Abstract
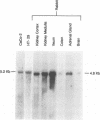
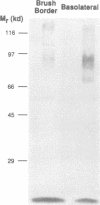
A cDNA clone encoding a rabbit ileal villus cell Na+/H+ exchanger was isolated and its complete nucleotide sequence was determined. The cDNA is 4 kb long and contains 322 bp of 5'-untranslated region, 2451 bp of open reading frame and 1163 bp of 3'-untranslated area, with 70%, 91% and 40% identity to the human sequence, respectively. Amino acid sequence deduced from the longest open reading frame indicated a protein of 816 residues (predicted Mr 90,716) which exhibits 95% amino acid identity to the human Na+/H+ exchanger. The two putative glycosylation sites in the human Na+/H+ exchanger are conserved in this protein, suggesting that it is a glycoprotein. Stable transfection of the cDNA into an Na+/H+ exchanger deficient fibroblast cell line, established Na+/H+ exchange. The Na+/H+ exchanger was stimulated by serum and a phorbol ester but not by 8-Br-cAMP. In Northern blot analysis, the cDNA hybridized to a 4.8 kb message in rabbit ileal villus cells, kidney cortex, kidney medulla, adrenal gland, brain and descending colon and to a 5.2 kb message in cultured human colonic cancer cell lines, HT29-18 and Caco-2. In immunoblotting, a polyclonal antibody raised against a fusion protein of beta-galactosidase and the C-terminal 158 amino acids of the human Na+/H+ exchanger identified a rabbit ileal basolateral membrane protein of 94 kd and only weakly interacted with the ileal brush border membrane. In immunocytochemical studies using ileal villus and crypt epithelial cells, the same antibody identified basolateral and not brush border epitopes. Restriction analysis of genomic DNA with a 462 bp PstI-AccI fragment of the rabbit Na+/H+ exchanger strongly suggests the existence of closely related Na+/H+ exchanger genes. The near identity of the basolateral Na+/H+ exchanger and the human Na+/H+ exchanger plus the ubiquitous expression of this message suggests that the ileal basolateral Na+/H+ exchanger is the 'housekeeping' Na+/H+ exchanger.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierman A. J., Tertoolen L. G., de Laat S. W., Moolenaar W. H. The Na+/H+ exchanger is constitutively activated in P19 embryonal carcinoma cells, but not in a differentiated derivative. Responsiveness to growth factors and other stimuli. J Biol Chem. 1987 Jul 15;262(20):9621–9628. [PubMed] [Google Scholar]
- Birnbaum M. J. Identification of a novel gene encoding an insulin-responsive glucose transporter protein. Cell. 1989 Apr 21;57(2):305–315. doi: 10.1016/0092-8674(89)90968-9. [DOI] [PubMed] [Google Scholar]
- Brosius F. C., 3rd, Alper S. L., Garcia A. M., Lodish H. F. The major kidney band 3 gene transcript predicts an amino-terminal truncated band 3 polypeptide. J Biol Chem. 1989 May 15;264(14):7784–7787. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Donowitz M., Cohen M. E., Gudewich R., Taylor L., Sharp G. W. Ca2+-calmodulin-, cyclic AMP- and cyclic GMP-induced phosphorylation of proteins in purified microvillus membranes of rabbit ileum. Biochem J. 1984 Apr 15;219(2):573–581. doi: 10.1042/bj2190573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmer E., Rood R. P., Wesolek J. H., Cohen M. E., Braithwaite R. S., Sharp G. W., Murer H., Donowitz M. Role of calcium and calmodulin in the regulation of the rabbit ileal brush-border membrane Na+/H+ antiporter. J Membr Biol. 1989 Jun;108(3):207–215. doi: 10.1007/BF01871735. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Steitz T. A., Goldman A. Identifying nonpolar transbilayer helices in amino acid sequences of membrane proteins. Annu Rev Biophys Biophys Chem. 1986;15:321–353. doi: 10.1146/annurev.bb.15.060186.001541. [DOI] [PubMed] [Google Scholar]
- Friedrich T., Sablotni J., Burckhardt G. Identification of the renal Na+/H+ exchanger with N,N'-dicyclohexylcarbodiimide (DCCD) and amiloride analogues. J Membr Biol. 1986;94(3):253–266. doi: 10.1007/BF01869721. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Rotin D., Mason M. J. Na+/H+ exchange and growth factor-induced cytosolic pH changes. Role in cellular proliferation. Biochim Biophys Acta. 1989 Jan 18;988(1):73–97. doi: 10.1016/0304-4157(89)90004-x. [DOI] [PubMed] [Google Scholar]
- Huet C., Sahuquillo-Merino C., Coudrier E., Louvard D. Absorptive and mucus-secreting subclones isolated from a multipotent intestinal cell line (HT-29) provide new models for cell polarity and terminal differentiation. J Cell Biol. 1987 Jul;105(1):345–357. doi: 10.1083/jcb.105.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huot S. J., Cassel D., Igarashi P., Cragoe E. J., Jr, Slayman C. W., Aronson P. S. Identification and purification of a renal amiloride-binding protein with properties of the Na+-H+ exchanger. J Biol Chem. 1989 Jan 15;264(2):683–686. [PubMed] [Google Scholar]
- Igarashi P., Aronson P. S. Covalent modification of the renal Na+/H+ exchanger by N,N'-dicyclohexylcarbodiimide. J Biol Chem. 1987 Jan 15;262(2):860–868. [PubMed] [Google Scholar]
- Knickelbein R. G., Aronson P. S., Dobbins J. W. Membrane distribution of sodium-hydrogen and chloride-bicarbonate exchangers in crypt and villus cell membranes from rabbit ileum. J Clin Invest. 1988 Dec;82(6):2158–2163. doi: 10.1172/JCI113838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knickelbein R., Aronson P. S., Schron C. M., Seifter J., Dobbins J. W. Sodium and chloride transport across rabbit ileal brush border. II. Evidence for Cl-HCO3 exchange and mechanism of coupling. Am J Physiol. 1985 Aug;249(2 Pt 1):G236–G245. doi: 10.1152/ajpgi.1985.249.2.G236. [DOI] [PubMed] [Google Scholar]
- Kopito R. R., Andersson M., Lodish H. F. Structure and organization of the murine band 3 gene. J Biol Chem. 1987 Jun 15;262(17):8035–8040. [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Montrose M. H., Knoblauch C., Murer H. Separate control of regulatory volume increase and Na+-H+ exchange by cultured renal cells. Am J Physiol. 1988 Jul;255(1 Pt 1):C76–C85. doi: 10.1152/ajpcell.1988.255.1.C76. [DOI] [PubMed] [Google Scholar]
- Pouysségur J., Sardet C., Franchi A., L'Allemain G., Paris S. A specific mutation abolishing Na+/H+ antiport activity in hamster fibroblasts precludes growth at neutral and acidic pH. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4833–4837. doi: 10.1073/pnas.81.15.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood R. P., Emmer E., Wesolek J., McCullen J., Husain Z., Cohen M. E., Braithwaite R. S., Murer H., Sharp G. W., Donowitz M. Regulation of the rabbit ileal brush-border Na+/H+ exchanger by an ATP-requiring Ca++/calmodulin-mediated process. J Clin Invest. 1988 Sep;82(3):1091–1097. doi: 10.1172/JCI113665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardet C., Counillon L., Franchi A., Pouysségur J. Growth factors induce phosphorylation of the Na+/H+ antiporter, glycoprotein of 110 kD. Science. 1990 Feb 9;247(4943):723–726. doi: 10.1126/science.2154036. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicentini L. M., Villereal M. L. Activation of Na+/H+ exchange in cultured fibroblasts: synergism and antagonism between phorbol ester, Ca2+ ionophore, and growth factors. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8053–8056. doi: 10.1073/pnas.82.23.8053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinman E. J., Dubinsky W. P., Dinh Q., Steplock D., Shenolikar S. Effect of limited trypsin digestion on the renal Na+-H+ exchanger and its regulation by cAMP-dependent protein kinase. J Membr Biol. 1989 Aug;109(3):233–241. doi: 10.1007/BF01870280. [DOI] [PubMed] [Google Scholar]
- Weiser M. M. Intestinal epithelial cell surface membrane glycoprotein synthesis. I. An indicator of cellular differentiation. J Biol Chem. 1973 Apr 10;248(7):2536–2541. [PubMed] [Google Scholar]
- Welsh M. J., Smith P. L., Fromm M., Frizzell R. A. Crypts are the site of intestinal fluid and electrolyte secretion. Science. 1982 Dec 17;218(4578):1219–1221. doi: 10.1126/science.6293054. [DOI] [PubMed] [Google Scholar]
- Williams S. A., Birnbaum M. J. The rat facilitated glucose transporter gene. Transformation and serum-stimulated transcription initiate from identical sites. J Biol Chem. 1988 Dec 25;263(36):19513–19518. [PubMed] [Google Scholar]