Two auxin influx carriers are required for auxin signaling that activates transcriptional control in lateral root development.
Abstract
Several members of the Lateral Organ Boundaries Domain (LBD)/Asymmetric Leaves2-Like (ASL) gene family have been identified to play important roles in Arabidopsis (Arabidopsis thaliana) lateral root (LR) development during auxin response, but their functional relationship with auxin transporters has not been established yet. Here, we show that the AUXIN1 (AUX1) and LIKE-AUXIN3 (LAX3) auxin influx carriers are required for auxin signaling that activates LBD16/ASL18 and LBD18/ASL20 to control LR development. The lax3 mutant phenotype was not significantly enhanced when combined with lbd16 or lbd18. However, LBD18 overexpression could rescue the defects in LR emergence in lax3 with concomitant expression of the LBD18 target genes. Genetic and gene expression analyses indicated that LBD16 and LBD18 act with AUX1 to regulate LR initiation and LR primordium development, and that AUX1 and LAX3 are needed for auxin-responsive expression of LBD16 and LBD18. LBD18:SUPERMAN REPRESSIVE DOMAIN X in the lbd18 mutant inhibited LR initiation and LR primordium development in response to a gravitropic stimulus and suppressed promoter activities of the cell cycle genes Cyclin-Dependent Kinase A1;1 and CYCLINB1;1. Taken together, these results suggest that LBD16 and LBD18 are important regulators of LR initiation and development downstream of AUX1 and LAX3.
Root branching, such as lateral root (LR) formation, is critical for the absorption of water and nutrients, and for the anchorage of plants in soil (Hochholdinger and Zimmermann, 2008; Dastidar et al., 2012). In recent years, intensive research on the molecular mechanisms of LR formation has been conducted with Arabidopsis (Arabidopsis thaliana; Lavenus et al., 2013). The developmental events of LR formation include priming, initiation, primordium development, and the emergence of LRs, which are primarily regulated by auxin (Dubrovsky et al., 2008, 2011; De Smet, 2012; Lavenus et al., 2013). The pericycle cells associated with a xylem pole are primed by auxin in the basal meristem to become pericycle founder cells, and the founder cells divide asymmetrically for LR initiation, generating small daughter cells flanked by long daughter cells (Parizot et al., 2008; Péret et al., 2009a,b; Lavenus et al., 2013). These cells undergo a series of anticlinal divisions to produce a few initial cells. Further anticlinal, periclinal, and tangential divisions of lateral root primordium (LRP) cells generate a dome-shaped LRP that continues to grow and emerge through the cortex and epidermal layers of the primary root via cell separation.
In the Arabidopsis root, auxin is transported acropetally through the central cylinder toward the root tip and columella cells (Friml et al., 2003). Basipetal auxin transport from the root tip toward the basal region plays an important role in the control of auxin homeostasis in the root meristem (Band et al., 2014). The AUXIN1 (AUX1)/LIKE-AUXIN1 (LAX1) gene family encoding major auxin influx carriers comprise four highly conserved genes: AUX1, LAX1, LAX2, and LAX3 (Péret et al., 2012; Swarup and Péret, 2012). AUX1 regulates the initiation of LR by basipetal auxin transport (Swarup et al., 2001; Marchant et al., 2002; De Smet et al., 2007). LAX2 regulates vascular patterning in cotyledons (Péret et al., 2012). LAX3 promotes LR emergence by affecting auxin influx of outer endodermis and cortex cells (Swarup et al., 2008). The aux1 lax3 double mutations effectively blocked LR formation, indicating that these two auxin influx carriers are critical for LR formation (Swarup et al., 2008). Auxin efflux carriers, PIN-FORMED (PIN) proteins, have also been demonstrated to play a role in LR formation (Benková et al., 2003; Laskowski et al., 2008; Marhavý et al., 2013; Péret et al., 2013).
Auxin-responsive transcriptional regulatory modules for LR formation have been identified in Arabidopsis. The GATA transcription factor23 specifies LR founder cell identity in an INDOLE-3-ACETIC ACID INDUCIBLE28 (IAA28)-dependent auxin signaling for LR priming (De Rybel et al., 2010). SOLITARY-ROOT (SLR)/IAA14-Auxin Response Factor7 (ARF7)-ARF19 and others regulate nuclear polarization of LR founder cells (De Rybel et al., 2010; Goh et al., 2012a). Two Aux/IAA-ARF modules, SLR/IAA14-ARF7-ARF19 and BODENLOS/IAA12-ARF5, control the LR initiation and patterning process (Fukaki et al., 2002; Vanneste et al., 2005; De Smet et al., 2010). LR emergence is distinctively modulated in the endodermis and the cortex and epidermis by two different Aux/IAA-ARF modules. SHORT HYPOCOTYL2/IAA3-ARF signaling operates in the endodermis, whereas SLR/IAA14-ARF7-ARF19 signaling functions in the cortex and epidermis (Goh et al., 2012b; Lavenus et al., 2013).
Several transcription factors, in particular, Lateral Organ Boundaries Domain (LBD)/Asymmetric Leaves2-Like (ASL) proteins, are regulated downstream of Aux/IAA-ARF modules during LR development (Okushima et al., 2007; Lee et al., 2009a; Berckmans et al., 2011; Goh et al., 2012a; Lee et al., 2013a; Lee and Kim, 2013). ARF7 and ARF19 directly activate LBD16/ASL18 and LBD29/ASL16 (Okushima et al., 2007). LBD16 and related LBDs are involved in the symmetry breaking of LR founder cells for LR initiation (Goh et al., 2012b). LBD18/ASL20 regulates LR formation in conjunction with LBD16 downstream of ARF7 and ARF19 (Lee et al., 2009a,b) and contributes to both the initiation and emergence of LRs (Berckmans et al., 2011; Lee et al., 2013a; Lee and Kim, 2013). LBD18 transcriptionally activates the E2Fa transcription factor, which regulates the asymmetric cell division for LR initiation (Berckmans et al., 2011). LBD18 promotes LR emergence by acting as a specific DNA-binding transcriptional activator that directly up-regulates EXPANSIN14 (EXP14) encoding a cell wall-loosening protein (Lee et al., 2013a). LBD18 indirectly up-regulates EXP17, which promotes LR emergence during the auxin response (Lee and Kim, 2013). Although the auxin-responsive transcriptional cascade of the Aux/IAA-ARFs and LBD genes has been well characterized, no functional evidence has been provided yet on how auxin transport proteins are linked to LBD gene expression to control LR development during the auxin response, and on the role of LBD18 in LR initiation and LRP development.
In this study, we investigated the connection between auxin influx carriers, LAX3 and AUX1, and two auxin-responsive LBD genes, LBD16 and LBD18, during LR development by conducting genetic analysis with various multiple mutants derived from lbd16, lbd18, lax3, and aux1 and gene expression analysis. Our molecular genetic analysis results suggested that LBD16 and LBD18 are linked to auxin signaling via AUX1 for LR initiation and LRP development, in part via LAX3 for LRP development, and that LBD18 acts downstream of LAX3 to control LR emergence in Arabidopsis. To confirm that LBD18 is involved in LR initiation and LRP development, we expressed LBD18:SUPERMAN REPRESSIVE DOMAIN X (SRDX), a dominant repressor of LBD18, under the control of its own promoter in wild-type or lbd18 mutant backgrounds and showed that LBD18:SRDX suppressed LR initiation events, periclinal divisions of primordium after LR initiation, and later stages of LRP development in response to a gravitropic stimulus. In addition, we identified a link between LBD18 and the regulation of cell cycle genes during LR initiation through the analyses of GUS expression under the cell cycle gene promoter Cyclin-Dependent Kinase A1;1 (CDKA1;1), CDKB1;1, or CYCLINB1;1 (CYCB1;1), a marker gene for LR initiation, in transgenic Arabidopsis expressing LBD18:SRDX, and of expression of these cell cycle genes by LBD18:GLUCOCORTICOID RECEPTOR (GR).
RESULTS
LR Phenotypes of Multiple Mutants Derived from lax3, lbd16, and lbd18 and Gene Expression Analysis
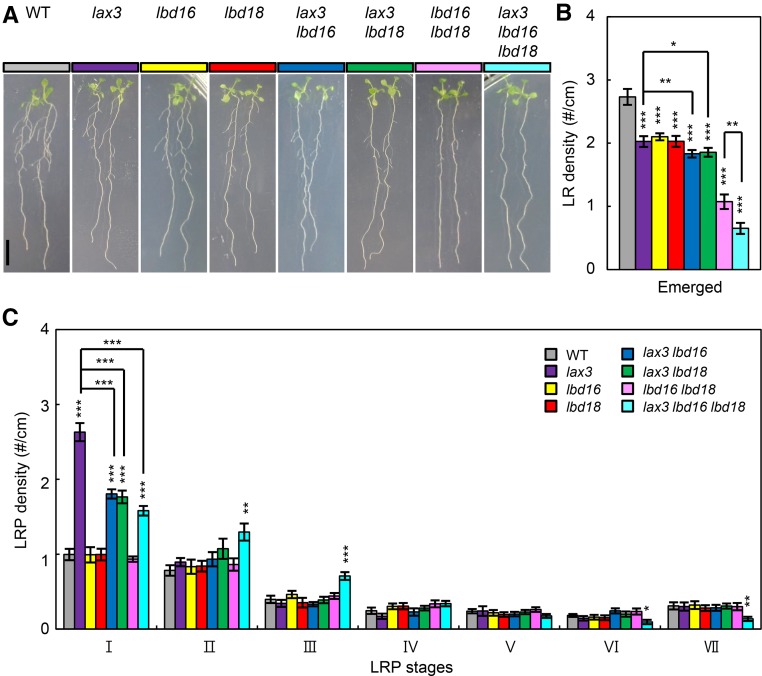
To investigate whether LBD16 and LBD18 are genetically linked to LAX3 in controlling LR development, we generated single, double, and triple mutants and analyzed LR phenotypes of these mutants. As shown in Figure 1, A and B, the mean ± se numbers of emerged LRs in lax3 lbd16 (1.83 ± 0.06) and lax3 lbd18 (1.86 ± 0.07) double mutants were slightly lower than in single mutants (lax3, 2.03 ± 0.08; lbd16, 2.1 ± 0.09; lbd18, 2.03 ± 0.08). The number of emerged LRs in lax3 lbd16 lbd18 triple mutants (0.65 ± 0.09) was considerably lower than that in lax3 lbd16 (1.83 ± 0.06) and lax3 lbd18 (1.86 ± 0.07) double mutants, but to a lesser degree than that of lbd16 lbd18 double mutants (1.07 ± 0.09). The process of LRP development is divided by eight stages defined by specific anatomical characteristics and cell divisions (Malamy and Benfey, 1997). It has been previously shown that the lax3 mutation significantly increases the LRP number at stage I (Swarup et al., 2008), whereas the numbers at all LRP developmental stages in lbd16 or lbd18 single and double mutants were similar to those in the wild type (Lee et al., 2009b). The LRP numbers of lax3 at stage I (2.63 ± 0.12) were mitigated in lax3 lbd16 (1.80 ± 0.06) and lax3 lbd18 (1.77 ± 0.08) double mutants, and in lax3 lbd16 lbd18 (1.58 ± 0.06) triple mutants (Fig. 1C). These results indicated that LR development in lax3 mutants is dependent on LBD16 and LBD18 function.
Figure 1.
Genetic analysis of lax3, lbd16, and lbd18 in LR development. A, LR phenotypes of 7-d-old seedlings of the wild type (WT; Columbia-0 [Col-0], gray), lax3 (purple), lbd16 (yellow), lbd18 (red), lax3 lbd16 (blue), lax3 lbd18 (green), lbd16 lbd18 (lilac), and lax3 lbd16 lbd18 (sky blue) are shown. B, Density of emerged LRs (LR no. per unit primary root length; #/cm). Error bars = se. n = 20. Asterisks denote statistical significance (*, P < 0.05; **, P < 0.01; and ***, P < 0.001). C, Density of primordia at given stages. Stages I to VII of primordia were based on the classification by Malamy and Benfey (1997). Error bars = se. n = 10. Asterisks denote statistical significance (**, P < 0.01; and ***, P < 0.001).
Quantitative reverse transcription (qRT)-PCR analyses showed that the lax3 mutation significantly reduced expression of LBD16 and LBD18 in response to auxin after a 4- or 8-h treatment (Fig. 2A). Moreover, auxin-induced expression of the LBD18 target genes, EXP14 and EXP17 (Lee et al., 2013a; Lee and Kim, 2013), in the lax3 mutant decreased compared with that in the wild type (Fig. 2B). However, LAX3 expression with auxin was not affected by lbd16 or lbd18 single or double mutations (Fig. 2C). These results indicated that LAX3 acts upstream of LBD16 and LBD18 during the auxin response. GUS staining of ProLBD16:GUS, ProLBD18:GUS (Lee et al., 2009b), and ProLAX3:GUS (Swarup et al., 2008) transgenic Arabidopsis demonstrated that LBD18 and LAX3 were coexpressed in the cortex and epidermis from stages I to VII during LRP development and after the emergence of LRs (Supplemental Fig. S1). Although strong GUS staining was detected in the LRPs and emerged LRs of ProLBD16:GUS and ProLBD18:GUS Arabidopsis, we did not detect GUS staining in the same tissues of ProLAX3:GUS Arabidopsis (Supplemental Fig. S1). The overlapping expression pattern of LBD18 and LAX3 in the overlaying tissues (Supplemental Fig. S1) and the decrease in the LBD18 transcript levels by the lax3 mutation (Fig. 2A) are consistent with the observation that GUS expression under the control of the LBD18 promoter was reduced in lax3 mutants compared with that in the wild type in the overlaying tissues as well as in the LRP without or with auxin treatment (Fig. 2, D–G). In contrast, we only noted reduced GUS expression under the control of the LBD16 promoter in lax3 mutants compared with that in the wild type in the LRP with auxin treatment (Fig. 2, H–K). These results indicated that LAX3 promotes LBD18 expression in the overlaying tissues.
Figure 2.
Expression analysis of LBD16, LBD18, EXP14, and EXP17 in lax3 and LAX3 in lbd16, lbd18, and lbd16 lbd18. A, LBD16 and LBD18 expression in lax3 in response to auxin. Seven-day-old seedlings were incubated with auxin (20 μm IAA) for 0, 4, or 8 h and subjected to qRT-PCR. Data are the mean ± se of three independent biological replications. Asterisks denote statistical significance (*, P < 0.05; and ***, P < 0.001). B, EXP14 and EXP17 expression in lax3 in response to auxin. Treatment and analysis of the samples were performed as described in A. C, LAX3 expression in the wild type (WT), lbd16, lbd18, and lbd16 lbd18 in response to auxin. Seven-day-old seedlings were incubated with auxin (20 μm IAA) for 8 h and subjected to qRT-PCR. Data are the mean ± se of three independent biological replications. Asterisks denote statistical significance (***, P < 0.001). D to K, GUS expression of ProLBD18:GUS (D and F), ProLBD18:GUS/lax3 (E and G), ProLBD16:GUS (H and J), and ProLBD16:GUS/lax3 transgenic Arabidopsis during LR development (I and K). Six-day-old light-grown seedlings were incubated without (D, E, H, and I) or with (F, G, J, and K) 20 μm IAA for 4 h and subjected to GUS staining. Bars = 50 μm.
LBD18 Induces LR Formation and Expression of the Target Genes, EXP14 and EXP17, in lax3 Mutants
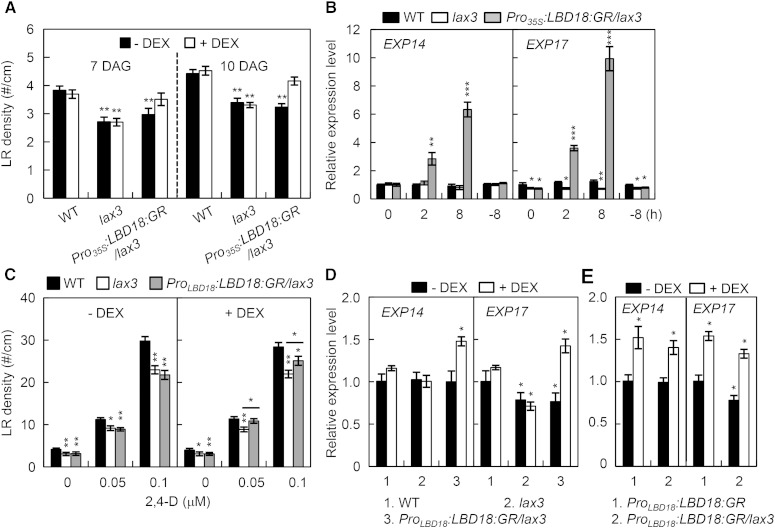
To demonstrate that LBD18 functions downstream of LAX3 for LR emergence, we constructed dexamethasone (DEX)-regulated LBD18-transgenic Arabidopsis in the lax3 mutant background (Pro35S:LBD18:GR/lax3) by crossing these transgenic plants and the lax3 mutant. As shown in Figure 3A, LR densities in Pro35S:LBD18:GR/lax3 were rescued to the wild-type levels by DEX treatment for both 7 and 10 d, as compared with what was observed in the absence of DEX and in both the presence and absence of DEX in the lax3 mutants. EXP14 and EXP17 are up-regulated by LBD18 during the auxin response (Lee et al., 2013a; Lee and Kim, 2013). Using qRT-PCR analysis, we showed that EXP14 and EXP17 expression was greatly induced in the lax3 mutants by DEX treatment of Pro35S:LBD18:GR/lax3 plants for 2 and 8 h (Fig. 3B). These results demonstrated that LBD18 controls LR formation downstream of LAX3. However, LR formation by LBD18 in the lax3 mutants under the control of the LBD18 promoter requires exogenous auxin treatment (Fig. 3C; Supplemental Fig. S2), indicating that lax3 mutation caused defects in LR formation, which could not be rescued by LBD18 normally expressed under its own promoter but could be complemented by ectopic overexpression of LBD18. We further showed that LBD18 expression under the control of the LBD18 promoter induced expression of EXP14 and EXP17 in lax3 mutants at levels similar to those that can be gained by LBD18 expression in the wild-type background (Fig. 3, D and E). These results together with genetic and gene expression analysis data (Figs. 1 and 2) demonstrated that LBD18 regulates expression of EXP14 and EXP17 downstream of LAX3 during the auxin response.
Figure 3.
Induction of LRs in lax3 mutants by LBD18:GR with DEX treatment. A, LR densities of Pro35S:LBD18:GR/lax3 plants. Plants were grown vertically for 7 or 10 d in the absence or presence of 10 μm DEX, and LR numbers per unit root length (#/cm) measured were plotted. Asterisks denote statistical significance (**, P < 0.01). DAG, Days after germination. B, Expression analysis of EXP14 and EXP17 in the wild type (WT), lax3, and Pro35S:LBD18:GR/lax3 plants. Seven-day-old seedlings were incubated with DEX for 2 or 8 h in the light and subjected to qRT-PCR. −8 h, Samples that were incubated with mock treatment for 8 h. Data are the mean ± se of three independent biological replications. Asterisks denote statistical significance (*, P < 0.05; **, P < 0.01; and ***, P < 0.001). C, The effects of auxin on LR densities of ProLBD18:LBD18:GR/lax3 plants. Plants were grown vertically for 7 d in the absence or presence of 10 μm DEX and 0, 0.05, or 0.1 μm auxin 2,4-dichlorophenoxyacetic acid (2,4-d), and LR numbers per unit root length (#/cm) measured were plotted. Asterisks denote statistical significance (*, P < 0.05; and **, P < 0.01). D, Expression analysis of EXP14 and EXP17 in the wild type, lax3, and Pro35S:LBD18:GR/lax3 plants. Plants were grown for 7 d in the absence or presence of 10 μm DEX and subjected to qRT-PCR. Data are the mean ± se of three independent biological replications. Asterisks denote statistical significance (*, P < 0.05). E, Expression analysis of EXP14 and EXP17 in Pro35S:LBD18:GR and Pro35S:LBD18:GR/lax3 plants. Plants were grown for 7 d in the absence or presence of 10 μm DEX and subjected to qRT-PCR. Data are the mean ± se of three independent biological replications. Asterisks denote statistical significance (*, P < 0.05).
LBD18 Up-Regulates POLYGALACTURONASE Expression as a Secondary Response
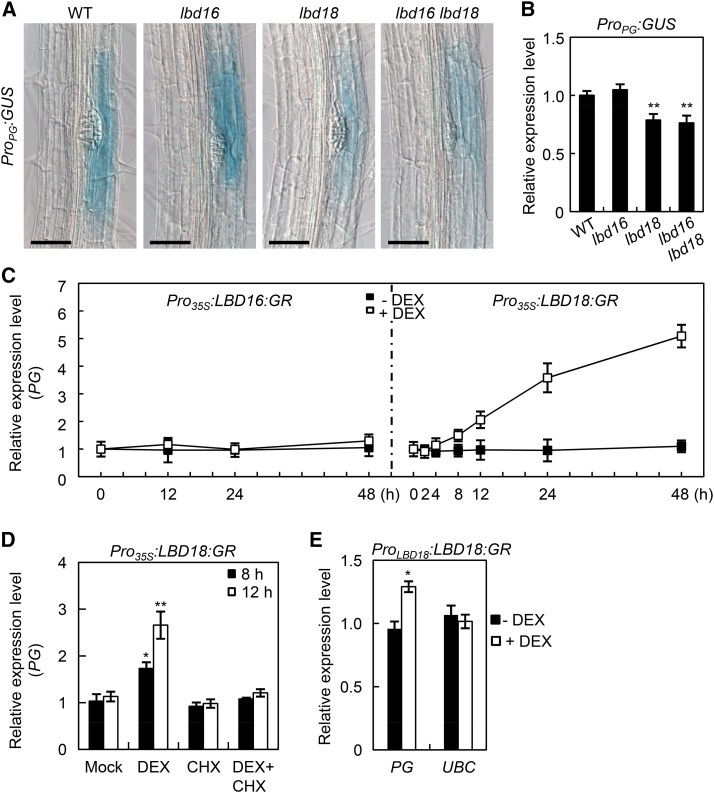
POLYGALACTURONASE (PG) is induced by auxin, and this depends on proper auxin influx regulated by LAX3 (Swarup et al., 2008). PG enzymes cleave demethylated pectin, a substrate enriched in the middle lamella of root cells overlaying LRP, and are important for cell separation during LR emergence (Hadfield and Bennett, 1998; Laskowski et al., 2006; González-Carranza et al., 2007). We investigated whether PG is regulated by LBD16 and/or LBD18. We generated ProPG:GUS in the lbd16, lbd18, or lbd16 lbd18 mutant backgrounds and conducted GUS expression analyses (Fig. 4A). GUS staining detected in overlaying tissues of the LRP was significantly reduced in lbd18 compared with that in the wild type, but not in lbd16 (Fig. 4A). GUS staining in lbd16 lbd18 double mutants was similar to that in lbd18 single mutants. Consistent with these observations, qRT-PCR analysis of the GUS transcripts confirmed that the lbd18 mutation decreased GUS expression of ProPG:GUS, but the lbd16 mutation did not (Fig. 4B). DEX treatment of Pro35S:LBD18:GR plants resulted in a sustained increase in the PG transcript levels after 4 h of treatment (Fig. 4C), whereas the same treatment of Pro35S:LBD16:GR plants did not alter the PG transcript levels until 48 h of treatment. PG expression in Pro35S:LBD18:GR plants was not induced by DEX treatment in the presence of cycloheximide after 8 and 12 h of incubation (Fig. 4D), indicating that PG is a secondary response gene of LBD18. We further demonstrated that LBD18 up-regulates PG expression in tissues where LBD18 is normally expressed (Fig. 4E).
Figure 4.
LBD18 activates PG expression. A, GUS expression analysis of ProPG:GUS in the wild type (WT), lbd16, lbd18, or lbd16 lbd18 double mutant backgrounds. Seven-day-old light-grown seedlings were subjected to GUS staining. Scale bars = 50 μm. B, qRT-PCR analysis of ProPG:GUS in the wild type, lbd16, lbd18, or lbd16 lbd18. Seven-day-old seedlings were subjected to qRT-PCR for GUS expression. Relative transcript levels are plotted. Data are the mean ± se of three independent biological replications. Asterisks denote statistical significance (**, P < 0.01). C, Time course expression analysis of PG in Pro35S:LBD16:GR and Pro35S:LBD18:GR plants. Seven-day-old plants were incubated without or with DEX for the indicated time and subjected to qRT-PCR for PG expression. Relative transcript levels are plotted. Data are the mean ± se of three independent biological replications. D, The effect of cycloheximide (CHX) treatment on DEX-induced expression of PG. Bars represent ses. Data are the mean ± se of three independent biological replications. Asterisks denote statistical significance (*, P < 0.05; and **, P < 0.01). E, Expression of PG following treatment with DEX in ProLBD18:LBD18:GR plants. ProLBD18:LBD18:GR seedlings were grown for 7 d in the absence (black squares) or presence (white squares) of DEX. Expression of PG was then analyzed by qRT-PCR and plotted as relative expression levels. Ubiquitin-Conjugating enzyme E2 (UBC) was used as a negative control. Asterisk denotes statistical significance (*, P < 0.05).
Analysis of LR Phenotypes of Multiple Mutants Derived from aux1, lax3, lbd16, and lbd18
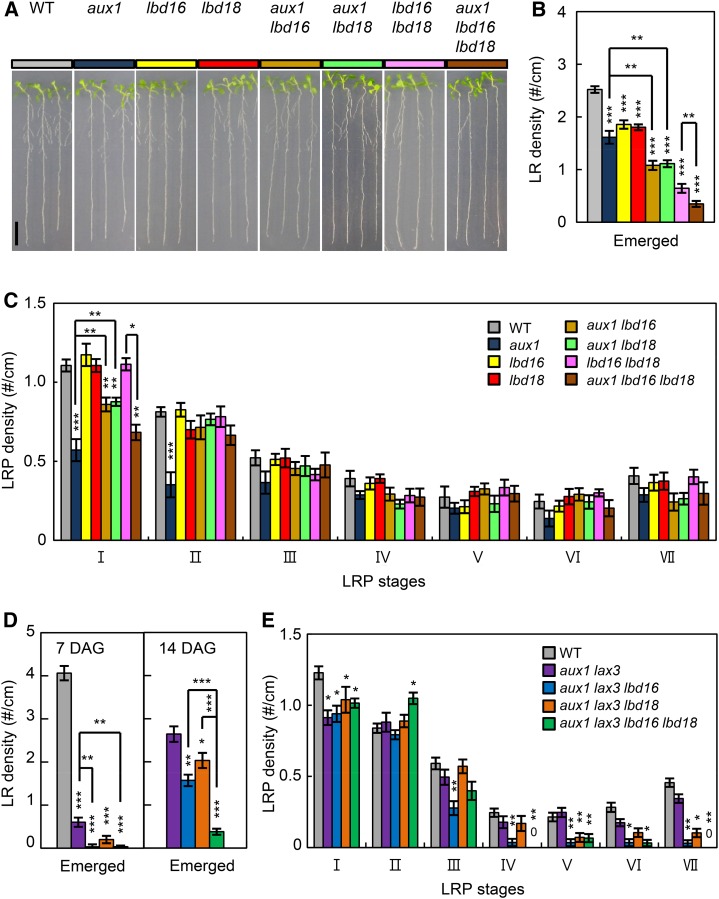
AUX1 facilitates LR initiation by promoting auxin accumulation in the root apex, and later LR emergence through the uptake in the LRP (Marchant et al., 2002; Swarup et al., 2008). Strong GUS expression in the LRPs and emerged LRs of ProLBD16:GUS and ProLBD18:GUS (Supplemental Fig. S1) overlaps with that of ProAux1:GUS (Marchant et al., 2002), indicating that a potential link may exist between LBD18 and AUX1 during LR development. To investigate a functional link between LBD16/LBD18 and AUX1 for LR development, we generated multiple mutants derived from lbd16, lbd18, and aux1-22 and analyzed LR initiation, LRP development, and LR emergence of these multiple mutants. As shown in Figure 5, A and B, the numbers of emerged LRs in aux1 lbd16 (1.08 ± 0.09), aux1 lbd18 (1.11 ± 0.07) double mutants, and aux1 lbd16 lbd18 (0.35 ± 0.06) triple mutants decreased additively compared with that of corresponding single mutants (aux1, 1.8 ± 0.06; lbd16, 1.61 ± 0.12; and lbd18, 1.86 ± 0.08) and double mutants (lbd16 lbd18, 0.65 ± 0.08), indicating that LBD16/LBD18 and AUX1 combinatorially contribute to LR emergence. In contrast, the numbers of primordia at the initiation stage I in aux1 lbd16 (0.86 ± 0.04) and aux1 lbd18 (0.88 ± 0.03) double mutants increased compared with that in aux1 (0.57 ± 0.07), but decreased compared with that in lbd16 (1.17 ± 0.07) or lbd18 (1.11 ± 0.04; Fig. 5C), indicating that the aux1 phenotype requires LBD16 and LBD18 gene function during LR initiation and at early stages of the LRP development. The aux1 lbd16 lbd18 triple mutants (0.68 ± 0.04) exhibited a further decreased LRP number compared with that of aux1 lbd16 or aux1 lbd18 mutants. At stage II, lbd16 and lbd18 mutations in aux1 rescued the LRP number reduced by aux1 mutation to the wild-type levels. Similar patterns were observed at stages II to VI, albeit the aux1 impact on the LRP numbers was small. These results indicated that LBD16 and LBD18 function together with AUX1 to control LR initiation and early LRP development.
Figure 5.
Genetic analysis of aux1, lax3, lbd16, and lbd18 in LR development. A, LR phenotypes of 7-d-old seedlings of the wild type (WT; Col-0, gray), aux1 (ocean blue), lbd16 (yellow), lbd18 (red), aux1 lbd16 (orange), aux1 lbd18 (pastel green), lbd16 lbd18 (lilac), and aux1 lbd16 lbd18 (indigo blue) are shown. B, Density of emerged LRs (LR no. per unit primary root length; #/cm). Error bars = se. n = 20. Asterisks denote statistical significance (**, P < 0.01; and ***, P < 0.001). C, Density of primordia at given stages. Stages I to VII of primordia were based on the classification by Malamy and Benfey (1997). Error bars = se. n = 10. Asterisks denote statistical significance (*, P < 0.05; **, P < 0.01; and ***, P < 0.001). D, LR densities (LR no. per unit primary root length; #/cm) of 7- or 14-d-old seedlings of the wild type, aux1 lax3, aux1 lax3 lbd16, aux1 lax3 lbd18, and aux1 lax3 lbd16 lbd18. Error bars = se. n = 20. Asterisks denote statistical significance (**, P < 0.01; and ***, P < 0.001). DAG, Days after germination. E, Density of primordia at given stages of 7-d-old seedlings of the wild type, aux1 lax3, aux1 lax3 lbd16, aux1 lax3 lbd18, and aux1 lax3 lbd16 lbd18. Error bars = se. n = 10. Asterisks denote statistical significance (*, P < 0.05; **, P < 0.01; and ***, P < 0.001).
To define a combinatorial role of AUX1 and LAX3 in LBD16 and LBD18 function during LR development, we further generated lbd16 and lbd18 mutations in aux1 lax3 double mutants. Double mutations in AUX1 and LAX3 dramatically reduced numbers of emerged LRs (0.06 ± 0.11) compared with that of single mutants (aux1, 1.8 ± 0.06; and lax3, 2.03 ± 0.08; Figs. 1B and 5, B and D), consistent with a previous report (Swarup et al., 2008). The lbd16 (0.03 ± 0.02 for 7 d; and 1.57 ± 0.13 for 14 d) or lbd18 (0.20 ± 0.09 for 7 d; and 2.03 ± 0.18 for 14 d) single mutation in aux1 lax3 double mutants further reduced the emerged LR numbers of aux1 lax3 mutants (0.60 ± 0.11 for 7 d; and 2.64 ± 0.18 for 14 d), and the lbd16 lbd18 (0.03 ± 0.02 for 7 d; and 0.38 ± 0.07 for 14 d) double mutations additively reduced the emerged LR number of aux1 lax3 mutants, which is due to the independent roles of AUX1, LBD16, and LBD18 during LR emergence (Fig. 5D; Supplemental Fig. S3). Numbers of primordia of aux1 lax3 double mutants at stage I were not significantly altered by lbd16 and lbd18 single or double mutations, whereas those numbers from stages III to VII were significantly reduced by the lbd16 mutation (Fig. 5E). The lbd18 or lbd16 lbd18 mutation greatly reduced the LRP numbers from stages V to VII. These results indicated that both LBD16 and LBD18 control LRP development in combination with LAX3 and AUX1 but at different stages after stage III and after stage V, respectively.
AUX1 and LAX3 Are Involved in Auxin-Responsive Expression of LBD16 and LBD18
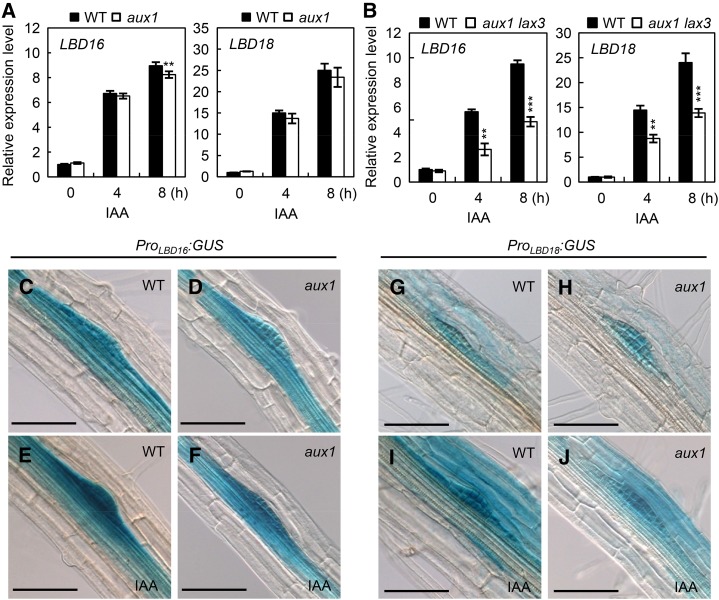
qRT-PCR analysis of auxin-responsive expression of LBD16 or LBD18 in aux1 mutants showed that the aux1 mutation did not alter LBD18 expression, but decreased LBD16 expression slightly after 8 h of auxin treatment (Fig. 6A). However, aux1 lax3 double mutations reduced the expression of LBD16 and LBD18 by approximately 50% in response to auxin after a 4- or 8-h treatment compared with those of wild-type and aux1 or lax3 mutants (Figs. 2A and 6B). These results show that both LAX3 and AUX1 are involved in auxin-responsive expression of LBD16 and LBD18. These qRT-PCR data are consistent with the observation that aux1 mutation reduced GUS expression in the primordium under the control of the LBD16 or LBD18 promoter in response to auxin compared with that in the wild type (Fig. 6, C–J).
Figure 6.
Expression of LBD16 or LBD18 in aux1 and aux1 lax3 compared with that in the wild type (WT) in response to auxin. A, LBD16 and LBD18 expression in aux1 in response to auxin. Seven-day-old seedlings were incubated with auxin (20 μm IAA) for 0, 4, or 8 h and subjected to qRT-PCR. Data are the mean ± se of three independent biological replications. Asterisks denote statistical significance (**, P < 0.01). B, LBD16 and LBD18 expression in aux1 lax3 double mutants in response to auxin. Treatment and analysis of the samples were performed as described in the legend of Figure 7A. C to J, GUS expression of ProLBD16:GUS (C and E), ProLBD16:GUS/aux1 (D and F), ProLBD18:GUS (G and I), and ProLBD18:GUS/aux1 plants (H and J). Six-day-old seedlings were incubated without (C, D, G, and H) or with (E, F, I, and J) 20 μm IAA for 4 h. Bars = 50 μm.
Expression of LBD18:SRDX in lbd18 Mutant Caused Inhibition of LR Initiations, Periclinal Divisions in the Initiated LRP, and Progression of LRP Development
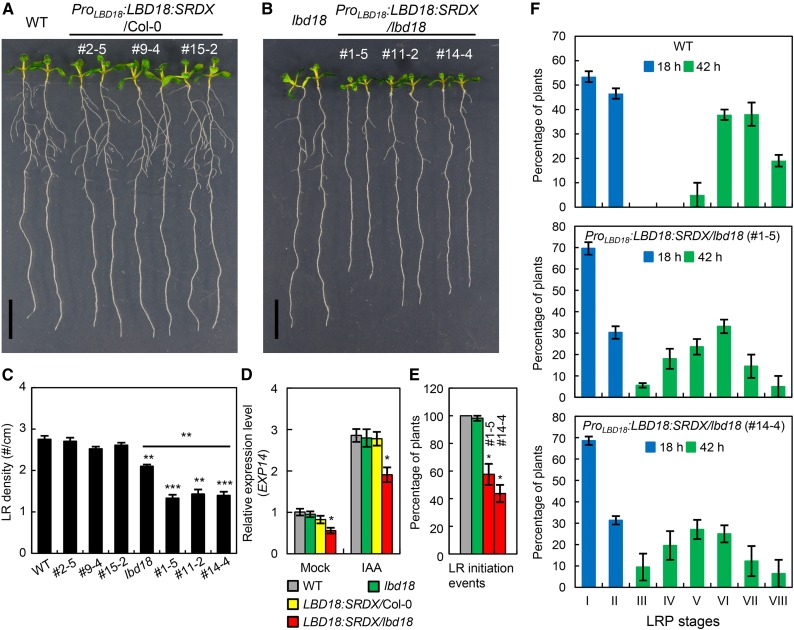
The present genetic analyses with multiple mutants consisting of aux1, lax3, and lbd18 indicated that LBD18 is involved in controlling LR initiation and LRP development. Our previous study showed that any alterations in the numbers of the LRPs from stages I to VII were not detected in lbd18 compared with those in the wild type (Lee et al., 2009b). To further investigate the role of LBD18 during LR initiation and LRP development, we constructed transgenic Arabidopsis expressing LBD18 fused to an SRDX repression domain under its own promoter and analyzed LR phenotypes. The SRDX chimeric repressors cause dominant repression of target genes, allowing the functional analysis of plant transcription factors with genetic redundancy (Hiratsu et al., 2003). This approach has been previously utilized to reveal a unique function of LBD16 in the polar nuclear migration prior to LR initiation (Goh et al., 2012a). A complete block of LR formation has been observed with ProLBD16:LBD16:SRDX transgenic plants in the wild-type background (Goh et al., 2012a). We also observed the same phenotype with ProLBD16:LBD16:SRDX transgenic plants in wild-type as well as in the lbd16 mutant backgrounds (Supplemental Fig. S4). In contrast, ProLBD18:LBD18:SRDX transgenic plants in the wild-type background displayed wild-type appearance with normal LR formation (Fig. 7A), whereas ProLBD18:LBD18:SRDX transgenic plants in the lbd18 mutant background exhibited more reduced LR formation compared with that of the lbd18 mutant (Fig. 7, B and C). Consistent with these observations, a significant dominant repression of EXP14, a direct target of LBD18 (Lee et al., 2013a), by LBD18:SRDX was found in both the absence and presence of auxin in the lbd18 mutant (Fig. 7D). None of eight ProLBD18:LBD18:SRDX lines analyzed showed a complete block of LR formation, unlike ProLBD16:LBD16:SRDX transgenic plants. Determination of LRP numbers at all developmental stages of ProLBD18:LBD18:SRDX lines showed wild-type distribution of LRPs (Supplemental Fig. S5). We then conducted an LR induction experiment, which allows the direct analysis of LR development kinetics as opposed to the analysis of the LRPs and LRs at a given plant developmental stage (Péret et al., 2012). We applied a gravitropic stimulus inducing initiation of LRs to wild-type and ProLBD18:LBD18:SRDX/lbd18 plants grown vertically for 30 h by rotating the agar plate through 90°. We then measured the number of newly developed LRPs on the convex side of the curves after 18 or 42 h of gravitropic induction and determined the relative distribution of the LRPs at stages I to VIII. We found that ProLBD18:LBD18:SRDX lines displayed 40% block of LR initiation events compared with those of the wild type and lbd18 mutant 18 h postgravitropic induction (pgi) in LR induction experiments with a gravitropic stimulus (Fig. 7E). Moreover, distribution of the LRPs at stages I to VIII showed that developmental transition from stage I to II was significantly inhibited 18 h pgi and LRP development was significantly delayed 42 h pgi, compared with that of the wild type (Fig. 7F). These results showed that LBD18:SRDX suppressed LR initiation events, periclinal divisions of primordium after LR initiation, and later stages of LRP development in response to a gravitropic stimulus. In contrast, LBD18:SRDX did not suppress auxin-induced LR initiations compared with the wild type and lbd18 mutant (Supplemental Fig. S6), indicating that exogenous auxin overrides dominant repression of LR initiation by LBD18:SRDX, activating LR initiation.
Figure 7.
Analysis of LR formation of ProLBD18:LBD18:SRDX in wild-type (WT) and lbd18 mutant backgrounds. A, Seven-day-old seedlings of ProLBD18:LBD18:SRDX Arabidopsis and the wild type (Col-0). #, Line number of ProLBD18:LBD18:SRDX/Col-0 plants. B, Seven-day-old seedlings of ProLBD18:LBD18:SRDX/lbd18 plants and the lbd18 single mutant. #, Line number of ProLBD18:LBD18:SRDX/lbd18 plants. C, LR densities of Col-0, lbd18, ProLBD18:LBD18:SRDX/Col-0, and ProLBD18:LBD18:SRDX/lbd18 plants. Error bars = se. n = 20. Asterisks denote statistical significance (**, P < 0.01; and ***, P < 0.001). D, Expression of EXP14 in wild-type (Col-0), lbd18, ProLBD18:LBD18:SRDX/Col-0 (line no.), and ProLBD18:LBD18:SRDX/lbd18 plants. Seven-day-old seedlings were incubated with mock or 20 μm IAA for 6 h, and subjected to qRT-PCR for EXP14 expression. Relative transcript levels are plotted. Data are the mean ± se of three independent biological replications. Asterisks denote statistical significance (*, P < 0.05). LBD18:SRDX/Col-0 = ProLBD18:LBD18:SRDX/Col-0 (line#2–5); LBD18:SRDX/lbd18 = ProLBD18:LBD18:SRDX/lbd18 (line#1–5). E, Analyses of LR initiation events of Col-0, lbd18, and two different lines of ProLBD18:LBD18:SRDX/lbd18 plants after synchronization with a gravitropic stimulus. Three-day-old Col-0, lbd18, and ProLBD18:LBD18:GR/lbd18 transgenic plants treated with DEX were subjected to a 90° gravitropic stimulus, and the numbers of LR initiation events were determined at 18 h pgi. Error bars = se. n > 85. #, Line number of transgenic plants. F, Analyses of LR development of the wild type and two different lines of ProLBD18:LBD18:SRDX/lbd18 plants after synchronization with a gravitropic stimulus.
LBD18 Regulates Expression of Cell Cycle Genes CDKA1;1 and CYCB1;1
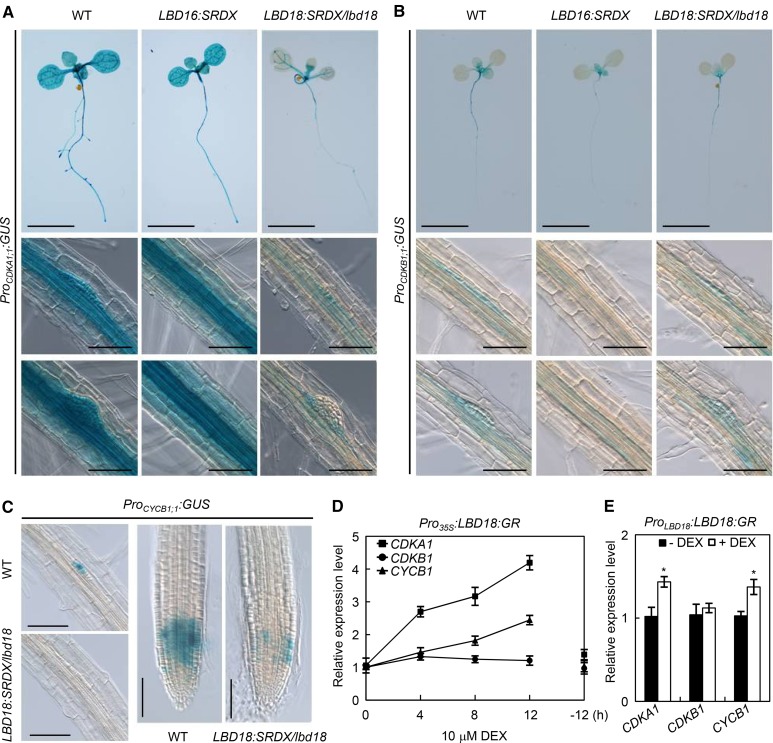
Several lines of evidence suggested that the regulated cell cycle is essential for asymmetric divisions of a subset of pericycle cells during LR initiation (De Smet, 2012). For example, progression of pericycles through the cell cycle is prevented by the treatment of auxin transport inhibitor in Arabidopsis seedlings or in a gain-of-function slr mutant blocking early auxin response (Fukaki et al., 2002; Himanen et al., 2002; Vanneste et al., 2005). Auxin activates many cell cycle-related genes, such as cyclins and CDKs, before pericycle division (Himanen et al., 2002). Various cell cycle genes have been identified to play a role in LR initiation (Himanen et al., 2002, 2004; Vanneste et al., 2005; Ren et al., 2008; Nieuwland et al., 2009; Sanz et al., 2011). To identify a link between LBD18 function and the regulation of cell cycle during LR initiation and LR development, we first made multiple mutants, lbd16 and/or lbd18, crossed to transgenic Arabidopsis harboring the promoter of cell cycle genes, CDKA1;1, CDKB1;1, or CYCB1;1, fused to the GUS reporter gene (Hemerly et al., 1993; Doerner et al., 1996; de Almeida Engler et al., 1999). However, none of the mutations in LBD16, LBD18, or LBD16 and LBD18 altered GUS expression in the LRPs due to the genetic redundancy with other LBD gene family members (Supplemental Fig. S7). We then constructed transgenic Arabidopsis expressing LBD18:SRDX under its own promoter and harboring CDKA1;1, CDKB1;1, or CYCB1;1-GUS in the lbd18 mutant background, and found that LBD18:SRDX greatly repressed GUS expression in the CDKA1;1 promoter but slightly suppressed GUS expression under the CDKB1;1 promoter (Fig. 8, A and B). LBD18:SRDX completely removed GUS expression under the CYCB1;1 promoter in the primordium and greatly reduced GUS staining in the root tip (Fig. 8C). LBD16:SRDX greatly reduced GUS expression under the CDKB1;1 promoter in the root (Fig. 8B). However, LBD16:SRDX did not affect GUS expression under the CDKA1;1 promoter (Fig. 8A). These results showed that LBD18:SRDX repressed the promoter activities of cell cycle genes, CDKA1;1 and CYCB1;1. We further showed that LBD18:GR up-regulates expression of CDKA1;1 and CYCB1;1, but not that of CDKB1:1, under the control of the Cauliflower mosaic virus 35S promoter (Fig. 8D) and under the control of the LBD18 promoter (Fig. 8E). These results indicated that LBD18 plays a role in the regulation of cell cycle genes. Cycloheximide treatment of Pro35S:LBD18:GR transgenic plants in the presence of DEX prevented DEX-inducible expression of CDKA1;1 and CYCB1;1 (Supplemental Fig. S8), demonstrating that induction of these cell cycle genes by LBD18 is a secondary response requiring new protein synthesis.
Figure 8.
GUS expression of Arabidopsis plants harboring the promoter of cell cycle genes fused to GUS reporter and ProLBD16:LBD16:SRDX or ProLBD18:LBD18:SRDX in lbd18. A and B, GUS expression of CDKA1;1::GUS (A) or CDKB1;1::GUS (B) in Col-0, ProLBD16:LBD16:SRDX, and ProLBD18:LBD18:SRDX/lbd18 plants. The lower two rows show GUS staining of the LRP. C, GUS expression of CYCB1;1::GUS in Col-0 and ProLBD18:LBD18:SRDX/lbd18 plants. GUS staining of the LRP at stage II and the primary root tip is shown. Bars = 1 cm (in seedlings) or 50 μm (in LRPs and root tip). D, Time course expression analysis of CDKA1;1, CDKB1;1, and CYCB1;1 genes following treatment with DEX in Pro35S:LBD18:GR plants. Seven-day-old Pro35S:LBD18:GR seedlings were incubated with 10 μm DEX for the indicated times, and subjected to qRT-PCR analysis for CDKA1;1, CDKB1;1, and CYCB1;1 gene expression; −12 h, Samples incubated with mock for 12 h. E, Expression of CDKA1;1, CDKB1;1, and CYCB1;1 following treatment with DEX in ProLBD18:LBD18:GR plants. ProLBD18:LBD18:GR seedlings were grown for 7 d in the absence (black squares) or presence (white squares) of DEX. Expression of CDKA1;1, CDKB1;1, and CYCB1;1 was then analyzed by qRT-PCR and plotted as relative expression levels. Asterisks denote statistical significance (*, P < 0.05).
DISCUSSION
Emerged LR densities of lax3 lbd16 and lax3 lbd18 were similar to those of lax3, lbd16, or lbd18 single mutants (Fig. 1B). However, the lax3 mutation in lbd16 lbd18 double mutants significantly reduced the emerged LR density to some extent, but not in an additive manner (Fig. 1B). These results indicate that, although LAX3 and LBD16 or LBD18 function largely in a linear pathway to control LR emergence, LAX3 also acts in parallel with LBD16 and LBD18 to some extent during LR emergence. Auxin-inducible expression of LBD16 and LBD18 and LBD18-regulated target genes, EXP14 and EXP17, significantly decreased in lax3 mutants as compared with the wild type (Fig. 2, A and B). In contrast, double mutations in LBD16 and LBD18 did not affect the expression of LAX3 in response to auxin (Fig. 2C). Altogether, genetic and gene expression analysis results indicate that LAX3 acts in part upstream of LBD16 and LBD18 during the auxin response, whereas LBD16 and LBD18 are not critically involved in auxin-responsive LAX3 expression. Consistent with this, overexpression of LBD18 in lax3 fully restored the defect in LR emergence of lax3 to the wild-type levels with concomitant overexpression of LBD18 target genes, EXP14 and EXP17 (Fig. 3, A and B). However, in the case of using the LBD18 promoter to express LBD18 in lax3, the defect in LR formation in lax3 was rescued to some extent, although only in the presence of auxin. This result indicates that lax3 mutation produced some defects in LR formation that are caused by other components and/or transcription factors acting downstream of LAX3 as well as the defect in LBD18 function under the LBD18 promoter. Auxin activates the signaling pathway, inducing expression of certain transcription factor genes along with which LBD18 acts to promote LR formation to a certain degree in the absence of lax3 function. We further showed that LBD18 up-regulates expression of EXP14 and EXP17 in lax3 mutants under the control of the LBD18 promoter (Fig. 3, D and E), indicating that LBD18 can regulate its target gene expression in the absence of LAX3 function in tissues where LBD18 is normally expressed. Expression of PG, encoding a cell wall remodeling enzyme, whose expression is dependent upon LAX3 function (Swarup et al., 2008), was down-regulated in the overlaying tissues of the LRP in lbd18 and up-regulated by LBD18 expression under the control of the Cauliflower mosaic virus 35S promoter and under the control of the LBD18 promoter (Fig. 4). Taken together, these results demonstrated that LBD18 modulates its target gene expression downstream of LAX3 to promote LR emergence. Overlapping expression patterns of LAX3 and LBD18 in the overlaying tissues of the LRP (Supplemental Fig. S1) and reduced GUS expression under the control of the LBD18 promoter by lax3 mutation in the same tissue (Fig. 2, D–G) are consistent with the notion that LAX3 induces LBD18 expression by promoting auxin influx into the cortex and epidermis to facilitate LR emergence. We noted significant auxin-inducible expression of LBD16, LBD18, EXP14, and EXP17 in lax3, which may be due to involvement of other auxin transporters. Two additional LAX genes, LAX1 and LAX2 (Kerr and Bennett, 2007; Ugartechea-Chirino et al., 2010), could be involved redundantly in auxin-responsive expression of LBD16 and LBD18. Several studies have demonstrated that PINs are involved in LR development (Benková et al., 2003; Laskowski et al., 2008; Marhavý et al., 2013; Péret et al., 2013). However, it remains to be determined whether other LAXs and PINs are involved in auxin-responsive expression of LBD16 and LBD18 to control LR development.
It has been reported that the increased number of primordia at stage I in lax3 (Fig. 1C) was due to accumulation of leaf-derived auxin at supraoptimal levels in the root pericycle, thus stimulating the ectopic initiation of new primordia in lax3, but not due to the defects in LR initiation (Fig. 5C; Swarup et al., 2008). We found that lbd18 mutation reduced the primordium number at stage I, which was exaggerated by lax3 mutation (Fig. 1C). This result indicates involvement of LBD18 in LR initiation. A previous study showed that lbd18 mutation did not affect the distribution of the LRPs at all developmental stages, but significantly reduced the number of emerged LRs as compared with the wild type, indicating that the emergence of LRs in the lbd18 mutant is a rate-limiting step in LR formation compared with the wild type (Lee et al., 2009b). The present genetic analysis results with multiple mutants of aux1 or lax3 provided the evidence that LBD18 is involved in LR initiation and LRP development in addition to playing a role in LR emergence (Figs. 1 and 5). This notion was further confirmed by the analysis of LR development kinetics using transgenic Arabidopsis expressing LBD18:SRDX under its own promoter in the lbd18 mutant background (Fig. 7). We found that LBD18 target gene expression and LR formation by LBD18:SRDX were not affected in wild-type plants when compared with that in the lbd18 mutant background, indicating that wild-type LBD18 homodimers (Lee et al., 2013b) might have higher DNA-binding affinity to the target promoter and/or higher transcriptional activity than LBD18:SRDX homodimers and thus can overcome dominant repression by LBD18:SRDX.
A model integrating the present data and previous reports (Marchant et al., 2002; Okushima et al., 2007; Swarup et al., 2008; Lee et al., 2009a,b, 2013a; Berckmans et al., 2011; Goh et al., 2012a; Lee and Kim, 2013) into a gene regulatory pathway for LR development is presented in Figure 9. LBD16 plays a role in the polar nuclear migration in the LR founder cell after LR priming (Goh et al., 2012a), whereas LBD18 is involved in asymmetric anticlinal division during LR initiation and periclinal divisions of the LRP initiated in the xylem pole pericycle cells and in the progression of LRP development through the regulation of cell cycle genes such as CDKA1;1 and CYCB1;1 (Figs. 7 and 8). LBD18 also activates E2Fa expression for LR initiation (Berckmans et al., 2011). LBD16 and LBD18 function together to regulate LRP development with AUX1 (Figs. 5 and 6). LAX3 activates the SLR/IAA14-ARF7-ARF19 signaling module (Swarup et al., 2008), thus inducing LBD18 expression (Figs. 1–3). LBD18 in the endodermis and cortex in turn directly activates EXP14 and indirectly activates EXP17 and other EXPs (Kim and Lee, 2013; Lee et al., 2013a; Lee and Kim, 2013) for cell wall loosening, and induces PG expression at a later time (Fig. 4) for cell wall remodeling, which facilitates local cell separation. PG enzymatically modifies the structures of the cell wall, rendering it more responsive to wall loosening mediated by expansions (Cosgrove, 2000; Péret et al., 2009a, 2009b). These molecular events help promote the emergence of LRP through the outer cell layers of the primary root. As ARF7 induces expression of ARF19 and LBD16 in response to auxin signal, and subsequently ARF19 induces LBD18 expression (Okushima et al., 2007; Lee et al., 2009a), the ARF7/ARF19-LBD16/LBD18 transcriptional network via the AUX1/LAX3 auxin influx carriers plays a key role in controlling LR development from polar nuclear migration of LR founder cells to initiation, primordium development, and emergence of LR during the auxin response.
Figure 9.
A model showing the molecular signaling network of LBD16 and LBD18 via the auxin influx carriers AUX1 and LAX3 during LR development. The column shaded with lines indicates the xylem. The thick arrow and dotted arrow indicate direct and indirect activation of target genes, respectively. P, Pericycle; En, endodermis; C, cortex; Ep, epidermis.
MATERIALS AND METHODS
Plant Growth and Tissue Treatment
Arabidopsis (Arabidopsis thaliana; Col-0) seedlings were grown and treated as described previously (Park et al., 2002). For the treatment of hormone and chemicals, plants were grown in a 16-h photoperiod on 3MM Whatman filter paper (GE Healthcare) on top of agar plates, and the filter paper with the seedlings was then transferred to a plate containing plant hormone or chemicals (20 μm for IAA, 10 μm for DEX, or 50 μm cycloheximide) and incubated for a given period of time with gentle shaking in the light at 23°C. The light intensity was approximately 120 μmol m−2 s−1 and was provided by three wavelength daylight color fluorescent bulbs (Kumho Electric Co.).
Plant Materials
All plants used were the Arabidopsis Col-0 ecotype. The lax3 and aux1-21 Arabidopsis mutants and the ProPG:GUS line were provided by Dr. Malcolm Bennett (University of Nottingham) and confirmed via genotyping prior to usage (Swarup et al., 2008). The lbd16, lbd18, or lbd16 lbd18 mutants (female) were crossed with lax3, aux1-21, and aux1 lax3 mutants (male) to generate lax3 lbd16, lax3 lbd18, lax3 lbd16 lbd18, aux1 lbd16, aux1 lbd18, aux1 lbd16 lbd18, aux1 lax3 lbd16, aux1 lax3 lbd18, and aux1 lax3 lbd16 lbd18 mutants, and the homozygous lines were isolated based on PCR genotyping and agrotropic phenotype of aux1-21 (Swarup et al., 2008; Lee et al., 2009b). Transgenic mutant plants, ProPG:GUS/lbd16, ProPG:GUS/lbd18, and ProPG:GUS/lbd16lbd18, were generated by crossing lbd16, lbd18, and lbd16 lbd18 mutants (female) with the ProPG:GUS line (male), and the homozygous lines were isolated. The lbd16, lbd18, or lbd16 lbd18 mutants and ProLBD16:LBD16:SRDX or ProLBD18:LBD18:SRDX/lbd18 transgenic Arabidopsis (female) were crossed with the ProCDKA1;1:GUS, ProCDKB1;1:GUS, or ProCYCB1;1:GUS line (male) to generate ProCDKA1;1:GUS, ProCDKB1;1:GUS, and ProCYCB1;1:GUS reporters in lbd16, lbd18, and lbd16 lbd18 mutant backgrounds and in ProLBD16:LBD16:SRDX and ProLBD18:LBD18:SRDX/lbd18 transgenic Arabidopsis. Homozygous lines were isolated by genotyping for lbd16, lbd18, lbd16 lbd18, ProLBD16:LBD16:SRDX, and ProLBD18:LBD18:SRDX/lbd18 and by PCR detection of genomic DNA for the CDKA1;1::GUS, CDKB1;1::GUS, or CYCB1;1::GUS transgenes. Pro35S:LBD18:GR and ProLBD18:LBD18:GR in the lax3 mutant background (Pro35S:LBD18:GR/lax3 and ProLBD18:LBD18:GR/lax3) were generated by crossing Pro35S:LBD18:GR and ProLBD18:LBD18:GR (female) with lax3 (male), and the homozygous lines were isolated. ProLBD16:GUS and ProLBD18:GUS transgenic Arabidopsis in aux1 or lax3 mutant backgrounds (ProLBD16:GUS/aux1 and ProLBD18:GUS/aux1 or ProLBD16:GUS/lax3 and ProLBD18:GUS/lax3) were generated by crossing ProLBD16:GUS and ProLBD18:GUS (female) with aux1 or lax3 (male). Homozygous lines were isolated by genotyping for ProLBD16:GUS, ProLBD18:GUS, and lax3 or agrotropic phenotype of aux1-21. Primer sequences used in this study are shown in Supplemental Table S1.
Plasmid Construction and Arabidopsis Transformation
To generate ProLBD16:LBD16:SRDX, ProLBD16:LBD16:SRDX/lbd16, ProLBD18:LBD18:SRDX, and ProLBD18:LBD18:SRDX/lbd18 plants, the ProLBD16:LBD16 and ProLBD18:LBD18 sequences were isolated by PCR from the existing ProLBD16:LBD16:GR and ProLBD18:LBD18:GR plasmids (Lee et al., 2013a) using the primers with SRDX sequence (NH2-LDLDLELRLGFA-COOH) and subcloned into pDONR221 (Invitrogen) by the Gateway BP recombination reaction, yielding pDONR-ProLBD16:LBD16:SRDX and pDONR-ProLBD18:LBD18:SRDX, respectively. This construct was subcloned into pB7WG vector (destination vector, Vlaams Instituut voor Biotechnologie) by the Gateway LR recombination reaction, yielding ProLBD16:LBD16:SRDX and ProLBD18:LBD18:SRDX. ProLBD16:LBD16:SRDX and ProLBD18:LBD18:SRDX plasmids were then introduced into Arabidopsis, lbd16, or lbd18 mutants by Agrobacterium tumefaciens-mediated transformation, respectively. T3 homozygous transformants were prepared and amplified. All constructs were confirmed via DNA sequencing prior to plant transformation. Primer sequences used in this study are shown in Supplemental Table S1.
RNA Isolation, Reverse Transcription-PCR, and qRT-PCR Analysis
Following treatment, Arabidopsis plants were immediately frozen in liquid nitrogen and stored at −80°C. Total RNA was isolated from frozen Arabidopsis using TRI Reagent (Molecular Research Center). For reverse transcription (RT)-PCR analysis, total RNA was isolated using an RNeasy plant mini kit (Qiagen) and subjected to RT-PCR analysis with the Access RT-PCR System (Promega) according to the manufacturer’s instructions. Real-time RT-PCR was carried out using a QuantiTect SYBR Green RT-PCR kit (Qiagen) in a CFX96 real-time system using a C1000 thermal cycler (Bio-Rad) as described previously (Jeon et al., 2010). All real-time RT-PCR analyses were conducted in triplicate biological replications and subjected to statistical analysis. RT-PCR conditions and primer sequences are shown in Supplemental Table S1.
Microscopy and Histochemical GUS Assays
For whole-mount visualization, the seedlings were then cleared in 80% (v/v) ethanol for 24 h and mounted in 90% (v/v) glycerol and observed under the Leica DM2500 microscope with differential interference contrast according to Malamy and Benfey (1997). Histochemical assays for GUS activity were performed with 5-bromo-4-chloro-3-indolyl glucuronide, as described previously (Jefferson and Wilson, 1991). Samples were observed using a DM2500 microscope (Leica) at 200- or 400-fold magnification with differential interference contrast.
Statistical Analysis
Quantitative data were subjected to statistical analysis for every pairwise comparison, using the software for Student’s t test (Predictive Analytics Software for Windows, version 20.0).
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: LBD16 (At2g42430), LBD18 (At2g45420), AUX1 (At2g38120), LAX3 (At1g77690), PG (At5g14650), CDKA1;1 (At3g48750), CDKB1;1 (At3g54180), CYCB1;1 (At4g37490), EXP14, (At5g56320), and EXP17 (At4g01630).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. GUS expression of ProLBD16:GUS, ProLBD18:GUS, and ProLAX3:GUS transgenic Arabidopsis during LR development.
Supplemental Figure S2. LR densities of wild-type, lax3, and ProLBD18:LBD18:GR/lax3 plants without or with DEX.
Supplemental Figure S3. LR phenotypes of aux1 lax3, aux1 lax3 lbd16, aux1 lax3 lbd18, and aux1 lax3 lbd16 lbd18 mutants.
Supplemental Figure S4. Analysis of LR formation of ProLBD16:LBD16:SRDX transgenic plants in Col-0 and lbd16 mutant backgrounds.
Supplemental Figure S5. Analysis of the numbers of primordia at different stages of LRP development in 7-d-old wild-type (Col-0), lbd18, ProLBD18:LBD18:SRDX/Col-0, and ProLBD18:LBD18:SRDX/lbd18 plants.
Supplemental Figure S6. Differential interference contrast images of LR initiations of wild-type (Col-0), lbd18, and ProLBD18:LBD18:SRDX/lbd18 plants without or with auxin.
Supplemental Figure S7. Analysis of GUS expression of transgenic Arabidopsis harboring the promoter of cell-cycle genes fused to GUS in lbd16, lbd18, or lbd16 lbd18 mutant backgrounds.
Supplemental Figure S8. The effect of cycloheximide treatment on DEX-induced expression of CDKA1;1 or CycB1;1 in Pro35S:LBD18:GR transgenic Arabidopsis.
Supplemental Table S1. Oligonucleotides and PCR conditions.
Supplementary Material
Acknowledgments
We thank Dr. Bennett for lax3, aux1-21, LAX3:GUS, and PG:GUS seeds and Dr. De Veylder for the CDKA1;1:GUS, CDKB1;1:GUS, and CYCB1;1:GUS seeds, and Arabidopsis Biological Resource Center for transfer DNA insertion mutants.
Glossary
- LR
lateral root
- LRP
lateral root primordium
- Col-0
Columbia-0
- qRT
quantitative reverse transcription
- pgi
postgravitropic induction
- RT
reverse transcription
Footnotes
This work was supported by the Next-Generation BioGreen 21 Program (grant no. PJ01104701), Rural Development Administration, Republic of Korea (grant to J.K.), Basic Science Research Programs through the National Research Foundation of Korea funded by the Ministry of Education (grant no. 2013R1A6A3A01062668), and Chonnam National University, 2013.
References
- Band LR, Wells DM, Fozard JA, Ghetiu T, French AP, Pound MP, Wilson MH, Yu L, Li W, Hijazi HI, et al. (2014) Systems analysis of auxin transport in the Arabidopsis root apex. Plant Cell 26: 862–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, Friml J (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602 [DOI] [PubMed] [Google Scholar]
- Berckmans B, Vassileva V, Schmid SPC, Maes S, Parizot B, Naramoto S, Magyar Z, Alvim Kamei CL, Koncz C, Bögre L, et al. (2011) Auxin-dependent cell cycle reactivation through transcriptional regulation of Arabidopsis E2Fa by lateral organ boundary proteins. Plant Cell 23: 3671–3683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ. (2000) Expansive growth of plant cell walls. Plant Physiol Biochem 38: 109–124 [DOI] [PubMed] [Google Scholar]
- Dastidar MG, Jouannet V, Maizel A (2012) Root branching: mechanisms, robustness, and plasticity. Wiley Interdiscip Rev Dev Biol 1: 329–343 [DOI] [PubMed] [Google Scholar]
- De Rybel B, Vassileva V, Parizot B, Demeulenaere M, Grunewald W, Audenaert D, Van Campenhout J, Overvoorde P, Jansen L, Vanneste S, et al. (2010) A novel aux/IAA28 signaling cascade activates GATA23-dependent specification of lateral root founder cell identity. Curr Biol 20: 1697–1706 [DOI] [PubMed] [Google Scholar]
- De Smet I. (2012) Lateral root initiation: one step at a time. New Phytol 193: 867–873 [DOI] [PubMed] [Google Scholar]
- De Smet I, Lau S, Voss U, Vanneste S, Benjamins R, Rademacher EH, Schlereth A, De Rybel B, Vassileva V, Grunewald W, et al. (2010) Bimodular auxin response controls organogenesis in Arabidopsis. Proc Natl Acad Sci USA 107: 2705–2710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smet I, Tetsumura T, De Rybel B, Frei dit Frey N, Laplaze L, Casimiro I, Swarup R, Naudts M, Vanneste S, Audenaert D, et al. (2007) Auxin-dependent regulation of lateral root positioning in the basal meristem of Arabidopsis. Development 134: 681–690 [DOI] [PubMed] [Google Scholar]
- Doerner P, Jørgensen JE, You R, Steppuhn J, Lamb C (1996) Control of root growth and development by cyclin expression. Nature 380: 520–523 [DOI] [PubMed] [Google Scholar]
- Dubrovsky JG, Napsucialy-Mendivil S, Duclercq J, Cheng Y, Shishkova S, Ivanchenko MG, Friml J, Murphy AS, Benková E (2011) Auxin minimum defines a developmental window for lateral root initiation. New Phytol 191: 970–983 [DOI] [PubMed] [Google Scholar]
- Dubrovsky JG, Sauer M, Napsucialy-Mendivil S, Ivanchenko MG, Friml J, Shishkova S, Celenza J, Benková E (2008) Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. Proc Natl Acad Sci USA 105: 8790–8794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida Engler J, De Vleesschauwer V, Burssens S, Celenza JL Jr, Inzé D, Van Montagu M, Engler G, Gheysen G (1999) Molecular markers and cell cycle inhibitors show the importance of cell cycle progression in nematode-induced galls and syncytia. Plant Cell 11: 793–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, Hamann T, Offringa R, Jürgens G (2003) Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 426: 147–153 [DOI] [PubMed] [Google Scholar]
- Fukaki H, Tameda S, Masuda H, Tasaka M (2002) Lateral root formation is blocked by a gain-of-function mutation in the SOLITARY-ROOT/IAA14 gene of Arabidopsis. Plant J 29: 153–168 [DOI] [PubMed] [Google Scholar]
- Goh T, Joi S, Mimura T, Fukaki H (2012a) The establishment of asymmetry in Arabidopsis lateral root founder cells is regulated by LBD16/ASL18 and related LBD/ASL proteins. Development 139: 883–893 [DOI] [PubMed] [Google Scholar]
- Goh T, Kasahara H, Mimura T, Kamiya Y, Fukaki H (2012b) Multiple AUX/IAA-ARF modules regulate lateral root formation: the role of Arabidopsis SHY2/IAA3-mediated auxin signalling. Philos Trans R Soc Lond B Biol Sci 367: 1461–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Carranza ZH, Elliott KA, Roberts JA (2007) Expression of polygalacturonases and evidence to support their role during cell separation processes in Arabidopsis thaliana. J Exp Bot 58: 3719–3730 [DOI] [PubMed] [Google Scholar]
- Hadfield KA, Bennett AB (1998) Polygalacturonases: many genes in search of a function. Plant Physiol 117: 337–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemerly AS, Ferreira P, de Almeida Engler J, Van Montagu M, Engler G, Inzé D (1993) cdc2a expression in Arabidopsis is linked with competence for cell division. Plant Cell 5: 1711–1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himanen K, Boucheron E, Vanneste S, de Almeida Engler J, Inzé D, Beeckman T (2002) Auxin-mediated cell cycle activation during early lateral root initiation. Plant Cell 14: 2339–2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himanen K, Vuylsteke M, Vanneste S, Vercruysse S, Boucheron E, Alard P, Chriqui D, Van Montagu M, Inzé D, Beeckman T (2004) Transcript profiling of early lateral root initiation. Proc Natl Acad Sci USA 101: 5146–5151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiratsu K, Matsui K, Koyama T, Ohme-Takagi M (2003) Dominant repression of target genes by chimeric repressors that include the EAR motif, a repression domain, in Arabidopsis. Plant J 34: 733–739 [DOI] [PubMed] [Google Scholar]
- Hochholdinger F, Zimmermann R (2008) Conserved and diverse mechanisms in root development. Curr Opin Plant Biol 11: 70–74 [DOI] [PubMed] [Google Scholar]
- Jefferson RA, Wilson KJ (1991) The GUS gene fusion system. Plant Mol Biol Manual B14: 1–33 [Google Scholar]
- Jeon J, Kim NY, Kim S, Kang NY, Novák O, Ku SJ, Cho C, Lee DJ, Lee EJ, Strnad M. , et al. (2010) A subset of cytokinin two-component signaling system plays a role in cold temperature stress response in Arabidopsis. J Biol Chem 285: 23371–23386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr ID, Bennett MJ (2007) New insight into the biochemical mechanisms regulating auxin transport in plants. Biochem J 401: 613–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Lee HW (2013) Direct activation of EXPANSIN14 by LBD18 in the gene regulatory network of lateral root formation in Arabidopsis. Plant Signal Behav 8: e22979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski M, Biller S, Stanley K, Kajstura T, Prusty R (2006) Expression profiling of auxin-treated Arabidopsis roots: toward a molecular analysis of lateral root emergence. Plant Cell Physiol 47: 788–792 [DOI] [PubMed] [Google Scholar]
- Laskowski M, Grieneisen VA, Hofhuis H, Hove CA, Hogeweg P, Marée AF, Scheres B (2008) Root system architecture from coupling cell shape to auxin transport. PLoS Biol 6: e307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavenus J, Goh T, Roberts I, Guyomarc’h S, Lucas M, De Smet I, Fukaki H, Beeckman T, Bennett M, Laplaze L (2013) Lateral root development in Arabidopsis: fifty shades of auxin. Trends Plant Sci 18: 450–458 [DOI] [PubMed] [Google Scholar]
- Lee DJ, Park JW, Lee HW, Kim J (2009a) Genome-wide analysis of the auxin-responsive transcriptome downstream of iaa1 and its expression analysis reveal the diversity and complexity of auxin-regulated gene expression. J Exp Bot 60: 3935–3957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HW, Kim J (2013) EXPANSINA17 up-regulated by LBD18/ASL20 promotes lateral root formation during the auxin response. Plant Cell Physiol 54: 1600–1611 [DOI] [PubMed] [Google Scholar]
- Lee HW, Kim MJ, Kim NY, Lee SH, Kim J (2013a) LBD18 acts as a transcriptional activator that directly binds to the EXPANSIN14 promoter in promoting lateral root emergence of Arabidopsis. Plant J 73: 212–224 [DOI] [PubMed] [Google Scholar]
- Lee HW, Kim MJ, Park MY, Han KH, Kim J (2013b) The conserved proline residue in the LOB domain of LBD18 is critical for DNA-binding and biological function. Mol Plant 6: 1722–1725 [DOI] [PubMed] [Google Scholar]
- Lee HW, Kim NY, Lee DJ, Kim J (2009b) LBD18/ASL20 regulates lateral root formation in combination with LBD16/ASL18 downstream of ARF7 and ARF19 in Arabidopsis. Plant Physiol 151: 1377–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy JE, Benfey PN (1997) Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124: 33–44 [DOI] [PubMed] [Google Scholar]
- Marchant A, Bhalerao R, Casimiro I, Eklöf J, Casero PJ, Bennett M, Sandberg G (2002) AUX1 promotes lateral root formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. Plant Cell 14: 589–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marhavý P, Vanstraelen M, De Rybel B, Zhaojun D, Bennett MJ, Beeckman T, Benková E (2013) Auxin reflux between the endodermis and pericycle promotes lateral root initiation. EMBO J 32: 149–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwland J, Maughan S, Dewitte W, Scofield S, Sanz L, Murray JAH (2009) The D-type cyclin CYCD4;1 modulates lateral root density in Arabidopsis by affecting the basal meristem region. Proc Natl Acad Sci USA 106: 22528–22533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okushima Y, Fukaki H, Onoda M, Theologis A, Tasaka M (2007) ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis. Plant Cell 19: 118–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parizot B, Laplaze L, Ricaud L, Boucheron-Dubuisson E, Bayle V, Bonke M, De Smet I, Poethig SR, Helariutta Y, Haseloff J, et al. (2008) Diarch symmetry of the vascular bundle in Arabidopsis root encompasses the pericycle and is reflected in distich lateral root initiation. Plant Physiol 146: 140–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JY, Kim HJ, Kim J (2002) Mutation in domain II of IAA1 confers diverse auxin-related phenotypes and represses auxin-activated expression of Aux/IAA genes in steroid regulator-inducible system. Plant J 32: 669–683 [DOI] [PubMed] [Google Scholar]
- Péret B, De Rybel B, Casimiro I, Benková E, Swarup R, Laplaze L, Beeckman T, Bennett MJ (2009a) Arabidopsis lateral root development: an emerging story. Trends Plant Sci 14: 399–408 [DOI] [PubMed] [Google Scholar]
- Péret B, Larrieu A, Bennett MJ (2009b) Lateral root emergence: a difficult birth. J Exp Bot 60: 3637–3643 [DOI] [PubMed] [Google Scholar]
- Péret B, Middleton AM, French AP, Larrieu A, Bishopp A, Njo M, Wells DM, Porco S, Mellor N, Band LR, et al. (2013) Sequential induction of auxin efflux and influx carriers regulates lateral root emergence. Mol Syst Biol 9: 699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péret B, Swarup K, Ferguson A, Seth M, Yang Y, Dhondt S, James N, Casimiro I, Perry P, Syed A, et al. (2012) AUX/LAX genes encode a family of auxin influx transporters that perform distinct functions during Arabidopsis development. Plant Cell 24: 2874–2885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H, Santner A, del Pozo JC, Murray JA, Estelle M (2008) Degradation of the cyclin-dependent kinase inhibitor KRP1 is regulated by two different ubiquitin E3 ligases. Plant J 53: 705–716 [DOI] [PubMed] [Google Scholar]
- Sanz L, Dewitte W, Forzani C, Patell F, Nieuwland J, Wen B, Quelhas P, De Jager S, Titmus C, Campilho A, et al. (2011) The Arabidopsis D-type cyclin CYCD2;1 and the inhibitor ICK2/KRP2 modulate auxin-induced lateral root formation. Plant Cell 23: 641–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup K, Benková E, Swarup R, Casimiro I, Péret B, Yang Y, Parry G, Nielsen E, De Smet I, Vanneste S, et al. (2008) The auxin influx carrier LAX3 promotes lateral root emergence. Nat Cell Biol 10: 946–954 [DOI] [PubMed] [Google Scholar]
- Swarup R, Friml J, Marchant A, Ljung K, Sandberg G, Palme K, Bennett M (2001) Localization of the auxin permease AUX1 suggests two functionally distinct hormone transport pathways operate in the Arabidopsis root apex. Genes Dev 15: 2648–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup R, Péret B (2012) AUX/LAX family of auxin influx carriers-an overview. Front Plant Sci 3: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugartechea-Chirino Y, Swarup R, Swarup K, Péret B, Whitworth M, Bennett M, Bougourd S (2010) The AUX1 LAX family of auxin influx carriers is required for the establishment of embryonic root cell organization in Arabidopsis thaliana. Ann Bot (Lond) 105: 277–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste S, De Rybel B, Beemster GT, Ljung K, De Smet I, Van Isterdael G, Naudts M, Iida R, Gruissem W, Tasaka M, et al. (2005) Cell cycle progression in the pericycle is not sufficient for SOLITARY ROOT/IAA14-mediated lateral root initiation in Arabidopsis thaliana. Plant Cell 17: 3035–3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.