ABSTRACT
Genetically based modifications of hemoglobin (Hb) function that increase blood–O2 affinity are hallmarks of hypoxia adaptation in vertebrates. Among mammals, felid Hbs are unusual in that they have low intrinsic O2 affinities and reduced sensitivities to the allosteric cofactor 2,3-diphosphoglycerate (DPG). This combination of features compromises the acclimatization capacity of blood–O2 affinity and has led to the hypothesis that felids have a restricted physiological niche breadth relative to other mammals. In seeming defiance of this conjecture, the snow leopard (Panthera uncia) has an extraordinarily broad elevational distribution and occurs at elevations above 6000 m in the Himalayas. Here, we characterized structural and functional variation of big cat Hbs and investigated molecular mechanisms of Hb adaptation and allosteric regulation that may contribute to the extreme hypoxia tolerance of the snow leopard. Experiments revealed that purified Hbs from snow leopard and African lion exhibited equally low O2 affinities and DPG sensitivities. Both properties are primarily attributable to a single amino acid substitution, β2His→Phe, which occurred in the common ancestor of Felidae. Given the low O2 affinity and reduced regulatory capacity of feline Hbs, the extreme hypoxia tolerance of snow leopards must be attributable to compensatory modifications of other steps in the O2-transport pathway.
KEY WORDS: Felidae, Oxygen affinity, Amino acid substitution, Blood-oxygen transport, Allosteric regulation
Highlighted Article: Snow leopards are high-altitude specialists that survive and function in low-oxygen conditions but, surprisingly, they possess characteristically feline hemoglobins with low-oxygen affinities and diminished capacities for allosteric regulatory control.
INTRODUCTION
The O2-transport properties of vertebrate red blood cells exhibit a high degree of plasticity in adjusting to changes in metabolic demands and/or environmental O2 availability (Nikinmaa, 1990; Weber and Fago, 2004). At high altitude, O2 uptake may be compromised by the low ambient O2 tension (PO2) (Bouverot, 1985; Weber, 2007). As a general rule, the tolerance to low PO2 at high altitude is associated with an increase in hemoglobin (Hb)–O2 affinity that raises arterial O2 saturation (Storz et al., 2010a; Weber, 2007). In most mammals, reversible changes in Hb–O2 affinity are mediated by regulatory adjustments in the erythrocytic concentration of the organic phosphate 2,3-diphosphoglycerate (DPG) (Mairbaurl and Weber, 2012; Weber, 2007), a potent allosteric regulator of Hb–O2 affinity (Benesch and Benesch, 1967; Bunn, 1980, 1971). In human Hb, negatively charged DPG electrostatically binds in the symmetric cationic cleft between the paired β-subunits, including strong interactions with positively charged β82Lys and β2His and weaker interactions with the β-chain N-termini and β143His (Richard et al., 1993). As this positively charged cleft is accessible for DPG binding in the low-affinity T quaternary structure of the tetrameric (α2β2) Hb but not in the high-affinity R structure, changes in red blood cell DPG levels alter Hb–O2 affinity by shifting the T–R allosteric equilibrium of the Hb (Weber, 2007). Although Hb–O2 affinity is also allosterically regulated by chloride ions (Cl−), the Cl− concentration does not change appreciably in red blood cells (Nikinmaa, 1990). In addition to changes in the concentration of allosteric cofactors, adaptation of mammalian species to high altitude often involves genetically based modifications of the Hb structure that increase Hb's intrinsic O2 affinity or hamper the protein's ability to bind DPG or Cl− (Storz and Moriyama, 2008; Weber and Fago, 2004; Weber, 2007).
Intriguingly, the red blood cells of two mammalian lineages, ruminant artiodactyls and carnivores in the suborder Feliformia (cats, hyenas, mongooses, civets and allies) contain exceedingly low concentrations of DPG (<1.0 mmol l−1 versus 4–10 mmol l−1 in other mammals) (Bunn et al., 1974; Scott et al., 1977). Moreover, previous studies indicate that the adult Hbs of both groups have independently evolved dramatically reduced O2 affinities and suppressed sensitivities to DPG (Bunn et al., 1974; Bunn, 1980, 1971; Scott et al., 1976, 1977; Taketa, 1973), features that may limit the capacity to regulate Hb–O2 affinity.
In mammals with DPG-insensitive Hbs, the reduced plasticity in blood–O2 affinity may limit physiological niche breadth (Bunn, 1980; Kay, 1977; Scott et al., 1976, 1977). Those taxa that have secondarily lost the capacity to regulate Hb–O2 affinity through changes in erythrocytic DPG concentration ‘…are restricted to a much narrower range of possible habitats and/or life-styles', according to Kay (1977). Consistent with this idea, domestic cats are notoriously intolerant of environmental hypoxia (Campbell, 1927; Reeves et al., 1963).
As a denizen of the Himalayan alpine, the snow leopard, Panthera uncia, defies conventional wisdom about restricted physiological tolerances among cats in general, and dispels the notion that susceptibility to hypoxic stress is a characteristic feline trait. Snow leopards are most common above the tree line at elevations between 3500 and 5000 m (Sunquist and Sunquist, 2002), although they have been recorded at elevations above 6000 m (where PO2 is less than 50% of the sea level value). However, they are not restricted to these zones; in parts of their range including the Gobi Desert of Mongolia, they also occur at low elevations (1500–2000 m) (Janečka et al., 2011). In the Himalaya, the home ranges of individual snow leopards can span >2000 m in elevation. The remarkably broad elevational distribution of snow leopards and their ability to tolerate severe ambient hypoxia prompt questions about possible mechanisms of biochemical adaptation in O2 transport. Here, we addressed these questions by determining the amino acid sequences of the α- and β-subunits of adult Hbs from a number of species of the cat family (Felidae) and by comparing functional properties of snow leopard Hbs with those of a representative lowland species of the same genus, the African lion, Panthera leo, to investigate possible mechanisms of biochemical adaptation to high-altitude hypoxia.
List of abbreviations
- DPG
2,3-diphosphoglycerate
- Hb
hemoglobin
- IEF
isoelectric focusing
- n
cooperativity (Hill) coefficient
- P50
O2 tension at half-saturation
- PO2
O2 tension
RESULTS
Low levels of structural variation among felid Hbs
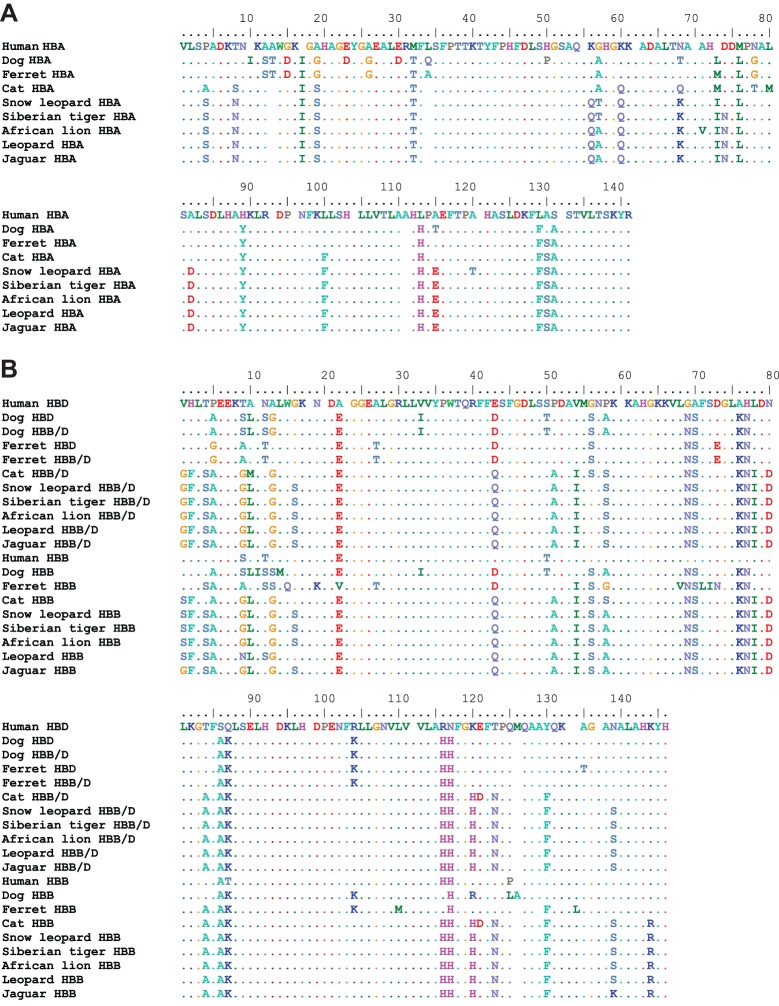
We examined structural variation in the adult-expressed Hb isoforms of five big cat species in the genus Panthera: snow leopard, P. uncia (Schreber 1775); African lion, P. leo (Linnaeus 1758); tiger, P. tigris (Linnaeus 1758); leopard, P. pardus (Linnaeus 1758); and jaguar, P. onca (Linnaeus 1758). Our sequencing results revealed that all five big cat species share the same complement of four adult-expressed Hb genes found in the domestic cat (Gaudry et al., 2014), including a tandemly linked pair of α-globin genes that are identical in amino acid sequence, and a tandemly linked pair of β-type globin genes (HBB/D and HBB) that are distinguished from one another by four to eight amino acid substitutions (Fig. 1). The β-type HBB/D gene is a chimeric fusion gene, which originated via unequal crossing over between the tandemly linked δ- and β-globin genes HBD and HBB, respectively (Gaudry et al., 2014) (Fig. 2). This recombination event occurred in the stem lineage of carnivores (Fig. 2), so the same fusion gene is shared with members of the carnivoran suborder Caniformia (Gaudry et al., 2014). The other β-globin gene is a non-chimeric HBB gene, which is orthologous to the adult β-globin gene of humans and most other mammals (Hoffmann et al., 2008; Opazo et al., 2008a,b, 2009). Remarkably, orthologs of HBB/D and HBB in snow leopard, tiger and African lion are completely invariant at the amino acid level (Fig. 1B). In each of these three species, the same four substitutions (at sites β1, β56, β58 and β144) distinguish the HBB/D and HBB paralogs (Fig. 1B). HBA orthologs of snow leopard and African lion differ at a total of four sites, α57, α71, α74 and α120, and these are the only HBA sites that vary among species in the genus Panthera (Fig. 1A).
Fig. 1.
Multiple sequence alignment of adult-expressed α- and β-type globins of cats and other eutherian mammals. (A) HBA α-globin genes and (B) HBB/D and HBB β-type globin genes.
Fig. 2.
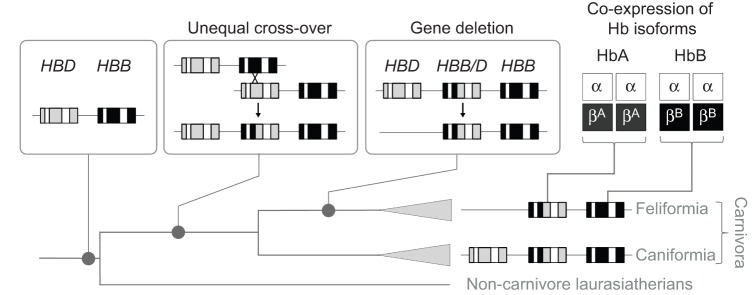
History of structural changes in the adult-expressed HBD and HBB β-globin genes of feliform carnivores. The ancestor of laurasiatherian mammals [the supraordinal group that includes carnivores, as well as bats, pangolins, eulipotyphlans (shrews, moles and hedgehogs), perissodactyls and cetartiodactyls] possessed a tandemly linked pair of HBD and HBB genes. In the stem lineage of carnivores, an unequal cross-over event produced a duplication in the chromosome now containing a chimeric HBB/D fusion gene, flanked by the parental HBD and HBB genes on the 5′ and 3′ sides, respectively (Gaudry et al., 2014). In the common ancestor of felids (or possibly in the common ancestor of all feliform carnivores), the 5′ HBD gene was deleted after divergence from the ancestor of caniform carnivores. Consequently, adult cats co-express two structurally distinct hemoglobin (Hb) isoforms: HbA (which incorporates β-chain products of the chimeric HBB/D gene) and HbB (which incorporates β-chain products of the HBB gene). The two isoforms share identical α-chain subunits.
In analogy with the domestic cat, products of each of the two β-globin genes HBB/D and HBB are incorporated into two distinct Hb isoforms, HbA and HbB, respectively, which share the same α-chain subunit encoded by the two identical HBA genes (Abbasi and Braunitzer, 1985; Taketa et al., 1968) (Fig. 2). The tandemly linked HBB/D and HBB genes share the same β2His→Phe substitution as in the domestic cat (Fig. 1B), as a result of a history of interparalog gene conversion (Gaudry et al., 2014). Thus, the feline HbA and HbB isoforms are all missing a major residue (Hisβ2) involved in DPG binding. HBB has an additional substitution at the adjacent N-terminal residue position, β1Val→Ser, which is acetylated in the native HbB. Overall, our survey of sequence variation revealed very little structural variation among the adult-expressed Hbs of big cat species in the genus Panthera (Fig. 1).
Hb isoform composition of big cat red blood cells
We characterized the Hb isoform composition of red blood cells from the five species of big cats that comprise the genus Panthera (snow leopard, tiger, African lion, leopard and jaguar). Isoelectric focusing (IEF) analysis resolved the hemolysates of each species into three major bands and one minor (more cathodic) band with identical mobilities across species (Fig. 3A). Similar to the case of the domestic cat (Hamilton and Edelstein, 1974), ion-exchange chromatography separated lion and snow leopard hemolysates into two distinct peaks, representing native HbA and HbB isoforms in an almost equimolar ratio (49:51 and 47:53, for lion and snow leopard, respectively). On IEF gels, each of these two peaks resolved into two bands with mobilities corresponding to two of the three major bands of the hemolysate (Fig. 3B).
Fig. 3.
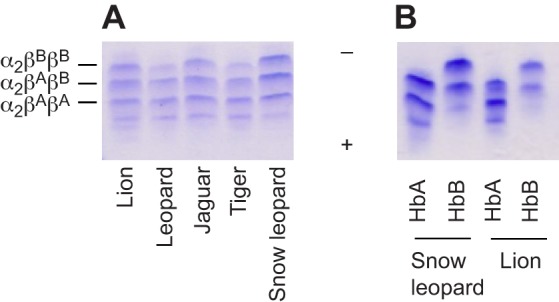
Isoelectric focusing gels (pH 3–9) and isoHb multiplicity in big cats. (A) Red blood cell lysates from lion, leopard, jaguar, tiger and snow leopard, and (B) purified HbA and HbB components from snow leopard and lion, showing highly similar patterns. The putative α- and β-subunit composition of each of the three major bands is indicated, with βA and βB corresponding to the products of genes HBB/D and HBB, respectively. Plus sign, cathode; minus sign, anode.
The fact that the HBB/D and HBB paralogs differ by only four amino acid residues (at positions β1, β56, β58 and β144) suggests that the β-chain products of both genes may be incorporated in the same tetrameric Hb. This is supported by the finding of three major bands of each feline hemolysate on IEF gels (Fig. 3). Each band may represent symmetric α2βAβA and α2βBβB Hb tetramers along with a stable asymmetric hybrid α2βAβB tetramer of intermediate mobility (where βA and βB indicate the products of genes HBB/D and HBB, respectively) (Fig. 3). Thus, feline red blood cells appear to contain the two parental symmetric isoforms HbA and HbB as well as their hybrid molecule. Feline Hbs are characterized by a relatively high tetramer–dimer dissociation constant (Hamilton and Edelstein, 1974), which may further favor hybrid tetrameric Hb assemblies in the red blood cell. Overall, the possession of structurally similar isoHb components among the Panthera species, as evident from the IEF patterns (Fig. 3), is consistent with the remarkably low level of interspecific variation in α- and β-globin primary structures (Fig. 1).
Oxygenation properties of big cat Hbs
To examine possible differences in the oxygenation properties of big cat Hbs, we measured O2-equilibrium curves for purified HbA and HbB isoforms of lion and snow leopard under standardized conditions of pH and anion concentrations. For comparison, O2-equilibrium curves were also measured for human Hb under identical buffer conditions (Fig. 4). Under cofactor-free (stripped) conditions, these experiments revealed uniformly low Hb–O2 affinities (i.e. high P50, the O2 tension at half-saturation) for both isoforms compared with human Hb (Fig. 4, Table 1). Hill coefficients of both lion and snow leopard isoforms (Table 1) indicated normal cooperative O2 binding. O2-equilibrium curves of HbA and HbB isoforms from lion (Fig. 4A) and snow leopard (Fig. 4B) were highly similar and were almost completely unresponsive to the addition of DPG, in contrast to those of human Hb, as shown by the similar P50 values obtained in the absence and presence of DPG (Fig. 4, Table 1). However, in both lion and snow leopard, the O2 affinities of HbA and HbB were markedly reduced in the presence of 0.1 mol l−1 Cl−, indicating that these Hbs are sensitive to Cl− (Fig. 5, Table 1).
Fig. 4.
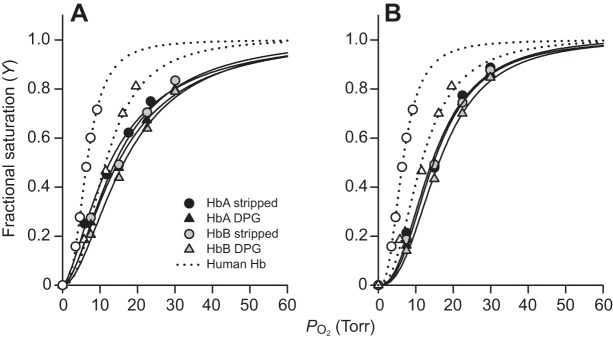
Hb–O2 equilibrium curves of big cats. Hb–O2 equilibrium curves of African lion (A) and snow leopard (B) HbA and HbB are insensitive to 2,3-diphosphoglycerate (DPG) and are right-shifted compared with those of human adult Hb (open symbols, dotted lines). Data were obtained at a heme concentration of 0.3 mmol l−1, 37°C, in 0.1 mol l−1 Hepes buffer, pH 7.4, in the absence (circles) and presence of DPG (triangles), at a 2-fold molar excess of DPG over tetrameric Hb. Non-linear regression of the O2-saturation data according to the sigmoidal Hill equation is shown for each of the conditions used. 1 Torr≈133 Pa.
Table 1.
O2 affinity (P50) and cooperativity (Hill) coefficients (n) of African lion and snow leopard HbA and HbB and of human Hb
Fig. 5.
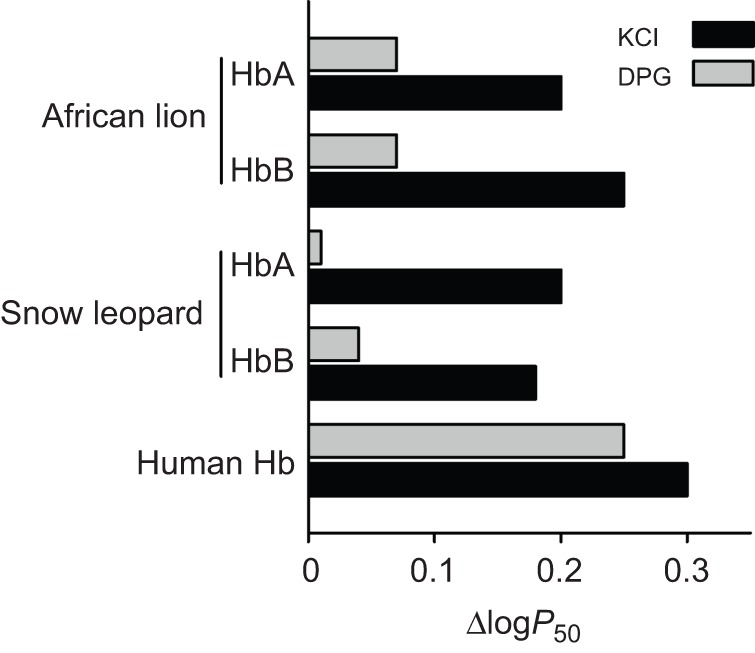
Allosteric regulation of Hb–O2 affinity (P50) by DPG and Cl− ions in African lion and snow leopard HbA and HbB. Sensitivity to DPG and Cl− anions is indexed by the difference in log-transformed P50 values (reported in Table 1) measured in the presence and absence (stripped) of each ionic cofactor. Human Hb–O2 affinity with a high sensitivity to both DPG and Cl− is shown as a comparison.
DISCUSSION
Structural and functional variation of feline Hbs
Consistent with the low level of structural variation among big cat Hbs, O2-equilibrium experiments revealed no appreciable differences in oxygenation properties between the purified HbA and HbB isoforms isolated from either snow leopard or African lion (Table 1), demonstrating that the four amino acid substitutions that distinguish the HBB/D and HBB paralogs do not have any functionally important net effect. This finding also indicates that, if present, hybrid Hb tetramers of the type α2βAβB would have the same O2-binding properties as the parental α2βAβA and α2βBβB Hbs. Moreover, the experiments did not reveal any appreciable differences in Hb–O2 affinity between the high-altitude, hypoxia-adapted snow leopard and the predominantly lowland African lion, as isoform-specific P50 values for the two species were very similar across all treatments (Table 1). As shown in Fig. 1, the amino acid sites that differ between the Hbs of snow leopard and African lion are among the only sites that vary in the Hbs of all big cats. Thus, our examination of structural and functional variation of felid Hbs indicates that all members of the genus Panthera possess functionally similar Hbs.
A single amino acid substitution is associated with suppressed DPG sensitivity and reduced O2 affinity of big cat Hbs
The low intrinsic O2 affinities, suppressed DPG sensitivities and large Cl− effects of the big cat Hbs (Table 1) relative to human Hb can be considered characteristic features of feline Hbs, as they are consistent with previously described properties of cat Hbs (Baumann and Haller, 1975; Hamilton and Edelstein, 1974; León-Velarde et al., 1996; Parer et al., 1970; Taketa et al., 1971; Taketa, 1973). Specifically, the suppressed DPG sensitivities of snow leopard and African lion HbA and HbB isoforms (Figs 4, 5) are chiefly attributable to the single β2His→Phe substitution shared by both HBB/D and HBB genes (and also shared by the corresponding orthologs of domestic cat and other big cat species) (Fig. 1B) (Gaudry et al., 2014). As the β1Val→Ser substitution in the HBB gene only affects the HbB isoform, and as our experiments revealed no appreciable differences in DPG sensitivity between the HbA and HbB isoforms of snow leopard or lion (Table 1, Fig. 5), it appears that replacing β1Val with acetylated Ser does not impair DPG binding beyond that induced by β2His→Phe alone. This interpretation agrees with the minor role of the β-subunit N-terminus in DPG binding (Richard et al., 1993) compared with the original model for DPG binding proposed by Arnone (1972). In addition to impairing DPG binding, the β2His→Phe substitution that exchanges a basic residue with an apolar one also contributes to the reduced intrinsic O2 affinity typical of feline Hb isoforms by moving the β-chain N-termini towards the center of the Hb molecule, thereby stabilizing the T-state conformation (Perutz et al., 1993; Safo and Abraham, 2001) even when the heme is fully ligated, as found for domestic cat Hb (Balasubramanian et al., 2014). Similarly, the low O2 affinity and DPG insensitivity of bovine Hb is also attributable to amino acid substitutions in the β-subunit N-termini (β1Val deleted and β2His→Met), which stabilize the low-affinity T state and prevent DPG access to the central cavity (Perutz et al., 1993; Safo and Abraham, 2001). Although amino acid substitutions replacing the key β2His with uncharged residues may cause a high Hb–O2 affinity as a result of weakened DPG binding in species adapted to high altitudes (Weber and Fago, 2004; Weber, 2007), we find the opposite functional effect of the same substitution (i.e. the Hb–O2 affinity is reduced) when it is combined with a more hydrophobic N-terminal segment of the β-subunit.
Allosteric regulatory control of feline Hbs
Although feline Hbs have severely reduced capacities for allosteric regulatory control by DPG (Fig. 5), HbA and HbB are both strongly modulated by Cl− ions, as indicated by the increased Hb P50 in the presence of 0.1 mol l−1 Cl− (Table 1, Fig. 5). Although Cl− ions present in red blood cells will affect P50 in vivo, this may not fully compensate for the reduced DPG regulatory capacity, as the Cl− concentration typically exhibits only minor fluctuations mainly caused by the influx and efflux of bicarbonate ions (Nikinmaa, 1990).
It is clear that the loss of DPG sensitivity caused by the β2His→Phe substitution effectively eliminates a mechanism of phenotypic plasticity that plays a well-documented role in the acclimatization response to hypoxia in many other eutherian mammals (MacArthur, 1984; Tufts et al., 2013; Weber and Fago, 2004). The reduced intrinsic O2 affinity of feline Hb clearly compensates for the loss of DPG-mediated regulation of O2 binding, otherwise the constitutively elevated blood–O2 affinity would impair O2 unloading to the cells of respiring tissues. This is evidenced by the fact that human Hb mutants with suppressed DPG sensitivity are invariably associated with erythrocytosis (often at clinically pathological levels) caused by inadequate tissue oxygenation (Percy et al., 2009).
Hypoxia tolerance of snow leopards is not associated with specialized Hb adaptations
The results of our experiments clearly demonstrate that the ability of snow leopards to tolerate high-altitude hypoxia cannot be explained by the evolution of Hbs with specialized oxygenation properties. Snow leopards possess typical feline Hbs, characterized by a low intrinsic O2 affinity and a restricted capacity for allosteric regulatory control. In fact, Hbs of all felid species appear to have remarkably similar respiratory properties, despite extensive variation in body size, from 2 kg domestic cats to 220 kg African lions.
Studies of Hb function in high-altitude birds and mammals typically reveal distinctive increases in Hb–O2 affinity relative to closely related, similar-sized lowland taxa (Natarajan et al., 2013; Projecto-Garcia et al., 2013; Storz et al., 2009, 2010b; Storz and Moriyama, 2008; Tufts et al., 2015; Weber, 2007). In a few cases there is no evidence for differentiation in Hb function between species or conspecific populations that are native to different elevations (Cheviron et al., 2014; Natarajan et al., 2015; Revsbech et al., 2013; Storz et al., 2007). The snow leopard represents one more example of a high-altitude species that tolerates chronic altitudinal hypoxia in spite of the fact that it possesses Hbs that are no different from those of its closest lowland relatives. In parts of Nepal, India and Bhutan the distribution of snow leopards, leopards and tigers is partitioned by altitude. Leopards and tigers are known to occur at elevations up to 3000 m, but the snow leopard is the only big cat species that is a permanent resident of the high alpine. Given the low O2 affinity and reduced regulatory capacity of feline Hbs, the ability of snow leopards to tolerate the severe hypoxia of their high-altitude environment must be attributable to compensatory modifications of other convective and conductive steps in the O2-transport pathway.
In humans, theoretical analyses indicate that changes in ventilation, pulmonary diffusion capacity and tissue diffusion capacity exert strong influences on O2 consumption and exercise performance at high altitude (Wagner, 1996). Under severe hypoxia, tissue diffusion capacity is a limiting factor for O2 consumption when blood P50 is low (e.g. in species with high Hb–O2 affinity). By contrast, in snow leopards and other species that have a low Hb–O2 affinity, venous blood would be largely desaturated under conditions of severe hypoxia compared with humans (Cambier et al., 2004), so an increase in tissue diffusion capacity would lead to comparatively smaller improvements in the overall level of tissue oxygenation. However, an increase in lung size (which increases the surface area for pulmonary O2 diffusion) and an enhanced hypoxic ventilatory response could increase arterial PO2 and thus improve exercise performance under hypoxia regardless of blood P50, as in some high-altitude birds (Scott and Milsom, 2006). Because hypoxia-induced hyperventilation can cause respiratory hypocapnia/alkalosis, it is typically beneficial for high-altitude vertebrates to maintain a higher ventilation rate in spite of the reduced blood PCO2 and increased pH (Scott and Milsom, 2007). Thus, an enhanced capacity to increase ventilation under severe hypoxia could help compensate for the low Hb–O2 affinity of snow leopards living at high altitude. Similarly, humans that are indigenous to the Tibetan Plateau do not possess Hbs with specialized oxygenation properties, and their ability to tolerate severe hypoxia appears to be partly attributable to the maintenance of high resting ventilation and a brisk hypoxic ventilatory response (Beall et al., 1997; Hackett et al., 1980; Zhuang et al., 1993).
MATERIALS AND METHODS
Blood sample collection
The collection of blood samples was approved by the Clinical Research and Review Committee of the College of Veterinary Medicine and Biomedical Science, Texas A&M University (permit CRRC no. 10-44). We obtained blood samples from captive, adult animals representing five big cat species, including four snow leopards (P. uncia, San Francisco Zoo, USA and Philadelphia Zoo, USA), two African lions (P. leo, San Francisco Zoo), one tiger (P. tigris, Center for Animal Research and Education, Bridgeport, TX, USA), one common leopard (P. pardus, Feline Conservation Center, Rosamond, CA, USA) and one jaguar (P. onca, Feline Conservation Center). The individual snow leopards that we examined (studbook numbers 1650, 1850, 2662, and 2573) were descendants of wild-caught animals derived from high-altitude regions of Kazakhstan, Kyrgyzstan, China and Russia (Blomqvist, 2008). For each individual animal, approximately 7 ml of blood was collected by means of standard venipuncture. All blood samples were collected by qualified veterinarians during the course of routine medical examinations.
Processing of blood samples
Following blood collection, samples were shipped to the laboratory on an ice pack and were processed immediately after being received. Red blood cells were isolated using the following protocol. A 20 µl volume of blood was suspended in 80 µl of 0.85% NaCl saline solution, gently mixed, and centrifuged at 3000 g for 5 min. The supernatant was removed and 100 µl of the saline solution was added and samples were incubated on ice for 15 min, followed by centrifugation at 10,000 g for 30 min. Isolated red blood cells were stored at −80°C until analysis. DNA was extracted from blood samples using the Qiagen Gentra PureGene Blood Kit (Qiagen, Venlo, The Netherlands) following the manufacturer’s recommendations.
PCR and molecular cloning
We used the domestic cat genome reference sequence to design paralog-specific primers for the HBA, HBB/D and HBB genes of the five big cat species. Following DNA isolation from samples of each individual specimen, we PCR-amplified full-length HBA, HBB/D and HBB genes using the following paralog-specific primers: HBA-29F: CACCTTCTGGTCCCGACAC, HBA-1032R: TGAACACGGTTCAGCACATT, HBB/D-264F: CATACCCTTGAAGGTGGACA, HBB/D-1871R: TGGCTGTCATCATTCAGACC, HBB-92F: TGGGCATAAAAGGAAGAGCA and HBB-1633R: GCAGGATCTGTTTCCCACAT. The HBA gene was amplified using the following PCR conditions: 1× Qiagen Type-it Master Mix (HotStarTaq Plus DNA Polymerase, 3.0 mmol l−1 MgCl2, 200 µmol l−1 dNTPs), 0.480 µmol l−1 of each primer and 15 ng of DNA in a 50 µl reaction volume. The cycling conditions included a 95°C, 5 min activation step, followed by 10 cycles of 95°C for 30 s, 65–55°C for 30 s (1°C reduction per cycle) and 72°C for 2.0 min, and 20 cycles of 95°C for 30 s, 55°C for 30 s and 72°C for 2.0 min, followed by a final extension of 7 min. PCR amplicons were purified using the Qiagen QIAquick PCR Purification Kit and cloned with the NEB PCR Cloning Kit (New England Biolabs, Ipswich, MA, USA) following manufacturer recommendations. The HBB/D and HBB genes were amplified using the following PCR conditions: Applied Biosystems AmpliTaq Gold 1×360 PCR Buffer (Life Technologies, Valencia, CA, USA), 0.2 units of AmpliTaq Gold taq, 2.0 mmol l−1 MgCl2, 200 µmol l−1 dNTPs, 0.480 µmol l−1 of each primer and 15 ng of DNA in a 50 µl reaction volume. The cycling conditions included a 95°C, 10 min activation step, followed by 10 cycles of 95°C for 30 s, 65–55°C for 30 s (1°C reduction per cycle) and 72°C for 1.5 min, and 20 cycles of 95°C for 30 s, 55°C for 30 s and 72°C for 1.5 min, followed by a final extension of 10 min. The cloning was conducted in two independent replicates. We randomly selected a total of 16 colonies per individual for each gene (i.e. eight colonies from each cloning replicate). These were PCR amplified in 25 µl reactions with the following conditions: NEB LongAmp 1× PCR Buffer (contained 2.0 mmol l−1 MgSO4), 2.5 units of LongAmp taq, 300 µmol l−1 dNTPs and 0.300 µmol l−1 of each primer. Cycling conditions included a 94°C, 2 min activation step, followed by 30 cycles of 94°C for 15 s, 57°C for 15 s and 68°C for 1.5 min, followed by a final 68°C extension of 5 min. All PCR products were cleaned and sequenced using ABI BigDye chemistry (Beckman Coulter, Pasadena, CA, USA). We cloned and sequenced the full complement of adult-expressed α- and β-type globin genes in each individual that was used as a subject for the experimental analysis of Hb function and isoform composition (see supplementary material Table S1). We generated data for the remaining individuals via direct sequencing of diploid PCR products. All newly generated sequences were submitted to GenBank under accession nos KR818795–KR818811 (see supplementary material Table S1).
We augmented our newly generated sequence data for the five big cat species with globin sequences annotated from genome assemblies of domestic cat and Siberian tiger (P. tigris altaica). For comparative purposes, we also included the full complement of adult α- and β-like genes from human and two caniform carnivores, dog (Canis lupus familiaris, Canidae) and ferret (Mustela putorius furo, Mustelidae).
Characterization of Hb isoform composition
Frozen blood samples from each specimen were lysed as previously described (Revsbech et al., 2013) and Hb multiplicity was analyzed by IEF (pH 3–9) on polyacrylamide gels (Phastgel, GE Healthcare Biosciences AB, Uppsala, Sweden). For lion and snow leopard hemolysates, separation of Hb isoforms and simultaneous removal (stripping) of endogenous DPG were achieved by anion-exchange chromatography using an Äkta Pure chromatography system and a Hi-Trap Q 5 ml column (both GE Healthcare) equilibrated with 20 mmol l−1 Tris-HCl buffer pH 8.26, 0.5 mmol l−1 EDTA, and eluted using a 0–0.3 mol l−1 NaCl gradient over 80 min, at a flow rate of 1 ml min−1. Fractions containing HbA and HbB were pooled, concentrated by ultrafiltration (heme concentration >1 mmol l−1), dialyzed against 10 mmol l−1 Hepes buffer pH 7.6 to remove NaCl in the elution buffer and stored at −80°C in aliquots. Heme concentration was measured spectrophotometrically using published extinction coefficients (van Assendelft and Zijlstra, 1975). The purity of separate Hb isoforms was checked by IEF. For comparison of functional properties, human Hb was prepared and stripped of DPG as described elsewhere (Weber, 1992), dialyzed against 10 mmol l−1 Hepes buffer pH 7.6 and stored at −80°C.
Measurement of Hb–O2 equilibria
O2 equilibrium curves of isolated Hbs from snow leopard, African lion and human were measured using 5 µl samples in 0.1 mol l−1 Hepes buffer pH 7.4, 0.5 mmol l−1 EDTA using a thin-layer chamber technique described in detail elsewhere (Cheviron et al., 2014; Damsgaard et al., 2013; Storz et al., 2009; Tufts et al., 2015; Weber, 1992). For each curve, measures of O2 affinity (P50, the PO2 at which Hb is half-saturated with O2) and cooperativity (Hill) coefficients n were calculated by non-linear regression of four to six saturation steps using the sigmoidal Hill equation Y=PO2n/(P50n+PO2n), where Y is the fractional saturation (Tufts et al., 2015). Experiments were performed at 37°C and at a heme concentration of 0.3 mmol l−1 in the absence (stripped) and presence of 0.1 mol l−1 KCl and DPG (2-fold molar excess over Hb tetramer), that approximate standard physiological levels within red blood cells of most mammals. Cl− and DPG were added separately or in combination, to isolate the allosteric effects of each cofactor on Hb–O2 affinity (expressed as logP50 differences with and without cofactor) (Tufts et al., 2015). By contrast, previous studies of cat Hbs (Bunn, 1971; Hamilton and Edelstein, 1974; Taketa, 1973) measured O2 equilibria using buffer solutions that contained Cl− ions and inorganic phosphates, which potentially perturb DPG binding to Hb (Weber, 1992).
Supplementary Material
Acknowledgements
We thank G. R. Scott and two anonymous reviewers for helpful suggestions that improved the manuscript. We thank R. Jenkins and J. Jencek (San Francisco Zoo), K. Hinshaw and J. Blough (Philadelphia Zoo), J. Maynard (Feline Conservation Center) and H. Krahn (Center for Animal Research and Education) for providing blood samples, and B. Palmer (Denver Zoo) for providing pedigree information for captive snow leopards. We thank E. Ellebæk Petersen (Aarhus University) for excellent technical support and R. Jackson (Snow Leopard Conservancy) for additional assistance with the snow leopard initiative.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
J.E.J. and T.A. carried out the cloning and sequencing; F.G.H., J.E.J., and J.F.S. analyzed the sequence data; S.S.E.N. and S.D.A. carried out the isohemoglobin separations and O2-equilibrium experiments; S.S.E.N., S.D.A. and A.F. analyzed the functional data; F.G.H. and R.E.W. critically revised the manuscript and helped with data interpretation; J.E.J., J.F.S. and A.F. conceived the research; J.F.S. and A.F. designed the experiments, analysed the data and wrote the manuscript. All authors contributed critically to manuscript writing and approved the final version.
Funding
This work was supported by the Danish Council for Independent Research, Natural Sciences (grant no. 10-084-565 to A.F.), the Carlsberg Foundation (grant no. 2013-01-0178 to A.F.), the National Institutes of Health (NIH grant no. HL087216 to J.F.S.), the Faculty of Science and Technology, Aarhus University (to R.E.W.), the Snow Leopard Conservancy (to J.E.J.) and the Faculty Development Fund of Duquesne University (to J.E.J.). Deposited in PMC for release after 12 months.
Supplementary material
Supplementary material available online at http://jeb.biologists.org/lookup/suppl/doi:10.1242/jeb.125369/-/DC1
References
- Abbasi A. and Braunitzer G. (1985). The primary structure of hemoglobins from the domestic cat (Felis catus, Felidae). Biol. Chem. Hoppe-Seyler 366, 699-704. 10.1515/bchm3.1985.366.2.699 [DOI] [PubMed] [Google Scholar]
- Arnone A. (1972). X-ray diffraction study of binding of 2,3-diphosphoglycerate to human deoxyhaemoglobin. Nature 237, 146-149. 10.1038/237146a0 [DOI] [PubMed] [Google Scholar]
- Balasubramanian M., Sathya Moorthy P., Neelagandan K., Ramadoss R., Kolatkar P. R. and Ponnuswamy M. N. (2014). Structure of liganded T-state haemoglobin from cat (Felis silvestris catus), a low oxygen-affinity species, in two different crystal forms. Acta Crystallogr. D Biol. Crystallogr. 70, 1898-1906. 10.1107/S139900471400916X [DOI] [PubMed] [Google Scholar]
- Baumann R. and Haller E. A. (1975). Cat haemoglobins A and B: Differences in the interaction with Cl−, phosphate and CO2. Biochem. Biophys. Res. Commun. 65, 220-227. 10.1016/S0006-291X(75)80082-9 [DOI] [PubMed] [Google Scholar]
- Beall C. M., Strohl K. P., Blangero J., Williams-Blangero S., Almasy L. A., Decker M. J., Worthman C. M., Goldstein M. C., Vargas E., Villena M. et al. (1997). Ventilation and hypoxic ventilatory response of Tibetan and Aymara high altitude natives. Am. J. Phys. Anthropol. 104, 427-447. [DOI] [PubMed] [Google Scholar]
- Benesch R. and Benesch R. E. (1967). The effect of organic phosphates from the human erythrocyte on the allosteric properties of hemoglobin. Biochem. Biophys. Res. Commun. 26, 162-167. 10.1016/0006-291X(67)90228-8 [DOI] [PubMed] [Google Scholar]
- Blomqvist L. (2008). International Pedigree Book for Snow Leopards, Uncia uncia, pp. 1-175. Helsinki: Helsinki Zoo. [Google Scholar]
- Bouverot P. (1985). Adaptation to Altitude-Hypoxia in Vertebrates, pp. 1-176, Berlin: Springer Verlag. [Google Scholar]
- Bunn H. F. (1971). Differences in the interaction of 2,3-diphosphoglycerate with certain mammalian hemoglobins. Science 172, 1049-1050. 10.1126/science.172.3987.1049 [DOI] [PubMed] [Google Scholar]
- Bunn H. F. (1980). Regulation of hemoglobin-function in mammals. Amer. Zool. 20, 199-211. 10.1093/icb/20.1.199 [DOI] [Google Scholar]
- Bunn H. F., Seal U. S. and Scott A. F. (1974). Role of 2,3-diphosphoglycerate in mediating hemoglobin function of mammalian red cells. Ann. N. Y. Acad. Sci. 241, 498-512. 10.1111/j.1749-6632.1974.tb21906.x [DOI] [PubMed] [Google Scholar]
- Cambier C., Wierinckx M., Clerbaux T., Detry B., Liardet M.-P., Marville V., Frans A. and Gustin P. (2004). Haemoglobin oxygen affinity and regulating factors of the blood oxygen transport in canine and feline blood. Res. Vet. Sci. 77, 83-88. 10.1016/j.rvsc.2004.02.004 [DOI] [PubMed] [Google Scholar]
- Campbell J. A. (1927). Further observations on oxygen acclimatisation. J. Physiol. 63, 325-342. 10.1113/jphysiol.1927.sp002407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheviron Z. A., Natarajan C., Projecto-Garcia J., Eddy D. K., Jones J., Carling M. D., Witt C. C., Moriyama H., Weber R. E., Fago A. et al. (2014). Integrating evolutionary and functional tests of adaptive hypotheses: A case study of altitudinal differentiation in hemoglobin function in an Andean sparrow, Zonotrichia capensis. Mol. Biol. Evol. 31, 2948-2962. 10.1093/molbev/msu234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsgaard C., Storz J. F., Hoffmann F. G. and Fago A. (2013). Hemoglobin isoform differentiation and allosteric regulation of oxygen binding in the turtle, Trachemys scripta. Am. J. Physiol. Regul. Integr. Comp. Physiol. 305, R961-R967. 10.1152/ajpregu.00284.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudry M. J., Storz J. F., Butts G. T., Campbell K. L. and Hoffmann F. G. (2014). Repeated evolution of chimeric fusion genes in the β-globin gene family of laurasiatherian mammals. Genome Biol. Evol. 6, 1219-1234. 10.1093/gbe/evu097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett P. H., Reeves J. T., Reeves C. D., Grover R. F. and Rennie D. (1980). Control of breathing in Sherpas at low and high altitude. J. Appl. Physiol. 49, 374-379. [DOI] [PubMed] [Google Scholar]
- Hamilton M. N. and Edelstein S. J. (1974). Cat Hemoglobin: pH dependence of cooperativity and ligand binding. J. Biol. Chem. 249, 1323-1329. [PubMed] [Google Scholar]
- Hoffmann F. G., Opazo J. C. and Storz J. F. (2008). New genes originated via multiple recombinational pathways in the β-globin gene family of rodents. Mol. Biol. Evol. 25, 2589-2600. 10.1093/molbev/msn200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janečka J. E., Munkhtsog B., Jackson R. M., Naranbaatar G., Mallon D. P. and Murphy W. J. (2011). Comparison of noninvasive genetic and camera-trapping techniques for surveying snow leopards. J. Mammal 92, 771-783. 10.1644/10-MAMM-A-036.1 [DOI] [Google Scholar]
- Kay F. R. (1977). 2,3-diphosphoglycerate, blood oxygen dissociation and the biology of mammals. Comp. Biochem. Physiol. A Physiol. 57, 309-316. 10.1016/0300-9629(77)90197-9 [DOI] [Google Scholar]
- León-Velarde F., de Muizon C., Palacios J. A., Clark D. and Monge C. (1996). Hemoglobin affinity and structure in high-altitude and sea-level carnivores from Peru. Comp. Biochem. Physiol. A Physiol. 113, 407-411. 10.1016/0300-9629(95)02083-7 [DOI] [PubMed] [Google Scholar]
- MacArthur R. A. (1984). Seasonal changes in hematological and respiratory properties of muskrat (Ondatra zibethicus) blood. Can. J. Zool. 62, 537-545. 10.1139/z84-080 [DOI] [Google Scholar]
- Mairbaurl H. and Weber R. E. (2012). Oxygen transport by hemoglobin. Compr. Physiol. 2, 1463-1489. 10.1002/cphy.c080113 [DOI] [PubMed] [Google Scholar]
- Natarajan C., Inoguchi N., Weber R. E., Fago A., Moriyama H. and Storz J. F. (2013). Epistasis among adaptive mutations in deer mouse hemoglobin. Science 340, 1324-1327. 10.1126/science.1236862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan C., Hoffmann F. G., Lanier H. C., Wolf C. J., Cheviron Z. A., Spangler M. L., Weber R. E., Fago A. and Storz J. F. (2015). Intraspecific polymorphism, interspecific divergence, and the origins of function-altering mutations in deer mouse hemoglobin. Mol. Biol. Evol. 32, 978-997. 10.1093/molbev/msu403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikinmaa M. (1990). Vertebrate Red Blood Cells, pp. 1-262. Berlin: Springer-Verlag. [Google Scholar]
- Opazo J. C., Hoffmann F. G. and Storz J. F. (2008a). Differential loss of embryonic globin genes during the radiation of placental mammals. Proc. Natl. Acad. Sci. USA 105, 12950-12955. 10.1073/pnas.0804392105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opazo J. C., Hoffmann F. G. and Storz J. F. (2008b). Genomic evidence for independent origins of β-like globin genes in monotremes and therian mammals. Proc. Natl. Acad. Sci. USA 105, 1590-1595. 10.1073/pnas.0710531105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opazo J. C., Sloan A. M., Campbell K. L. and Storz J. F. (2009). Origin and ascendancy of a chimeric fusion gene: the β/δ-globin gene of paenungulate mammals. Mol. Biol. Evol. 26, 1469-1478. 10.1093/molbev/msp064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parer J. T., Hoversland A. S. and Metcalfe J. (1970). Comparative studies of the respiratory functions of mammalian blood. VI. Young lion and tiger. Respir. Physiol. 10, 30-37. 10.1016/0034-5687(70)90024-1 [DOI] [PubMed] [Google Scholar]
- Percy M. J., Butt N. N., Crotty G. M., Drummond M. W., Harrison C., Jones G. L., Turner M., Wallis J. and McMullin M. F. (2009). Identification of high oxygen affinity hemoglobin variants in the investigation of patients with erythrocytosis. Haematologica 94, 1321-1322. 10.3324/haematol.2009.008037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perutz M. F., Fermi G., Poyart C., Pagnier J. and Kister J. (1993). A novel allosteric mechanism in haemoglobin. J. Mol. Biol. 233, 536-545. 10.1006/jmbi.1993.1530 [DOI] [PubMed] [Google Scholar]
- Projecto-Garcia J., Natarajan C., Moriyama H., Weber R. E., Fago A., Cheviron Z. A., Dudley R., McGuire J. A., Witt C. C. and Storz J. F. (2013). Repeated elevational transitions in hemoglobin function during the evolution of Andean hummingbirds. Proc. Natl. Acad. Sci. USA 110, 20669-20674. 10.1073/pnas.1315456110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves J. T., Grover E. B. and Grover R. F. (1963). Circulatory responses to high altitude in cat and rabbit. J. Appl. Physiol. 18, 575-579. [DOI] [PubMed] [Google Scholar]
- Revsbech I. G., Tufts D. M., Projecto-Garcia J., Moriyama H., Weber R. E., Storz J. F. and Fago A. (2013). Hemoglobin function and allosteric regulation in semi-fossorial rodents (family Sciuridae) with different altitudinal ranges. J. Exp. Biol. 216, 4264-4271. 10.1242/jeb.091397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard V., Dodson G. G. and Mauguen Y. (1993). Human deoxyhaemoglobin-2,3-diphosphoglycerate complex low-salt structure at 2.5 Å resolution. J. Mol. Biol. 233, 270-274. 10.1006/jmbi.1993.1505 [DOI] [PubMed] [Google Scholar]
- Safo M. K. and Abraham D. J. (2001). The X-ray structure determination of bovine carbonmonoxy hemoglobin at 2.1 Å resolution and its relationship to the quaternary structures of other hemoglobin crystal forms. Protein Sci. 10, 1091-1099. 10.1110/ps.48301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott G. R. and Milsom W. K. (2006). Flying high: a theoretical analysis of the factors limiting exercise performance in birds at altitude. Respir. Physiol. Neurobiol. 154, 284-301. 10.1016/j.resp.2006.02.012 [DOI] [PubMed] [Google Scholar]
- Scott G. R. and Milsom W. K. (2007). Control of breathing and adaptation to high altitude in the bar-headed goose. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R379-R391. 10.1152/ajpregu.00161.2007 [DOI] [PubMed] [Google Scholar]
- Scott A. F., Bunn H. F. and Brush A. H. (1976). Functional aspects of hemoglobin evolution in the mammals. J. Mol. Evol. 8, 311-316. 10.1007/BF01739256 [DOI] [PubMed] [Google Scholar]
- Scott A. F., Bunn H. F. and Brush A. H. (1977). Phylogenetic distribution of red cell 2,3 diphosphoglycerate and its interaction with mammalian hemoglobins. J. Exp. Zool. 201, 269-288. 10.1002/jez.1402010211 [DOI] [PubMed] [Google Scholar]
- Storz J. F. and Moriyama H. (2008). Mechanisms of hemoglobin adaptation to high altitude hypoxia. High Alt. Med. Biol. 9, 148-157. 10.1089/ham.2007.1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz J. F., Baze M., Waite J. L., Hoffmann F. G., Opazo J. C. and Hayes J. P. (2007). Complex signatures of selection and gene conversion in the duplicated globin genes of house mice. Genetics 177, 481-500. 10.1534/genetics.107.078550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz J. F., Runck A. M., Sabatino S. J., Kelly J. K., Ferrand N., Moriyama H., Weber R. E. and Fago A. (2009). Evolutionary and functional insights into the mechanism underlying high-altitude adaptation of deer mouse hemoglobin. Proc. Natl. Acad. Sci. USA 106, 14450-14455. 10.1073/pnas.0905224106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz J. F., Scott G. R. and Cheviron Z. A. (2010a). Phenotypic plasticity and genetic adaptation to high-altitude hypoxia in vertebrates. J. Exp. Biol. 213, 4125-4136. 10.1242/jeb.048181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz J. F., Runck A. M., Moriyama H., Weber R. E. and Fago A. (2010b). Genetic differences in hemoglobin function between highland and lowland deer mice. J. Exp. Biol. 213, 2565-2574. 10.1242/jeb.042598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunquist M. E. and Sunquist F. (2002). Wild Cats of the World, pp. 1-462. Chicago: University of Chicago Press. [Google Scholar]
- Taketa F. (1973). Structure of the Felidae hemoglobins and response to 2,3-diphosphoglycerate. Comp. Biochem. Physiol. B Comp. Biochem. 45, 813-823. 10.1016/0305-0491(73)90144-2 [DOI] [PubMed] [Google Scholar]
- Taketa F., Smits M. R. and Lessard J. L. (1968). Hemoglobin heterogeneity in the cat. Biochem. Biophys. Res. Comm. 30, 219-226. 10.1016/0006-291X(68)90438-5 [DOI] [PubMed] [Google Scholar]
- Taketa F., Mauk A. G. and Lessard J. L. (1971). Chain amino termini of the cat hemoglobins and the response to 2,3-diphosphoglycerate and adenosine triphosphate. J. Biol. Chem. 246, 4471-4476. [PubMed] [Google Scholar]
- Tufts D. M., Revsbech I. G., Cheviron Z. A., Weber R. E., Fago A. and Storz J. F. (2013). Phenotypic plasticity in blood-oxygen transport in highland and lowland deer mice. J. Exp. Biol. 216, 1167-1173. 10.1242/jeb.079848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufts D. M., Natarajan C., Revsbech I. G., Projecto-Garcia J., Hoffmann F. G., Weber R. E., Fago A., Moriyama H. and Storz J. F. (2015). Epistasis constrains mutational pathways of hemoglobin adaptation in high-altitude pikas. Mol. Biol. Evol. 32, 287-298. 10.1093/molbev/msu311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Assendelft O. W. and Zijlstra W. G. (1975). Extinction coefficients for use in equations for the spectrophotometric analysis of haemoglobin mixtures. Anal. Biochem. 69, 43-48. 10.1016/0003-2697(75)90563-1 [DOI] [PubMed] [Google Scholar]
- Wagner P. D. (1996). A theoretical analysis of factors determining V˙O2max at sea level and altitude. Respir. Physiol. 106, 329-343. 10.1016/S0034-5687(96)00086-2 [DOI] [PubMed] [Google Scholar]
- Weber R. E. (1992). Use of ionic and zwitterionic (Tris/BisTris and HEPES) buffers in studies on hemoglobin function. J. Appl. Physiol. 72, 1611-1615. [DOI] [PubMed] [Google Scholar]
- Weber R. E. (2007). High-altitude adaptations in vertebrate hemoglobins. Respir. Physiol. Neurobiol. 158, 132-142. 10.1016/j.resp.2007.05.001 [DOI] [PubMed] [Google Scholar]
- Weber R. E. and Fago A. (2004). Functional adaptation and its molecular basis in vertebrate hemoglobins, neuroglobins and cytoglobins. Respir. Physiol. Neurobiol. 144, 141-159. 10.1016/j.resp.2004.04.018 [DOI] [PubMed] [Google Scholar]
- Zhuang J., Droma T., Sun S., Janes C., McCullough R. E., McCullough R. G., Cymerman A., Huang S. Y., Reeves J. T. and Moore L. G. (1993). Hypoxic ventilatory responsiveness in Tibetan compared with Han residents of 3,658 m. J. Appl. Physiol. 74, 303-311. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.