VSM
vascular smooth muscle
In addition, because of the controversy surrounding the involvement of NO and PGs as endothelial-derived relaxation factors (EDRFs) in fish (see Olson and Villa, 1991; Kågström and Holmgren, 1997; Park et al., 2000; Jennings et al., 2004), we decided to investigate the possible role played by these two vasoactive agents in the concentration-dependent vasodilation induced by the endothelium-dependent calcium ionophore A23187. Several studies have indicated that A23187 is an endothelium-dependent vasodilator of fish vessels (Olson and Villa, 1991; Park et al., 2000; Jennings et al., 2004).
RESULTS
The average resting internal diameter (ID) of isolated coronary arterioles from steelhead trout (Oncorhynchus mykiss Walbaum 1792) used in this study (N=50) was 83.6±3.0 µm, which was 68% of maximal vessel diameter (123.0±2.2 µm).
Vasoactivity of recombinant IL-1β
At 10°C, rIL-1β caused a concentration-dependent dilation of isolated coronary microvessels, with increases in vessel diameter of approximately 4, 10 and 31% at 10−9, 10−8 and 10−7 mol l−1, respectively (Fig. 1). Vessels at 20°C also showed concentration-dependent dilation to rIL-1β. While the response at 20°C appeared to be somewhat blunted (no difference compared with the sham injection control at 10−9 mol l−1 and a maximal dilation of only 22% at 10−7 mol l−1), the degree of dilation at 10−8 and 10−7 mol l−1 rIL-1β was not significantly different between temperatures (Fig. 1).
Fig. 1.
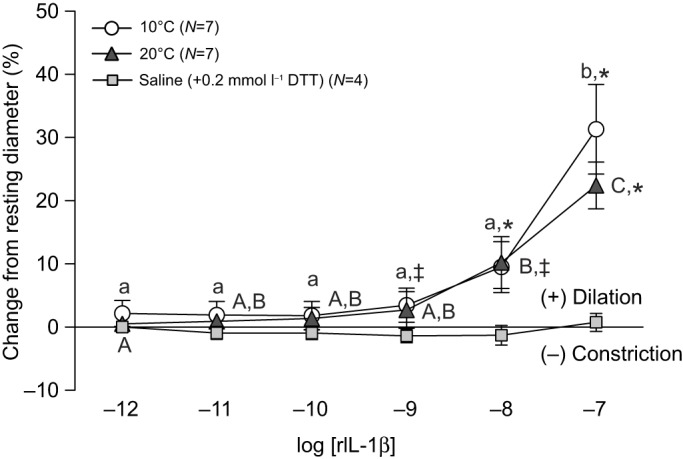
Vasomotor responses of trout coronary microvessels to increasing concentrations of recombinant interleukin-1β at two experimental temperatures. Vessels tested at 20°C were acutely warmed (over 1 h) from 10°C and then allowed to acclimate to 20°C (∼30 min) before rIL-1β injections were initiated. Dissimilar lower case letters indicate significant differences in the response to the various concentrations of rIL-1β in vessels tested at 10°C, whereas dissimilar capital letters indicate differences in the response to rIL-1β in vessels tested at 20°C. Significant differences between vessels exposed to rIL-1β (10 or 20°C) vs sham injections (at 10°C), at a particular concentration of rIL-1β, are indicated by *P<0.05 and ‡0.1>P≥0.05. Experimental temperature had no effects on vessel responses to rIL-1β. Note that the series of sham injections (i.e. saline with 0.2 mmol l−1 DTT) did not result in noticeable (i.e. ≤1%) changes in vessel ID. Thus, we considered any change in vessel ID greater than ∼3% (mean change plus 2 s.d. caused by the sham injection) to be biologically significant. All concentrations are in mol l−1.
Effectiveness of endothelial denudation
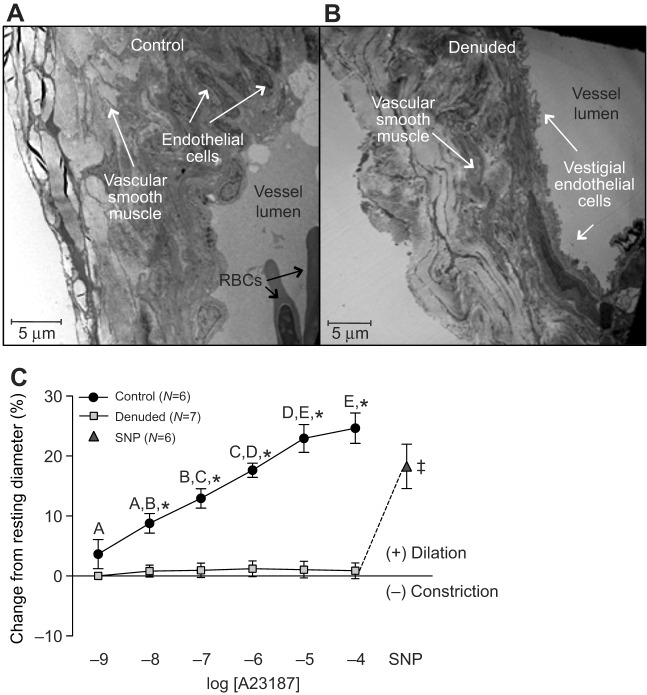
Based on a number of criteria, endothelial denudation of isolated coronary microvessels using the non-ionic detergent 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate (CHAPS, 0.4%) was a success. An intact endothelium was clearly visible in control vessels (Fig. 2A), whereas these cells were merely vestigial in vessels treated with CHAPS (Fig. 2B). The concentration-dependent dilation observed in intact isolated coronary microvessels with 10−9 to 10−4 mol l−1 of the endothelium-dependent calcium ionophore A23187 (maximum dilation, 24.6±6.2%) was abolished after treatment with CHAPS. Finally, direct stimulation of the smooth muscle in denuded vessels with the NO donor sodium nitroprusside (SNP, 10−4 mol l−1) elicited a significant (18.3±9.0%) vasodilator response (Fig. 2C). This latter result indicated that smooth muscle function was intact following denudation.
Fig. 2.
Trout coronary microvessel structure before and after endothelial denudation, and vasomotor responses to increasing concentrations of the calcium ionophore A23187. Denuded vessels were exposed to CHAPS (0.4%) by intraluminal injection (0.3 ml) for approximately 2 min. Intact endothelial cells are visible on the luminal surface of the control vessel (A), while in the treated vessel the endothelial cell layer is visibly reduced (B). See Materials and methods for details on TEM methodology. (C) Vasomotor responses of trout coronary microvessels to increasing concentrations of A23187. SNP (sodium nitroprusside, 10−4 mol l−1) was administered at the end of each trial to test for vascular smooth muscle viability in the denuded vessels. ‡ indicates a significant difference between denuded microvessels after exposure to 10−4 mol l−1 of A23187 versus SNP injection. Dissimilar letters indicate significant differences between concentrations of A23187, *P<0.05 between control (intact) and denuded vessels at a particular concentration of A23187. Note that the series of sham injections (saline containing ethanol) did not result in any noticeable (i.e. ≤0.5%) changes in vessel ID. Thus, we considered any change in vessel ID greater than 2% (mean change plus 2 s.d. caused by sham injection) to be biologically significant. All concentrations are in mol l−1.
Role of the endothelium, NO and prostaglandins in rIL-1β-induced dilation
Endothelial denudation of trout coronary microvessels using CHAPS (0.4%) completely abolished the rIL-1β-induced vasodilation (Fig. 3A). The non-selective NO synthase inhibitor N5-(1-iminoethyl)-l-ornithine, dihydrochloride (l-NIO, 10−4 mol l−1) had no effect on the vasodilator response of isolated trout coronary microvessels to increasing concentrations of rIL-1β (Fig. 3B). In contrast, pre-incubation with the non-selective cyclooxygenase (COX) inhibitor indomethacin (INDO, 10−4 mol l−1) greatly reduced the vasodilator effect caused by 10−7 mol l−1 rIL-1β (i.e. from approx. 18.5 to 4% of resting ID) (Fig. 3C).
Fig. 3.
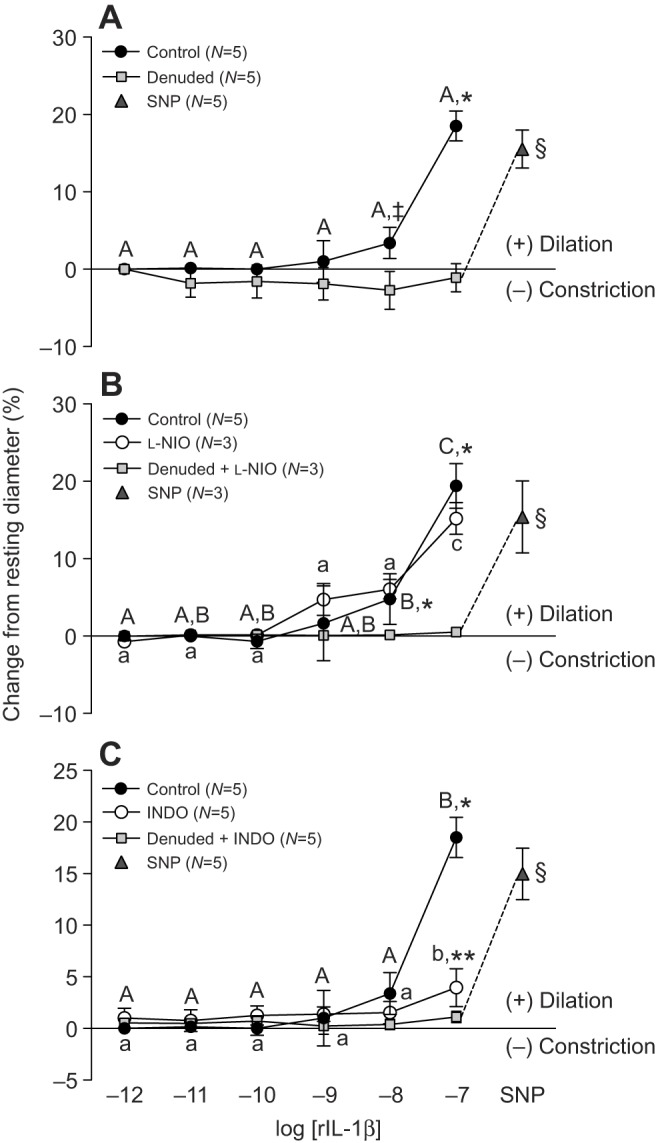
Effect of vessel denudation, l-NIO and indomethacin on trout coronary microvessel dilation in response to recombinant interleukin-1β. (A) Vessel denudation was achieved by exposing the intraluminal surface of the vessel to the non-ionic detergent CHAPS (0.4%). Vessels were incubated for 30 min with either the NOS inhibitor L-NIO (10−4 mol l−1) (B), or the COX inhibitor INDO (10−4 mol l−1) (C), before rIL-1β injections were initiated. Dissimilar capital letters indicate significant (P<0.05) differences in the response to the various concentrations of rIL-1β in control (intact) vessels, while dissimilar lower case letters indicate differences in the response to rIL-1β in vessels incubated with either L-NIO (B) or INDO (C). Significant differences between control (intact) and denuded vessels are indicated by *, while ** indicates a significant difference between vessels in the presence (open circles) versus absence (filled circles) of blocker (i.e. L-NIO or INDO) at a particular concentration of rIL-1β (P<0.05, except where indicated ‡, where 0.1>P≥0.05). Sodium nitroprusside (SNP, 10−4 mol l−1) was administered at the end of each trial to test for vascular smooth muscle viability in denuded vessels, and § indicates a significant difference between denuded microvessels after exposure to 10−7 mol l−1 rIL-1β (with or without blocker) versus after SNP injection. Note that a series of sham (saline containing DTT) injections did not result in any noticeable (i.e. ≤1%) change in vessel ID. Thus, we considered any change in vessel ID greater than 3% (mean change plus 2 s.d. caused by the sham saline injections) to be biologically significant. All concentrations are in mol l−1.
Role of NO and PGs in the calcium ionophore A23187 induced dilation
Pre-incubation of trout coronary microvessels with l-NIO (10−4 mol l−1) or INDO (10−4 mol l−1) completely abolished the vasodilator effect of A23187, and instead, resulted in a significant concentration-dependent vasoconstriction. This constriction with 10−7 to 10−4 mol l−1 A23187 ranged from 3.5 to 16% of resting ID for l-NIO-exposed vessels and from 4 to 9% of resting ID for those incubated with INDO (Fig. 4).
Fig. 4.
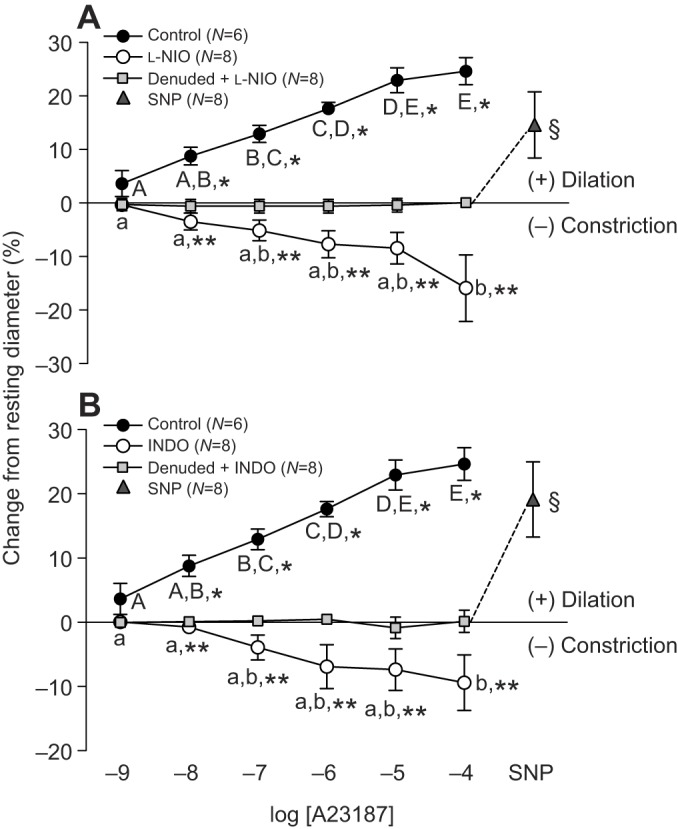
Effect of l-NIO and indomethacin on trout coronary microvessel dilation induced by the calcium ionophore A23187. Vessel denudation was achieved by exposing the intraluminal surface of the vessel to the non-ionic detergent CHAPS (0.4%). Vessels were incubated for 30 min with either the NOS inhibitor L-NIO (10−4 mol l−1) (A) or the COX inhibitor INDO (10−4 mol l−1) (B), before A23187 injections were initiated. Dissimilar capital letters indicate significant (P<0.05) differences in the response to the various concentrations of A23187 in control (intact) vessels, while dissimilar lower case letters indicate differences in the response to A23187 in vessels incubated with either L-NIO (A) or INDO (B). To improve clarity of the figure, letters are not included where differences were not significant. Significant differences between control (intact) and denuded vessels are indicated by *, while ** indicates significant differences between vessels in the presence (open circles) versus absence (filled circles) of blocker (i.e. l-NIO or INDO) at a particular concentration of A23187 (P<0.05). Sodium nitroprusside (SNP, 10−4 mol l−1) was administered at the end of each trial to test for vascular smooth muscle viability in denuded vessels, and § indicates a significant difference between denuded microvessels after exposure to 10−4 mol l−1 A23187 versus after SNP injection. Note that a series of sham saline (containing ethanol) injections did not result in a noticeable (i.e. ≤0.5%) change in vessel ID. Thus, we considered any change in vessel ID greater than 2% (the mean change plus 2 s.d. caused by the sham saline injections) to be biologically significant. All concentrations are in mol l−1.
DISCUSSION
Vasoactivity of recombinant IL-1β and the effect of temperature
This is the first study where the vasoactivity of trout (fish) rIL-1β has been investigated and we show that this cytokine caused a 20–30% dilation of isolated coronary microvessels that was independent of experimental temperature (10 vs 20°C) (Fig. 1). The magnitude of rIL-1β-induced vasodilation in trout coronary microvessels is consistent with what has been reported for mammalian vessels acutely exposed to this cytokine. IL-1β increased cerebral pial arteriolar diameter in a concentration-dependent manner in piglets, reaching a maximum dilation of approximately 19% from baseline (Shibata et al., 1996) and dilated the cerebral basilar artery in anaesthetized dogs by approximately 30% (Osuka et al., 1997). However, it is unclear what the effect of long-term exposure to this cytokine would have on trout coronary tone and blood flow. Long-term treatment with IL-1β induced vasospastic responses in the coronary vessels of pigs (Shimokawa et al., 1996) and there is a growing body of evidence that links IL-1β with reduced cerebral blood flow in mammals (Proescholdt et al., 2002; Maher et al., 2003; Jedrzejowska-Szypulka et al., 2009). This dependence of the vasoactive effects of IL-1β on time of exposure is not surprising given the broad spectrum of activities of this cytokine, and that many of its downstream actions occur over a prolonged period and involve other vasoactive substances such as IL-6 and endothelin-1 (Maher et al., 2003; Jedrzejowska-Szypulka et al., 2009).
In this study, we show that temperature (10 vs 20°C) did not have a significant effect on the dose-dependent response of trout coronary microvessels to rIL-1β (Fig. 1). Nonetheless, the lack of a temperature effect on the dilator response of trout isolated coronary microvessels does not preclude the possibility that the impacts of this cytokine on vasoactivity and blood flow are temperature dependent. The absence of a temperature effect on rIL-1β-induced coronary microvessel dilation could be related to the fact that dissection was performed at 4°C, prior to experiments at 10°C and 20°C. Amelio et al. (2013) showed that acute temperature changes abolished nitric-oxide-dependent modulation of the eel (Anguilla anguilla) heart's Frank–Starling mechanisms. Further, it is possible that 1 h of exposure to 20°C was not long enough to allow for sufficient physiological changes to occur that impact microvessel tone. An increase in IL-1β receptor density could increase the strength of the response of the vessels to rIL-1β, and Helwig and Leon (2011) report that while protein expression of soluble IL-1 subtype-1 receptor in the plasma of mice does not change immediately upon exposure to hyperthermia, it is significantly elevated after 24 h. Second, there is growing evidence that temperature can influence the expression of IL-1β mRNA in fish. In Atlantic cod, a gradual increase in water temperature (from 10 to 20°C; 1°C every 5 days) resulted in a 25-fold increase in plasma IL-1β mRNA expression (Pérez-Casanova et al., 2008). Trout injected with bacterial lipopolysaccharide show a significant increase in head kidney IL-1β transcript expression if subjected to a temperature increase from 13.5 to 16°C (Gräns et al., 2012). Finally, cultured trout head kidney leukocytes transferred to 22°C at the time of LPS exposure showed a 64% increase in IL-1β mRNA expression relative to cells kept at 14°C (Zou et al., 2000). Thus, while our results show that the concentration-dependent response of trout coronary microvessels to rIL-1β is not affected by exposure to acute high temperatures (20°C), it is very likely that the effects of IL-1β will be enhanced at high temperatures because of an increase in concentration of this cytokine.
Future studies should explore the effects of rIL-1β administration on isolated vessels and isolated cell cultures (e.g. endothelial and VSM cells) exposed to elevated temperatures for longer periods, but also from fish acclimated to high temperatures for various periods of time. Identifying the location of IL-1 receptors along the trout coronary vasculature (i.e. by immunohistochemistry techniques), ascertaining the influence of acclimation temperature on the density of these receptors, as well as determining in vivo plasma IL-1β protein levels under different thermal challenges will also be key to understanding the role of this cytokine in regulating vascular resistance.
Role of the endothelium, NO and prostaglandins in rIL-1β-induced dilation
From our results, it is apparent that rIL-1β-induced dilation of trout coronary vessels is endothelium dependent (e.g. see Fig. 2), and largely mediated by prostaglandins because INDO, but not l-NIO, decreased the vasodilator effect of this cytokine (Fig. 3). The involvement of PGs in IL-1β-mediated responses has been previously reported in fish. Hong et al. (2001, 2003) showed that while neither IL-1β nor COX-2 were constitutively expressed in rainbow trout, the expression of COX-2 mRNA increased after stimulation of both head kidney and macrophage cell lines with rIL-1β. Further, increased COX-2 transcript levels have been reported in the head kidney after in vivo exposure (intraperitoneal injection) of both trout (Hong et al., 2003) and sea bass (Dicentrarchus labrax) (Buonocore et al., 2005) to rIL-1β.
Role of NO and prostaglandins in calcium ionophore A23187-induced dilation
The existence of an endothelial NO system in the fish vasculature has been debated for a number of years, in large part because molecular approaches (i.e. molecular cloning and bioinformatic analysis of genomes) have not been able to identify an eNOS isoform (i.e. NOS3) in fish, only the nNOS (i.e. NOS1) and iNOS (i.e. NOS2) isoforms (Lepiller et al., 2009; Olson and Donald, 2009; González-Domenech and Muñoz-Chápuli, 2010; Fago et al., 2012). However: (1) a number of authors show that fish vascular beds are dilated by NO, and thus, provide evidence for an endothelial NO system in this taxa (Mustafa et al., 1997; Nilsson and Söderström, 1997; Mustafa and Agnisola, 1998); (2) several studies (Fritsche et al., 2000; McNeill and Perry, 2006; Wang et al., 2007; Amelio et al., 2008) have identified eNOS immunoreactivity in various fish tissues, including the vascular endothelium, when using heterologous mammalian eNOS antibodies (Olson and Donald, 2009; Imbrogno et al., 2014); and (3) Andreakis et al. (2011) suggest that a neuronal NOS (nNOS) isoform with an ‘endothelial-like’ consensus may cover some functional features typical of the eNOS isoform. As has previously been reported for mammals (Taniguchi et al., 1999; Oliveira et al., 2009), we showed that NO is involved in A23187-mediated vasodilation of isolated trout coronary arterioles (Fig. 4A), and that this effect was completely dependent on the presence of an intact endothelium. This latter aspect of our study is important because Jennings et al. (2004) described how nitrergic nerves in the dorsal aorta and intestinal vein of the Australian short-finned eel (Anguilla australis) provide NO-mediated vasodilation through nNOS, not eNOS, and we cannot exclude the possibility that some nitrergic axons were left intact after the vessel's dissection. Clearly, the results of this study using isolated trout coronary microvessels strongly support the hypothesis of Andreakis et al. (2011), and further experiments should be conducted to clarify the mechanism(s) (genes/proteins) through which NO regulates signaling between the endothelium and vascular smooth muscle in fishes.
The dilatory effect of A23187 on trout coronary microvessels was also abolished after blockade with the COX inhibitor INDO (Fig. 4B). While this result is in agreement with previous studies, which established a role for PGs in A23187-mediated vasodilation in fish vessels (Olson and Villa, 1991; Park et al., 2000; Jennings et al., 2004), it raises an interesting point. If INDO specifically blocks PG-related pathways and l-NIO specifically blocks the production of NO, then why did blocking only one of these two pathways still result in the complete elimination of a vasodilator response? Indeed, our results suggest that both PG and NO signaling cascades may be required to achieve dilation of trout coronary microvessels with A23187. The existence of a ‘cross-talk’ (i.e. interaction) between NO and PG pathways has been speculated many times based on studies on mammalian cells (Salvemini et al., 1993; Vassalle et al., 2003; Mollace et al., 2005). In fact, Salvemini et al. (1993) showed that inhibition of endogenous NO release by NOS inhibitors markedly attenuated the release of prostaglandin E2 (PGE2). If a similar relationship exists between the NO and PG pathways in the trout coronary microvasculature, it is possible that the blockade of either pathway is sufficient to allow the A23187 endothelium-dependent vasodilation to be overridden by the simultaneous release of an endothelium-dependent constriction factor; possibly explaining the endothelium-dependent vasoconstriction observed in Fig. 4.
Endothelium-dependent vasoconstriction responses to A23187 have been previously reported in mammals. Lüscher et al. (1992) demonstrated that the calcium ionophore A23187 is capable of stimulating the release of endothelin-1 by endothelial cells in certain blood vessels and A23187 (at 10−7 to 10−6 mol l−1) was shown by Shi et al. (2007) to cause an endothelium-dependent contraction of rat femoral arteries that was abolished by INDO, suggesting the release of a COX-dependent constriction factor. In trout, endothelin-1 induces a powerful concentration-dependent constriction of isolated coronary vessels (maximum dilation, ∼80% at 10−7 mol l−1) (Costa et al., 2015) and thus this vasoactive agent could potentially be involved in the A23187-induced constriction observed in this study.
In conclusion, this is the first study to demonstrate a functional link between the immune and cardiovascular systems in fishes. Administration of the pro-inflammatory cytokine rIL-1β dilated isolated coronary microvessels of steelhead trout in a concentration-dependent manner (maximum dilation, 31% at 10−7 mol l−1): an effect that was not influenced by incubation at high temperature (20°C), but was at least partially mediated by PG. We also present data suggesting that both endothelium-derived NO and prostaglandins are required for A23187-mediated vasodilation of trout coronary arterioles (fish vessels), and that this vasodilation normally overshadows the slight (5–15%) constriction induced by this agent. These are novel findings that contribute greatly to the field of fish cardiovascular physiology, and pave the way for future in vivo and in vitro experiments to examine the role of this cytokine and other factors in controlling blood flow and pressure in fish.
MATERIALS AND METHODS
Experimental animals
All procedures followed the guidelines published by the Canadian Council on Animal Care and were approved by the Institutional Animal Care Committee from Memorial University of Newfoundland (MUN). Adult steelhead trout were purchased from a cage-culture site on the south coast of Newfoundland (Canada) and held in seawater at 10±1.0°C in 6 m3 tanks at the Dr Joe Brown Aquatic Research Building (JBARB) (Department of Ocean Sciences, MUN) for a minimum of 5 weeks before experiments began. Fish were fed a maintenance ration (1.5% body mass−1 every 2 days) of commercial pellets (Skretting, Vancouver, BC, Canada) while held at the JBARB, and were maintained on a 12 h:12 h light:dark photoperiod.
Heart sampling
Fish were killed by a sharp blow to the head, and the heart was quickly removed and placed in ice-chilled 0.9% NaCl. The bulbus and the atrium were then removed and the ventricle transferred to a temperature-controlled dissection dish filled with cooled (4±0.5°C) physiological saline solution. The ventricle was then cut in half under a dissecting microscope (Model MZ9s; Leica Microsystems Inc., Concord, ON) to allow dissection of the arterioles (microvessels). The physiological saline solution contained (in mmol l−1) 155.0 NaCl, 4.7 KCl, 2.5 CaCl2·2H2O, 1.99 MgSO4·7H2O, 3.0 HEPES acid, 7.0 HEPES sodium salt, 1.0 NaH2PO4·H2O and 5.6 glucose. This saline, as well as high-KCl and zero-calcium physiological saline solutions (see below), were buffered to a pH of 7.8 at 10°C and filtered through a 0.22 µm cellulose acetate, low protein binding membrane (Corning® 430521, Corning Inc., Corning, NY, USA) prior to use.
Isolation and cannulation of coronary arterioles
The techniques for isolation and cannulation of trout coronary arterioles have been described previously (Costa et al., 2015). In brief, an unbranched arteriole of approximately 80–150 µm in external diameter and 1–1.5 mm in length was selected and carefully dissected free from the surface of the ventricle. After careful removal of any remaining extraneous tissue, the arteriole was transferred for cannulation to a custom-made (Technical Services, MUN) vessel bath (volume 2 ml) containing physiological saline at the experimental temperature (10°C). This vessel bath is made of titanium (to resist corrosion and ensure efficient heat transfer) and is equipped with a water jacket that allows temperature control of the saline solution within the bath throughout the experiment. The temperature of the physiological saline within the vessel bath was maintained by connecting the water jacket to a recirculating water bath (Isotemp Model 3016S, Thermo Fisher Scientific Inc., Waltham, MA, USA). The vessel chamber was, in turn, attached to a specially designed stage (Microcirculation Research Institute, Texas A&M University, College Station, TX, USA) equipped with micromanipulators (Model MMN-1, Narishige, East Meadow, NY, USA) that housed pipette holders and allowed for adjustments of pipette positioning in all three dimensions.
The arteriole was cannulated by pulling one end of the vessel onto the tip of a glass micropipette filled with physiological saline containing 1% albumin, and securely tying it to the pipette with 11-0 ophthalmic suture (Alcon Medical Safety Inc, Fort Worth, TX, USA). Any remaining blood was then flushed out of the vessel at low perfusion pressure (10 cmH2O or ∼7.5 mmHg), and the other end of the vessel was cannulated using a second micropipette and tied in place.
After cannulation was complete, the vessel stage was transferred from the dissecting microscope to an inverted microscope (Model DM1RB, Leica Microsystems Inc.) with a 20× objective, and coupled to a video camera (Sony CCD-Iris; Sony Corporation, Japan) and videomicrometer system (Model VIA-100; Boeckeler Instruments Inc., Tuscon, AZ) for continuous measurement of the vessel's internal diameter throughout the experiment. At this point, the glass pipettes were connected to wall-mounted, independent, adjustable pressure reservoirs filled with saline solution and pressure in the vessels was set to 20 cmH2O (∼15 mmHg) luminal pressure, without flow, by adjusting the height of the reservoirs. This pressure approximates the intraluminal pressure for trout coronary microvessels of this size range in vivo; based on: (1) mammalian studies that have estimated the intraluminal pressure of coronary arterioles in this size range to be approximately 50% of mean aortic pressure (Chilian et al., 1986; Kuo et al., 1988); and (2) values for dorsal aortic blood pressure in rainbow trout at rest that range from 38.8 to 42 cm of H2O (∼28.5 to 31 mmHg) (Olson et al., 1991; Gamperl et al., 1995; Perry and Reid, 2002). The vessel was then set to its resting in situ length (as measured with an eyepiece micrometer during dissection) by stretching the vessel using the pipette micromanipulators, and tested for leaks (any preparations with leaks were excluded from further investigation). At this point, the bath solution was replaced with fresh physiological saline and the vessel allowed approximately 30 min to develop spontaneous tone. However, vessels that did not regain tone were pre-contracted by replacing the bath with physiological saline containing 50 mmol l−1 KCl: the exact composition of this saline (in mmol l−1) was 110.0 NaCl, 50.0 KCl, 2.5 CaCl2·2H2O, 1.99 MgSO4·7H2O, 3.0 HEPES acid, 7.0 HEPES sodium salt, 1.0 NaH2PO4·H2O and 5.6 glucose. Preliminary experiments showed that this concentration of KCl produced a constriction of 60–70% of maximum diameter (within 10 min) that could be maintained for at least 120 min (data not shown). This degree of constriction was similar to that observed for trout coronary arterioles that developed spontaneous tone, and is considered in mammalian studies to be an appropriate level of spontaneous tone for similar-sized arterioles (Kuo et al., 1988, 1991; Hein and Kuo, 1998).
Recombinant interleukin-1β production and purification
Recombinant rainbow trout IL-1β was produced using Escherichia coli after cloning the cDNA encoding the predicted mature IL-1β peptide (Zou et al., 1999) into the pQE30 expression vector (Invitrogen, Carlsbad, CA, USA) (Hong et al., 2001). The recombinant protein was then purified using Ni-nitroloacetic acid metal affinity chromatography, checked for size and purity by SDS-PAGE and resuspended in buffer containing 20 mmol l−1 Tris-HCl (pH 8.0), 100 mmol l−1 NaCl, 100 mmol l−1 KCl, 5 mmol l−1 MgCl2, 5 mmol l−1 β-mercaptoethanol, 20% glycerol, 0.1% NP-40 (nonyl phenoxypolyethoxylethanol), 250 mmol l−1 imidazole. After storage at −80°C, the recombinant protein was further purified by passing it through a Detoxi-Gel endotoxin removing column (Thermo Scientific, Waltham, MA, USA), and size and purity verified again by SDS-PAGE (Ready Gels 10–20% Tris-HCl, Bio-Rad Laboratories Inc., Hercules, CA, USA). The final concentration of rIL-1β (mg ml−1) was determined by optical densitometry using the Bradford colorimetric method [Thermo Scientific Coomassie (Bradford) Protein Assay Kit 23200, Thermo Scientific, Waltham, MA, USA] and BSA (bovine serum albumin) as a standard.
Vasoactivity of recombinant interleukin-1β
Preliminary studies
Before testing the vasoactivity of rIL-1β in trout isolated coronary microvessels (i.e. performing concentration–response curves) a number of preliminary trials were performed to assess the vasoactivity of the buffer (i.e. vehicle) in which the protein was stored. The original buffer showed an extremely strong vasodilator effect (40–50% from resting ID), probably because of two components meant to maintain protein stability (i.e. imidazole and β-mercaptoethanol) (data not shown). Thus, a buffer exchange with a suitable, non-vasoactive protein stabilizer was necessary. The rIL-1β was subsequently dialyzed into microvessel saline solution (see above) containing 0.2 mmol l−1 dithiothreitol (DTT), for protein stability; this concentration of DTT showed very low vasoactivity (see Fig. 1). Dialysis was performed overnight at approximately 4–6°C using 10 mm Spectra/Por dialysis tubing (12,000–14,000 molecular mass cut off; Spectrum Laboratories Inc., Rancho Dominguez, CA, USA), and the concentration of rIL-1β was determined following the buffer exchange (i.e. post-dialysis) using the Bradford method. The rIL-1β in microvessel saline with 0.2 mmol l−1 DTT was then aliquoted into appropriate volumes (i.e. based on protein concentration post-dialysis) and kept at −80°C prior to use.
The effect of temperature
The concentration-dependent effect of rIL-1β on vessel vasoactivity was assessed by exposing vessels to increasing concentrations of rIL-1β (10−12 to 10−7 mol l−1) at 10 and 20°C, and measuring changes in the vessel's ID. To perform this experiment, we dissected two separate vessels from each ventricle, and randomized the order of test temperatures (10 vs 20°C) to ensure that the results were not affected by the post-dissection period. Briefly, a vessel was dissected free and cannulated as described above. After approximately 20 min, the vessel bath temperature was not changed (10°C) or was increased to 20°C over a period of 1 h by adjusting the temperature of the recirculating bath connected to the water jacket of the vessel bath (see above). After this 1 h period, the bath solution was replaced with fresh saline at the appropriate temperature, and the vessel was then allowed approximately 20 min to develop spontaneous tone. Vessels that did not regain tone were pre-contracted by replacing the saline in the bath with saline containing 50 mmol l−1 KCl.
Recombinant IL-1β was sequentially injected into the vessel bath in order to achieve the desired final concentration, with the volume injected (20–300 µl) varying depending on rIL-1β concentration after dialysis. Changes in the vessel's ID were measured 10 min after injection, and the next amount added to the bath immediately thereafter. The range of rIL-1β concentrations chosen for this study was based on previous mammalian and trout rIL-1β studies (Lin et al., 1997; Hong et al., 2001, 2003; Peddie et al., 2001). The highest concentration used was equivalent to the IL-1β dosage that induced heat-stroke-like cardiovascular symptoms in the rat (Lin et al., 1997), after taking into consideration trout blood volume (∼35 ml kg−1) (Kiceniuk and Jones, 1977; Olson, 2011).
Once the final measurement was complete on vessels at 10 or 20°C, the vessel was washed three times with physiological saline. The saline in the bath was then replaced with physiological saline without calcium (i.e. 0 mmol l−1 vs the normal 2.5 mmol l−1), and a single extraluminal injection of adenosine (final bath concentration 10−4 mol l−1) was given in order to obtain the vessel's maximum diameter. Adenosine was used because of its very strong vasodilator effect on trout coronary arterioles (Costa et al., 2015). The time required to obtain the vessel's maximum diameter was approximately 30 min. The zero-calcium physiological saline consisted of (in mmol l−1) 155.0 NaCl, 4.7 KCl, 1.99 MgSO4·7H2O, 3.0 HEPES acid, 7.0 HEPES sodium salt, 1.0 NaH2PO4·H2O and 5.6 glucose.
In order to assess whether any change in vessel responsiveness was due to the acute increase in temperature, a preliminary series of injections with increasing concentrations (10−9 to 10−4 mol l−1) of adenosine were made in vessels acutely warmed (present study, N=3), and compared with the response of vessels at 10°C (Costa et al., 2015). Similar to the trout's isolated coronary microvessels at 10°C, vessels at 20°C showed a very clear concentration-dependent vasodilation in response to adenosine; the maximum vasodilation was approximately 70% from resting ID compared with 60% at 10°C (data not shown).
Role of the endothelium, NO and prostaglandins in rIL-1β-induced dilation
Vessel denudation
In order to examine the role of the endothelium, and of potential endothelium-derived relaxation factors (i.e. NO and PGs) in rIL-1β-induced vasodilation, we needed to remove (denude) the endothelial cell layer of some coronary arterioles while leaving the vascular smooth muscle intact and functional. There are a number of studies where physical denudation of the vascular endothelium of fish vessels has been performed (Farrell and Johansen, 1995; Miller and Vanhoutte, 2000; Park et al., 2000). However, the vessels used in the latter studies were considerably larger than the ones used in the present research (80–150 µm external diameter), and the use of physical methods proved impossible. Thus, we decided on a chemical denudation approach that has been successfully used in mammalian microvascular studies (Ishizaka and Kuo, 1996; Gamperl et al., 2002).
Removal of the endothelial cells was achieved using the non-ionic detergent, 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate (CHAPS). Briefly, after setting the vessel to its in situ length and resting intraluminal pressure (see above), the valves connecting the micropipettes to the pressure reservoirs were closed. This allowed for access to the inside of the micropipette (through the opposite side of the pipette holder) and for the injection of 0.3 ml of CHAPS (0.4%) using P10 tubing (Intramedic Clay Adams, Becton Dickson) inserted as close as possible to the tip of the pipette. After 2 min of exposure to CHAPS, the vessel was carefully re-pressurized and a height differential of 4 cm of H2O between the two pressure reservoirs was used for 2 min to flush the CHAPS solution out of the vessels. After this ‘washing-out’ period, the pressure was equalized by bringing both pressure reservoirs back to 20 cm of H2O (∼15 mmHg) and the preparation set to no-flow conditions by closing the valve on the side that the CHAPS solution was injected.
The success of this method of denudation was evaluated by: (1) examining microvessel morphology/structure before and after denudation using transmission electron microscopy (TEM) (see below and Fig. 2A,B); and (2) exposing vessels to increasing concentrations of the endothelium-dependent calcium ionophore A23187, which causes a concentration-dependent relaxation of teleost blood vessels (Olson and Villa, 1991; Park et al., 2000; Jennings et al., 2004) and measuring changes in the vessel's ID at 10 min post-injection before and after denudation (Fig. 2C). To ensure that vascular smooth muscle function was not compromised by CHAPS treatment, the vessel's response to a single concentration of SNP (10−4 mol l−1) was also examined following the series of A23187 injections (10−9 to 10−4 mol l−1) (Fig. 2C).
Role of the endothelium
To examine the role of the vascular endothelium in the vasoactivity of rIL-1β, vessels were exposed to increasing concentrations of rIL-1β (10−12 to 10−7 mol l−1) before and after endothelial denudation. The physiological saline in the vessel bath was exchanged three times to remove any residual amounts of rIL-1β from the previous injection series (before CHAPS exposure) and the vessels were allowed up to 20 min to return to their initial resting diameter after denudation before the second series of rIL-1β injections was performed. Once the final measurement was complete (post-denudation), the vessels were again washed and pre-constricted with 50 mmol l−1 KCl. Then, a single injection of SNP (10−4 mol l−1) was made in order to ensure that smooth muscle vasodilator function was intact. Only vessels responsive (i.e. vasodilation) to SNP were included in the study. Finally, the vessels were washed three times with physiological saline, then the saline in the bath was replaced with physiological saline without calcium and a single injection of adenosine (10−4 mol l−1) performed to obtain the vessel's maximum diameter (see above).
Role of NO and prostaglandins
To elucidate the role of the endothelium-derived vasodilators NO and PGs in rIL-1β induced vasodilation, trout coronary microvessels were pre-incubated with the non-selective NO synthase inhibitor N5-(1-iminoethyl)-l-ornithine, dihydrochloride (l-NIO, 10−4 mol l−1) or the non-selective cyclooxygenase (COX) inhibitor indomethacin (INDO, 10−4 mol l−1) before being exposed to increasing concentrations of rIL-1β (see above). These experiments were first performed on intact vessels, and again on the same vessels after the endothelium had been removed using CHAPS. At the end of each isolated vessel trial, a single injection of SNP (10−4 mol l−1) was used to ensure that the vasodilator capacity of the smooth muscle was intact following denudation. All drugs were administered extraluminally and vessels incubated with each antagonist (i.e. l-NIO or INDO) for at least 30 min before exposure to rIL-1β.
Role of NO and prostaglandins in calcium ionophore A23187-induced dilation
To elucidate the role of NO and PGs in vasodilation induced by the endothelium-derived calcium ionophore A23187, microvessels were pre-incubated with l-NIO (10−4 mol l−1) or INDO (10−4 mol l−1) and then exposed to increasing concentrations of A23187 (see above). These experiments were first performed on intact vessels, and again on the same vessels after the endothelium had been removed using CHAPS (0.4%). At the end of each isolated vessel trial, a single injection of SNP (10−4 mol l−1) was used to ensure that the vasodilator capacity of the smooth muscle was intact following denudation. Only vessels responsive (i.e. vasodilation) to SNP were included in the study. All drugs were administered extraluminally and vessels incubated with each antagonist (i.e. l-NIO and INDO) for at least 30 min before exposure to A23187.
Vasoactive agents and chemicals
The purinergic receptor agonist adenosine, the NO donor SNP, the endothelium-dependent vasodilator calcium ionophore A23187 and the non-ionic detergent CHAPS were all purchased from Sigma-Aldrich (Oakville, ON, Canada), whereas INDO and l-NIO were purchased from Cedarlane Corp. (Burlington, ON, Canada). All solutions were made fresh and kept on ice until needed. Adenosine and SNP were dissolved in the physiological saline solution, whereas A23187, INDO and l-NIO were dissolved in 99% ethanol. Note that a series of preliminary (sham) injections (with ethanol dissolved in saline at appropriate concentrations) was performed to ensure that the vehicle had no vasoactivity. These injections did not result in any measureable (i.e. ≤0.5%) changes in vessel ID (data not shown).
Transmission electron microscopy
Vessels used for TEM images were subjected to a similar protocol as vessels used in other parts of the study with the exception of exposure to A23187 or SNP. Vessels segments were then placed in Karnovsky fixative for 24 h and stored in 0.1 mol l−1 Na cacodylate buffer until processed. Vessels were osmicated and then dehydrated, using graded alcohol and acetone, followed by infiltration with EPON resin, embedded in embedding molds and polymerized overnight at 80°C. Blocks were initially trimmed and 0.5 µm sections cut on a Reichert ultra-cut S microtome. Sections were stained using toluidine blue and examine under a light microscope for correct orientation and depth for cutting ultra-thin sections. Thin sections were cut at 85 nm (mounted on a 300 mesh copper grid) and stained with uranyl acetate followed by lead citrate stain before image capture.
Data analysis
All measurements of vessel ID were normalized to the resting ID at 20 cmH2O luminal pressure (i.e. after vessels developed basal tone or were pre-constricted with 50 mmol l−1 KCl). Repeated-measures (RM) two-way ANOVAs, followed by Tukey's post hoc tests, were used to examine the effects of rIL-1β or A23187 concentration on microvessel ID under a number of conditions: (1) 10 vs 20°C (rIL-1β only); (2) intact versus denuded vessels; (3) and l-NIO or INDO alone, or in combination, with denudation. When the interaction between the two explanatory variables in a particular analysis was significant, separate RM one-way ANOVAs were used to test for differences in vessel ID within a treatment due to rIL-1β or A23187 concentration, while t-tests were used to identify differences in the effects of each treatment at a particular concentration of rIL-1β or A23187. A t-test was also used to test for smooth muscle reactivity in denuded vessels (endothelium removed) by comparing the vessel's ID after the last injection of either rIL-1β (10−7 mol l−1) or A23187 (10−4 mol l−1) and SNP (10−4 mol l−1).
All statistical analyses were performed using Sigma Plot 12.0 (Systat Software Inc. San Jose, CA) with P<0.05 as the level of statistical significance. All values presented in the text, tables and figures are expressed as means±1 s.e.m.
Acknowledgements
We thank Wenjuan Xu and Xin Xu (Hein Lab) for their excellent instruction in microvessel techniques, Dr David Heeley (Biochemistry Department, MUN) for assistance with selecting an appropriate (non-vasoactive) protein stabilizer, Dr Zou (SFIRC, Aberdeen) for advice with regards to the use of rIL-1β and Gordon Nash (Gamperl Lab) for his assistance with the rIL-1β purification protocol.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
I.A.S.F.C. was the main contributor to the conception, design and execution of the experiments, the interpretation of the findings, as well as the drafting and revision of the article. A.K.G. made a significant contribution to the conception and design of the experiments, the interpretation of the findings and revision of the article. T.W.H. contributed to the design of the experiments, the interpretation of the findings and revision of the article, while C.J.S. contributed to the design of the experiments and revision of the article.
Funding
This research was supported by a Natural Sciences and Engineering Research Council of Canada Discovery Grant [RGPIN249926] and Accelerator Supplement [RGPAS412325-2011] to A.K.G. a National Institutes of Health Grant [EY018420] to T.W.H., and a doctoral fellowship from Fundação para a Ciência e a Tecnologia, Portugal [SFRH/BD/27497/2006] to I.A.S.F.C. Deposited in PMC for release after 12 months.
References
- Amelio D., Garofalo F., Brunelli E., Loong A. M., Wong W. P., Ip Y. K., Tota B. and Cerra M. C. (2008). Differential NOS expression in freshwater and aestivating Protopterus dolloi (lungfish): heart vs kidney readjustments. Nitric Oxide 18, 1-10. 10.1016/j.niox.2007.10.004 [DOI] [PubMed] [Google Scholar]
- Amelio D., Garofalo F., Capria C., Tota B. and Imbrogno S. (2013). Effects of temperature on the nitric oxide-dependent modulation of the Frank-Starling mechanism: the fish heart as a case study. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 164, 356-362. 10.1016/j.cbpa.2012.10.037 [DOI] [PubMed] [Google Scholar]
- Andreakis N., D'Aniello S., Albalat R., Patti F. P., Garcia-Fernàndez J., Procaccini G., Sordino P. and Palumbo A. (2011). Evolution of the nitric oxide synthase family in metazoans. Mol. Biol. Evol. 28, 163-179. 10.1093/molbev/msq179 [DOI] [PubMed] [Google Scholar]
- Bataillard A. and Sassard J. (1994). Cardiovascular effects of human recombinant interleukin-1β in conscious rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 266, R1148-R1153. [DOI] [PubMed] [Google Scholar]
- Beasley D. and McGuiggin M. E. (1995). Interleukin 1 induces prostacyclin-dependent increases in cyclic AMP production and does not affect cyclic GMP production in human vascular smooth muscle cells. Cytokine 7, 417-426. 10.1006/cyto.1995.0057 [DOI] [PubMed] [Google Scholar]
- Berne R. M. and Levy M. N. (1997). Cardiovascular Physiology, 7th edn. pp. 171–194. Michigan: Mosby-Year Book Inc. [Google Scholar]
- Buonocore F., Forlenza M., Randelli E., Benedetti S., Bossù P., Meloni S., Secombes C. J., Mazzini M. and Scapigliati G. (2005). Biological activity of sea bass Dicentrarchus labrax L. recombinant interleukin-1b. Mar. Biotechnol. 7, 609-617. 10.1007/s10126-004-5131-5 [DOI] [PubMed] [Google Scholar]
- Chilian W. M., Eastham C. L. and Marcus M. L. (1986). Microvascular distribution of coronary vascular resistance in beating left ventricle. Am. J. Physiol. Heart Circ. Physiol. 251, H779-H788. [DOI] [PubMed] [Google Scholar]
- Chiu W.-T., Kao T.-Y. and Lin M.-T. (1995). Interleukin-1 receptor antagonist increases survival in rat heatstroke by reducing hypothalamic serotonin release. Neurosci. Lett. 202, 33-36. 10.1016/0304-3940(95)12203-6 [DOI] [PubMed] [Google Scholar]
- Combes A., Frye C. S., Lemster B. H., Brooks S. S., Watkins S. C., Feldman A. M. and McTiernan C. F. (2002). Chronic exposure to interleukin 1β induces a delayed and reversible alteration in excitation-contraction coupling of cultured cardiomyocytes. Pflugers Arch. 445, 246-256. 10.1007/s00424-002-0921-y [DOI] [PubMed] [Google Scholar]
- Costa I. A. S. F., Hein T. W. and Gamperl A. K. (2015). Cold-acclimation leads to differential regulation of the steelhead trout (Oncorhynchus mykiss) coronary microcirculation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 308, R743-R754. 10.1152/ajpregu.00353.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. (1997). Interleukin-1. Cytokine Growth Factor Rev. 8, 253-265. 10.1016/S1359-6101(97)00023-3 [DOI] [PubMed] [Google Scholar]
- Duling B. R., Gore R. W., Dacey R. G. Jr and Damon D. N. (1981). Methods for isolation canulation and in vitro study of single microvessels. Am. J. Physiol. Heart Circ. Physiol. 241, H108-H116. [DOI] [PubMed] [Google Scholar]
- Duncan D. J., Yang Z., Hopkins P. M., Steele D. S. and Harrison S. M. (2010). TNF-α and IL-1β increase Ca2+ leak from the sarcoplasmic reticulum and susceptibility to arrhythmia in rat ventricular myocytes. Cell Calcium 47, 378-386. 10.1016/j.ceca.2010.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fago A., Jensen F. B., Tota B., Feelisch M., Olson K. R., Helbo S., Lefevre S., Mancardi D., Palumbo A., Sandvik G. K. et al. (2012). Integrating nitric oxide, nitrite and hydrogen sulfide signaling in the physiological adaptations to hypoxia: a comparative approach. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 162, 1-6. 10.1016/j.cbpa.2012.01.011 [DOI] [PubMed] [Google Scholar]
- Farrell A. P. (2009). Environment, antecedents and climate change: lessons from the study of temperature physiology and river migration of salmonids. J. Exp. Biol. 212, 3771-3780. 10.1242/jeb.023671 [DOI] [PubMed] [Google Scholar]
- Farrell A. P. and Johansen J. A. (1995). Vasoactivity of the coronary artery of rainbow trout, steelhead trout, and dogfish: lack of support for non-prostanoid endothelium-derived relaxation factors. Can. J. Zool. 73, 1899-1911. 10.1139/z95-223 [DOI] [Google Scholar]
- Farrell A. P., Gamperl A. K., Hicks J. M. T., Shiels H. A. and Jain K. E. (1996). Maximum cardiac performance of rainbow trout (Oncorhynchus mykiss) at temperatures approaching their upper lethal limit. J. Exp. Biol. 199, 663-672. [DOI] [PubMed] [Google Scholar]
- Farrell A. P., Eliason E. J., Sandblom E. and Clark T. D. (2009). Fish cardiorespiratory physiology in an era of climate change. Can. J. Zool. 87, 835-851. 10.1139/Z09-092 [DOI] [Google Scholar]
- Fritsche R., Schwerte T. and Pelster B. (2000). Nitric oxide and vascular reactivity in developing zebrafish Danio rerio. Am. J. Physiol. Regul. Integr. Comp. Physiol. 279, R2200-R2207. [DOI] [PubMed] [Google Scholar]
- Gamperl A. K., Axelsson M. and Farrell A. P. (1995). Effects of swimming and environmental hypoxia on coronary blood flow in rainbow trout. Am. J. Physiol. Regul. Integr. Comp. Physiol. 269, R1258-R1266. [DOI] [PubMed] [Google Scholar]
- Gamperl A. K., Hein T. W., Kuo L. and Cason B. A. (2002). Isoflurane-induced dilation of porcine coronary microvessels is endothelium dependent and inhibited by glibenclamide. Anesthesiology 96, 1465-1471. 10.1097/00000542-200206000-00028 [DOI] [PubMed] [Google Scholar]
- Gamperl A. K., Swafford B. L. and Rodnick K. J. (2011). Elevated temperature, per se, does not limit the ability of rainbow trout to increase stroke volume. J. Thermal. Biol. 36, 7-14. 10.1016/j.jtherbio.2010.08.007 [DOI] [Google Scholar]
- Gollock M. J., Currie S., Petersen L. H. and Gamperl A. K. (2006). Cardiovascular and haematological responses of Atlantic cod (Gadus morhua) to acute temperature increase. J. Exp. Biol. 209, 2961-2970. 10.1242/jeb.02319 [DOI] [PubMed] [Google Scholar]
- González-Domenech C. M. and Muñoz-Chápuli R. (2010). Molecular evolution of nitric oxide synthases in metazoans. Comp. Biochem. Physiol. D 5, 295-301. 10.1016/j.cbd.2010.08.004 [DOI] [PubMed] [Google Scholar]
- Graff G. R. and Gozal D. (1999). Cardiorespiratory responses to interleukin-1β in adult rats: role of nitric oxide, eicosanoids and glucocorticoids. Arch. Physiol. Biochem. 107, 97-112. 10.1076/apab.107.2.97.4344 [DOI] [PubMed] [Google Scholar]
- Gräns A., Rosengren M., Niklasson L. and Axelsson M. (2012). Behavioural fever boosts the inflammatory response in rainbow trout Oncorhynchus mykiss. J. Fish Biol. 81, 1111-1117. 10.1111/j.1095-8649.2012.03333.x [DOI] [PubMed] [Google Scholar]
- Hein T. W. and Kuo L. (1998). LDLs impair vasomotor function of the coronary microcirculation: role of superoxide anions. Circ. Res. 83, 404-414. 10.1161/01.RES.83.4.404 [DOI] [PubMed] [Google Scholar]
- Helwig B. G. and Leon L. R. (2011). Tissue and circulating expression of IL-1 family members following heat stroke. Physiol. Genomics 43, 1096-1104. 10.1152/physiolgenomics.00076.2011 [DOI] [PubMed] [Google Scholar]
- Hirafuji M., Machida T., Tsunoda M., Miyamoto A. and Minami A. (2002). Docosahexaenoic acid potentiates interleukin-1beta induction of nitric oxide synthase through mechanism involving p44/42 MAPK activation in rat vascular smooth muscle cells. Br. J. Pharmacol. 136, 613-619. 10.1038/sj.bjp.0704768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J. W., Pottinger T. G. and Secombes C. J. (2002). Recombinant interleukin-1 beta activates the hypothalamic-pituitary-interrenal axis in rainbow trout, Oncorhynchus mykiss. J. Endocrinol. 175, 261-267. 10.1677/joe.0.1750261 [DOI] [PubMed] [Google Scholar]
- Hong S., Zou J., Crampe M., Peddie S., Scapigliati G., Bols N., Cunningham C. and Secombes C. J. (2001). The production and bioactivity testing of rainbow trout (Oncorhynchus mykiss) recombinant IL-1β. Vet. Immunol. Immunopathol. 81, 1-14. 10.1016/S0165-2427(01)00328-2 [DOI] [PubMed] [Google Scholar]
- Hong S., Peddie S., Campos-Pérez J. J., Zou J. and Secombes C. J. (2003). The effect of intraperitoneally administered recombinant IL-1β on immune parameters and resistance to Aeromonas salmonicida in the rainbow trout (Oncorhynchus mykiss). Dev. Comp. Immunol. 27, 801-812. 10.1016/S0145-305X(03)00056-9 [DOI] [PubMed] [Google Scholar]
- Imbrogno S., Capria C., Tota B. and Jensen F. B. (2014). Nitric oxide improves the hemodynamic performance of the hypoxic goldfish (Carassius auratus) heart. Nitric Oxide 42, 24-31. 10.1016/j.niox.2014.08.012 [DOI] [PubMed] [Google Scholar]
- Ishizaka H. and Kuo L. (1996). Acidosis-induced coronary arteriolar dilation is mediated by ATP-sensitive potassium channels in vascular smooth muscle. Circ. Res. 78, 50-57. 10.1161/01.RES.78.1.50 [DOI] [PubMed] [Google Scholar]
- Jedrzejowska-Szypulka H., Larysz-Brysz M., Kukla M., Snietura M. and Lewin-Kowalik J. (2009). Neutralization of interleukin-1β reduces vasospasm and alters cerebral blood vessel density following experimental subarachnoid hemorrhage in rats. Curr. Neurovasc. Res. 6, 95-103. 10.2174/156720209788185669 [DOI] [PubMed] [Google Scholar]
- Jennings B. L., Broughton B. R. S. and Donald J. A. (2004). Nitric oxide control of the dorsal aorta and the intestinal vein of the Australian short-finned eel Anguilla australis. J. Exp. Biol. 207, 1295-1303. 10.1242/jeb.00883 [DOI] [PubMed] [Google Scholar]
- Kågström J. and Holmgren S. (1997). VIP-induced relaxation of small arteries of the rainbow trout, Oncorhynchus mykiss, involves prostaglandin synthesis but not nitric oxide. J. Auton. Nerv. Syst. 63, 68-76. 10.1016/S0165-1838(96)00138-5 [DOI] [PubMed] [Google Scholar]
- Kanno K., Hirata Y., Imai T., Iwashina M. and Marumo F. (1994). Regulation of inducible nitric oxide synthase gene by interleukin-1β in rat vascular endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 267, H2318-H2324. [DOI] [PubMed] [Google Scholar]
- Kiceniuk J. W. and Jones D. R. (1977). The oxygen transport system in trout (Salmo gairdneri) during sustained exercise. J. Exp. Biol. 69, 247-260. [Google Scholar]
- Kuo L., Davis M. J. and Chilian W. M. (1988). Myogenic activity in isolated subepicardial and subendocardial coronary arterioles. Am. J. Physiol. Heart Circ. Physiol. 255, H1558-H1562. [DOI] [PubMed] [Google Scholar]
- Kuo L., Chilian W. M. and Davis M. J. (1991). Interaction of pressure- and flow-induced responses in porcine coronary resistance vessels. Am. J. Physiol. Heart Circ. Physiol. 261, H1706-H1715. [DOI] [PubMed] [Google Scholar]
- Leon L. R. (2006). The thermoregulatory consequences of heat stroke: are cytokines involved? J. Therm. Biol. 31, 67-81. 10.1016/j.jtherbio.2005.11.023 [DOI] [Google Scholar]
- Lepiller S., Franche N., Solary E., Chluba J. and Laurens V. (2009). Comparative analysis of zebrafish nos2a and nos2b genes. Gene 445, 58-65. 10.1016/j.gene.2009.05.016 [DOI] [PubMed] [Google Scholar]
- Lin M. T., Liu H. H. and Yang Y. L. (1997). Involvement of interleukin-1 receptor mechanisms in development of arterial hypotension in rat heatstroke. Am. J. Physiol. Heart Circ. Physiol. 273, 2072-2077. [DOI] [PubMed] [Google Scholar]
- Lüscher T. F., Yang Z., Kiowski W., Linder L., Dohi Y. and Diederich D. (1992). Endothelin-induced vasoconstriction and calcium antagonists. J. Hum. Hypertens 6 Suppl. 2, 3-8. [PubMed] [Google Scholar]
- Maher C. O., Anderson R. E., Martin H. S., McClelland R. L. and Meyer F. B. (2003). Interleukin-1β and adverse effects on cerebral blood flow during long-term global hypoperfusion. J. Neurosurg. 99, 907-912. 10.3171/jns.2003.99.5.0907 [DOI] [PubMed] [Google Scholar]
- Marceau F., Petitclerc E., DeBlois D., Pradelles P. and Poubelle P. E. (1991). Human interleukin-1 induces a rapid relaxation of the rabbit isolated mesenteric artery. Br. J. Pharmacol. 103, 1367-1372. 10.1111/j.1476-5381.1991.tb09795.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKean D. J., Huntoon C. and Bell M. (1994). Ligand-induced desensitization of interleukin 1 receptor-initiated intracellular signaling events in T helper lymphocytes. J. Exp. Med. 180, 1321-1328. 10.1084/jem.180.4.1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill B. and Perry S. F. (2006). The interactive effects of hypoxia and nitric oxide on catecholamine secretion in rainbow trout (Oncorhynchus mykiss). J. Exp. Biol. 209, 4214-4223. 10.1242/jeb.02519 [DOI] [PubMed] [Google Scholar]
- Miller V. M. and Vanhoutte P. M. (2000). Prostaglandins but not nitric oxide are endothelium-derived relaxing factors in the trout aorta. Acta Pharmacol. Sin. 21, 871-876. [PubMed] [Google Scholar]
- Mollace V., Muscoli C., Masini E., Cuzzocrea S. and Salvemini D. (2005). Modulation of prostaglandin biosynthesis by nitric oxide and nitric oxide donors. Pharmacol. Rev. 57, 217-252. 10.1124/pr.57.2.1 [DOI] [PubMed] [Google Scholar]
- Mustafa T. and Agnisola C. (1998). Vasoactivity of adenosine in the trout (Oncorhynchus mykiss) coronary system: involvement of nitric oxide and interaction with noradrenaline. J. Exp. Biol. 201, 3075-3083. [DOI] [PubMed] [Google Scholar]
- Mustafa T., Agnisola C. and Hansen J. K. (1997). Evidence for NO-dependent vasodilation in the trout (Oncorhynchus mykiss) coronary system. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 167, 98-104. 10.1007/s003600050052 [DOI] [Google Scholar]
- Nilsson G. E. and Söderström V. (1997). Comparative aspects on nitric oxide in brain and its role as a cerebral vasodilator. Comp. Biochem. Physiol. A Physiol. 118, 949-958. 10.1016/S0300-9629(97)00024-8 [DOI] [PubMed] [Google Scholar]
- Oliveira A. P. S., Lunardi C. N., Rodrigues G. J. and Bendhack L. M. (2009). Relaxation induced by calcium ionophore is impaired in carotid arteries from 2K-1C rats due to failed effect of nitric oxide on the smooth muscle cells. Vasc. Pharmacol. 50, 153-159. 10.1016/j.vph.2008.11.010 [DOI] [PubMed] [Google Scholar]
- Olson K. R. (2011). Physiology of resistance vessels. In Encyclopedia of Fish Physiology: From Genome to Environment (ed. Farrell A. P.), pp. 1104-1110. San Diego, CA: Academic Press. [Google Scholar]
- Olson K. R. and Donald J. A. (2009). Nervous control of circulation - the role of gasotransmitters, NO, CO, and H2S. Acta Histochem. 111, 244-256. 10.1016/j.acthis.2008.11.004 [DOI] [PubMed] [Google Scholar]
- Olson K. R. and Villa J. (1991). Evidence against nonprostanoid endothelium-derived relaxing factor(s) is trout vessels. Am. J. Physiol. Regul. Integr. Comp. Physiol. 260, R925-R933. [DOI] [PubMed] [Google Scholar]
- Olson K. R., Duff D. W., Farrell A. P., Keen J., Kellogg M. D., Kullman D. and Villa J. (1991). Cardiovascular effects of endothelin in trout. Am. J. Physiol. Heart Circ. Physiol. 260, H1214-H1223. [DOI] [PubMed] [Google Scholar]
- Osuka K., Suzuki Y., Watanabe Y., Dogan A., Takayasu M., Shibuya M. and Yoshida J. (1997). Vasodilator effects on canine basilar artery induced by intracisternal interleukin-1β. J. Cereb. Blood Flow Metab. 17, 1337-1345. 10.1097/00004647-199712000-00009 [DOI] [PubMed] [Google Scholar]
- Park K. H., Kim K.-H., Choi M.-S., Choi S.-H., Yoon J.-M. and Kim Y.-G. (2000). Cyclooxygenase-derived products, rather than nitric oxide, are endothelium-derived relaxing factor(s) in the ventral aorta of carp (Cyprinus carpio). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 127, 89-98. 10.1016/S0305-0491(00)00264-9 [DOI] [PubMed] [Google Scholar]
- Peddie S., Zou J., Cunningham C. and Secombes C. J. (2001). Rainbow trout (Oncorhynchus mykiss) recombinant IL-1beta and derived peptides induce migration of head-kidney leucocytes in vitro. Fish Shellfish Immun. 11, 697-709. 10.1006/fsim.2001.0348 [DOI] [PubMed] [Google Scholar]
- Peddie S., Zou J. and Secombes C. J. (2002). A biologically active IL-1beta derived peptide stimulates phagocytosis and bactericidal activity in rainbow trout, Oncorhynchus mykiss (Walbaum), head kidney leucocytes in vitro. J. Fish Dis. 25, 351-360. 10.1046/j.1365-2761.2002.00380.x [DOI] [Google Scholar]
- Pérez-Casanova J. C., Rise M. L., Dixon B., Afonso L. O. B., Hall J. R., Johnson S. C. and Gamperl A. K. (2008). The immune and stress responses of Atlantic cod to long-term increases in water temperature. Fish Shellfish Immun. 24, 600-609. 10.1016/j.fsi.2008.01.012 [DOI] [PubMed] [Google Scholar]
- Perry S. F. and Reid S. G. (2002). Cardiorespiratory adjustments during hypercarbia in rainbow trout Oncorhynchus mykiss are initiated by external CO2 receptors on the first gill arch. J. Exp. Biol. 205, 3357-3365. [DOI] [PubMed] [Google Scholar]
- Proescholdt M. G., Chakravarty S., Foster J. A., Foti S. B., Briley E. M. and Herkenham H. (2002). Intracerebroventricular but not intravenous interleukin-1β induces widespread vascular-mediated leukocyte infiltration and immune signal mRNA expression followed by brain-wide glial activation. Neuroscience 112, 731-749. 10.1016/S0306-4522(02)00048-9 [DOI] [PubMed] [Google Scholar]
- Radin M. J., Holycross B. J., Dumitrescu C., Kelley R. and Altschuld R. A. (2008). Leptin modulates the negative inotropic effect of interleukin-1β in cardiac myocytes. Mol. Cell. Biochem. 315, 179-184. 10.1007/s11010-008-9805-6 [DOI] [PubMed] [Google Scholar]
- Rossi V., Breviario F., Ghezzi P., Dejana E. and Mantovani A. (1985). Prostacyclin synthesis induced in vascular cells by interleukin-1. Science 229, 174-176. 10.1126/science.2409598 [DOI] [PubMed] [Google Scholar]
- Salvemini D., Misko T. P., Masferrer J. L., Seibert K., Currie M. G. and Needleman P. (1993). Nitric oxide activates cyclooxygenase enzymes. Proc. Natl. Acad. Sci. USA 90, 7240-7244. 10.1073/pnas.90.15.7240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiltz J. C. and Sawchenko P. E. (2002). Distinct brain vascular cell types manifest inducible cyclooxygenase expression as a function of the strength and nature of immune insults. J. Neurosci. 22, 5606-5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Feletou M., Ku D. D., Man R. Y. K. and Vanhoutte P. M. (2007). The calcium ionophore A23187 induces endothelium-dependent contractions in femoral arteries from rats with streptozotocin-induced diabetes. Br. J. Pharmacol. 150, 624-632. 10.1038/sj.bjp.0706999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata M., Parfenova H., Zuckerman S. L., Seyer J. M., Krueger J. M. and Leffler C. W. (1996). Interleukin-1β peptides induce cerebral pial arteriolar dilation in anesthetized newborn pigs. Am. J. Physiol. Regul. Integr. Comp. Physiol. 270, R1044-R1050. [DOI] [PubMed] [Google Scholar]
- Shimokawa H., Ito A., Fukumoto Y., Kadokami T., Nakaike R., Skata M., Takayanagi T., Egashira K. and Takeshita A. (1996). Chronic treatment with interleukin-1β induces coronary intimal lesions and vasospastic responses in pigs in vivo: the role of platelet-derived growth factor. J. Clin. Invest. 97, 769-776. 10.1172/JCI118476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi H., Tanaka Y., Hirano H., Tanaka H. and Shigenobu K. (1999). Evidence for a contribution of store-operated Ca2+ channels to NO-mediated endothelium-dependent relaxation of guinea-pig aorta in response to a Ca2+ ionophore, A23187. Nauyn-Schmiedeberg's Arch. Pharmacol. 360, 69-79. 10.1007/s002109900033 [DOI] [PubMed] [Google Scholar]
- Tsujino M., Hirata Y., Imai T., Kanno K., Eguchi S., Ito H. and Marumo F. (1994). Induction of nitric oxide synthase gene by interleukin-1β in cultured rat cardiocytes. Circulation 90, 375-383. 10.1161/01.CIR.90.1.375 [DOI] [PubMed] [Google Scholar]
- Vassalle C., Domenici C., Lubrano V. and L'Abbate A. (2003). Interaction between nitric oxide and cyclooxygenase pathways in endothelial cells. J. Vasc. Res. 40, 491-499. 10.1159/000074550 [DOI] [PubMed] [Google Scholar]
- Wang W.-S., Hung S.-W., Lin Y.-H., Tu C.-Y., Wong M.-L., Chiou S.-H. and Shieh M.-T. (2007). Purification and localization of nitric oxide synthases from hybrid tilapia (Nile tilapia×Mozambique tilapia). J. Aquat. Anim. Health 19, 168-178. 10.1577/H06-022.1 [DOI] [PubMed] [Google Scholar]
- Warner S. J., Auger K. R. and Libby P. (1987a). Human interleukin 1 induces interleukin 1 gene expression in human vascular smooth muscle cells. J. Exp. Med. 165, 1316-1331. 10.1084/jem.165.5.1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner S. J. C., Auger K. R. and Libby P. (1987b). Interleukin 1 induces interleukin 1. II. Recombinant human interleukin 1 induces interleukin 1 production by adult human vascular endothelial cells. J. Immunol. 139, 1911-1917. [PubMed] [Google Scholar]
- Zou J., Grabowski P. S., Cunningham C. and Secombes C. J. (1999). Molecular cloning of interleukin-1β from rainbow trout Oncorhynchus mykiss reveals no evidence of an ICE cut site. Cytokine 11, 552-560. 10.1006/cyto.1998.0470 [DOI] [PubMed] [Google Scholar]
- Zou J., Holland J., Pleguezuelos O., Cunningham C. and Secombes C. J. (2000). Factors influencing the expression of interleukin-1β in cultured rainbow trout (Oncorhynchus mykiss) leucocytes. Dev. Comp. Immunol. 24, 575-582. 10.1016/S0145-305X(99)00085-3 [DOI] [PubMed] [Google Scholar]