HDA6 affects the cellular patterning of Arabidopsis root epidermis by altering the histone acetylation status of two promoters.
Abstract
Cellular patterning in the Arabidopsis (Arabidopsis thaliana) root epidermis is dependent on positional information, the transmission of which involves histone acetylation. Here, we report that HISTONE DEACETYLASE6 (HDA6) has significant effects on this cellular patterning. Mutation of HDA6 led to ectopic hair cells in the nonhair positions of root epidermis in Arabidopsis, based on an analysis of paraffin sections stained with Toluidine Blue. While HDA6 was present throughout the root tip, epidermis-specific complementation with HDA6 could rescue the hda6 phenotype. Both transcript levels and expression patterns of ENHANCER OF TRIPTYCHON AND CAPRICE1 (ETC1) and GLABRA2 (GL2) in the root tip were affected in hda6. Consistent with these changes in expression, HDA6 directly bound to the promoter regions of ETC1 and GL2, and acetylation of histone H3 on these promoter regions and acetylation of histone H4 on the ETC1 promoter region was increased in the hda6 mutant. Taken together, these results indicate that HDA6 affects the cellular patterning of Arabidopsis root epidermis through altering the histone acetylation status of ETC1 and GL2 promoters and thereby affects the expression of these two components of the core transcription factor network determining epidermal cell fates. Our findings thus provide new insights into the role of histone acetylation in root epidermis cell patterning.
Pattern formation is an important event during the morphogenesis of a multicellular organism. In Arabidopsis (Arabidopsis thaliana), root epidermis is a well-established model system for studying pattern formation in plant development (Schiefelbein, 2003; Schiefelbein et al., 2009, 2014; Grebe, 2012). The root epidermis comprises hair cells and nonhair cells. These two types of epidermal cells have different cytoplasmic characteristics, and their fates are determined in a position-dependent manner: epidermal cells overlying two cortical cells (the H position) adopt the hair cell fate, while epidermal cells located over a single cortical cell (the N position) adopt the nonhair cell fate (Dolan et al., 1993; Galway et al., 1994; Berger et al., 1998).
The position-dependent cellular patterning of Arabidopsis root epidermis is regulated mainly by a system consisting of at least three levels. The first level is a GLABRA2 (GL2)-centered transcription factor network including three types of proteins: the Myb domain proteins WEREWOLF (WER), CAPRICE (CPC), TRIPTYCHON (TRY), and ENHANCER OF TRIPTYCHON AND CAPRICE1 (ETC1; Wada et al., 1997; Lee and Schiefelbein, 1999; Schellmann et al., 2002; Kirik et al., 2004); the basic helix-loop-helix proteins GL3 and ENHANCER OF GALBRA3 (EGL3; Bernhardt et al., 2003, 2005); and a WD-repeat protein, TRANSPARENT TESTA GLABRA (TTG; Galway et al., 1994; Walker et al., 1999; Supplemental Fig. S1). The genes encoding these proteins are referred to as pattern genes and determine the fates of epidermal cells in the N and H positions. Additional components functioning at this level include MYB23 (Kang et al., 2009), GEM (for GL2 expression modulator; Caro et al., 2007), MYC1 (Bruex et al., 2012), Zinc Finger Protein5 (An et al., 2012), and ADENOSINEDIMETHYL TRANSFERASE1A (Wieckowski and Schiefelbein, 2012). The second level of the root epidermis cellular patterning system is sensor pathways consisting of membrane-localized receptor-like kinases, such as SCRAMBLED (SCM; Kwak et al., 2005; Kwak and Schiefelbein, 2007, 2008) and BRASSINOSTEROID INSENSITIVE1 (Kuppusamy et al., 2009). These factors seem to sense and interpret the positional information. The third level of the system concerns the source of the positional information; at this level, JACKDAW functions in the cortical cells to affect epidermal cell patterning (Hassan et al., 2010). However, little is known about how the positional information is transmitted from cortical cells to the epidermal cells.
In our previous work, we found that treatment with trichostatin A (an inhibitor of histone deacetylases) can alter cellular patterning in the Arabidopsis root epidermis by affecting the histone acetylation status at promoters of the pattern genes (Xu et al., 2005). HISTONE DEACETYLASE18 (HDA18), a member of the HDAC (for histone deacetylase) family, affects epidermal cell patterning by regulating the transcription of a group of kinase genes through histone acetylation (Liu et al., 2013a). In a phenotype screen of single mutants from the Arabidopsis HDAC family, we found that the single mutant of HDA6 also displayed altered cellular patterning of root epidermis. HDA6 is a class I reduced potassium dependency3-like HDAC (Pandey et al., 2002) and has been reported to have HDAC enzyme activity (Earley et al., 2006). HDA6 is localized in the nucleus, supporting its involvement in transcriptional regulation (Earley et al., 2006; Wu et al., 2008). HDA6 is involved in many biological processes, including gene silencing (Murfett et al., 2001; Probst et al., 2004; Earley et al., 2006, 2010; Liu et al., 2012; Pontvianne et al., 2013), RNA-directed DNA methylation (Aufsatz et al., 2002, 2007), jasmonic acid response (Devoto et al., 2002; Wu et al., 2008; Zhu et al., 2011), and flowering (Wu et al., 2008; Yu et al., 2011).
Here, we demonstrate the involvement of HDA6 in affecting the cellular patterning in Arabidopsis root epidermis via various genetic approaches. We found evidence that HDA6 directly binds to the promoter regions of pattern genes ETC1 and GL2, which are involved in regulating epidermal root cell identity, and loss of HDA6 activity causes increased acetylation and expression of ETC1 and GL2, resulting in altered cellular patterning. In addition, we found subtle differences between the effects of HDA6 on ETC1 and GL2. Taken together with the role of HDA18, we conclude that histone acetylation plays indispensable roles in various aspects of the regulating system in determining the cellular pattern of Arabidopsis root epidermis.
RESULTS
HDA6 Is Required for Proper Cellular Patterning of Arabidopsis Root Epidermis
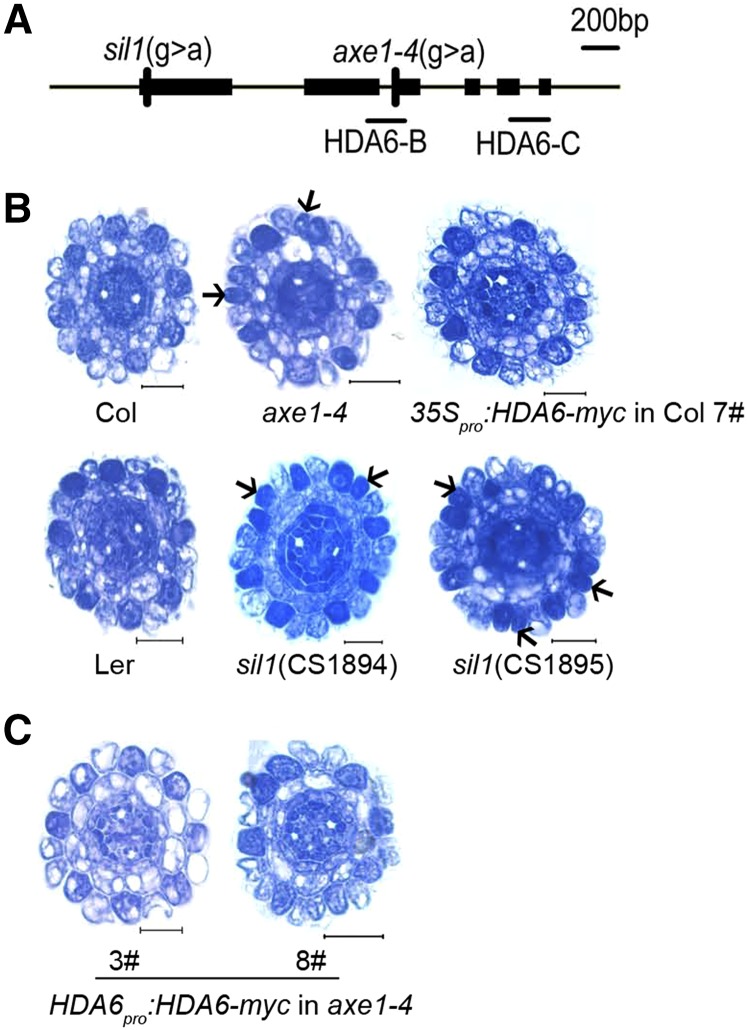
To test for HDA6 involvement in the cellular patterning of Arabidopsis root epidermis, we analyzed the available hda6 mutants, namely auxin gene expression mutants1-4 (axe1-4; Columbia [Col] background bearing DR5pro:GUS, a base substitution [G→A] at position 1,492 bp downstream of the ATG, at the second intron-exon junction; Murfett et al., 2001) and silencing1 (sil1; Landsberg erecta [Ler] background, CS1894 and CS1895, each having a G→A point mutation, 46 bp after the start codon and resulting in the replacement of Gly-16 by Arg; Probst et al., 2004). The expression of HDA6 in the root tip was reduced about 80% in the axe1-4 mutant compared with Col and was reduced only 10% to 15% in the sil1 mutant compared with Ler (Fig. 1A; Supplemental Fig. S2, A and B). Both axe1-4 and sil1 showed that the densely stained N-position cells were increased from around 2% to 3% in the wild type to around 12% to 17% in the mutant, indicative of ectopically developing hair cells at the N position in the epidermis (Fig. 1B; Table I).
Figure 1.
The hda6 mutant displays ectopic development of hair cells at the N position in the root epidermis. A, Schematic representation of hda6 point mutations. There are two sil1 mutant lines, CS1894 and CS1895, both with a point mutation 46 bp after the start codon that results in the replacement of Gly-16 by Arg. The axe1-4 mutant has a base substitution at the second intron-exon junction (1,492 bp downstream of the start codon). The short black lines indicate regions selected for quantitative reverse transcription (qRT)-PCR. B, Cross sections of root tips of Col, axe1-4, and overexpression line (top row, left to right), and Ler, sil1 (CS1894), and sil1 (CS1895; bottom row, left to right). Arrows indicate ectopically developing hair cells. Bars = 20 μm. C, Cross sections of root tips of hda6 complementation lines harboring a genomic HDA6 DNA fragment. Bars = 20 μm.
Table I. Cellular patterning in the root epidermis of hda6 mutants and transgenic plants.
At least 12 7-d-old seedlings were examined for each plant line. Values indicate means ± sd.
| Genotype |
H Position |
N Position |
||
|---|---|---|---|---|
| Developing Hair Cell | Developing Nonhair Cell | Developing Hair Cell | Developing Nonhair Cell | |
| % | ||||
| Col | 99.1 ± 1.4 | 0.9 ± 1.4 | 2.1 ± 2.9 | 97.9 ± 2.9 |
| axe1-4 | 100.0 ± 0.0 | 0.0 ± 0.0 | 13.4 ± 6.7a | 86.6 ± 6.7a |
| 35Spro:HDA6-myc in Col | 96.7 ± 5.2 | 3.3 ± 5.2 | 2.6 ± 3.9 | 97.4 ± 3.9 |
| HDA6pro:HDA6-myc in axe1-4 3# | 98.8 ± 2.2 | 1.2 ± 2.2 | 2.6 ± 4.0 | 97.4 ± 4.0 |
| HDA6pro:HDA6-myc in axe1-4 8# | 99.4 ± 1.0 | 0.6 ± 1.0 | 2.8 ± 4.2 | 97.2 ± 4.2 |
| Ler | 98.6 ± 2.5 | 1.4 ± 2.5 | 2.8 ± 4.2 | 97.2 ± 4.2 |
| sil1 (CS1894) | 98.9 ± 2.2 | 1.1 ± 2.2 | 12.3 ± 5.1a | 87.7 ± 5.1a |
| sil1 (CS1895) | 100.0 ± 0.0 | 0.0 ± 0.0 | 17.2 ± 5.6a | 82.8 ± 5.6a |
Differs significantly from the wild type (P < 0.01; Student’s t test).
To examine whether the altered cellular pattern in Arabidopsis was indeed caused by HDA6 mutation, we constructed complementation lines by transferring the genomic HDA6 sequence with a 9× Myc tag under the control of its native promoter into the axe1-4 mutant (Supplemental Fig. S3, A and C). Among the various lines, line 3 had an expression level of HDA6 similar to Col, while line 8 had nearly 8-fold increased HDA6 expression in the root tip (Supplemental Fig. S3B). Therefore, line 3 was used as a complementation line and line 8 as an overexpression line. Analysis of paraffin sections revealed that the altered cellular pattern in the root epidermis of axe1-4 was rescued in both lines (Fig. 1C; Table I), indicating that, on the one hand, HDA6 was indeed involved in root epidermis cellular patterning, and on the other hand, overexpression of HDA6 does not interfere with the cellular patterning phenotype (Fig. 1B; Table I; Supplemental Fig. S2A). Further confirming the lack of effect of overexpression, cellular patterning was not altered in the root epidermis of a 35Spro:HDA6-myc overexpression line in the Col background (Fig. 1B; Table I; Supplemental Fig. S2A).
HDA6 Is Expressed throughout the Arabidopsis Root Tip But Affects the Cellular Patterning in Epidermis
According to root gene expression data provided by AREX LITE (http://www.arexdb.org/; Brady et al., 2007), HDA6 is expressed in all root tip cells (Supplemental Fig. S4, B and C). To confirm the expression pattern of HDA6 at both the RNA and protein levels in Arabidopsis root tips, we constructed transgenic lines HDA6pro:EGFP (for enhanced GFP) and HDA6pro:HDA6-EGFP in the Col background (Supplemental Fig. S4A). EGFP signal was detected in all tissues of the root tip, including N-position and H-position cells of the root epidermis of both lines (Fig. 2). These results indicate that both HDA6 RNA and HDA6 protein are present ubiquitously in Arabidopsis root tips, which is consistent with the previous information.
Figure 2.
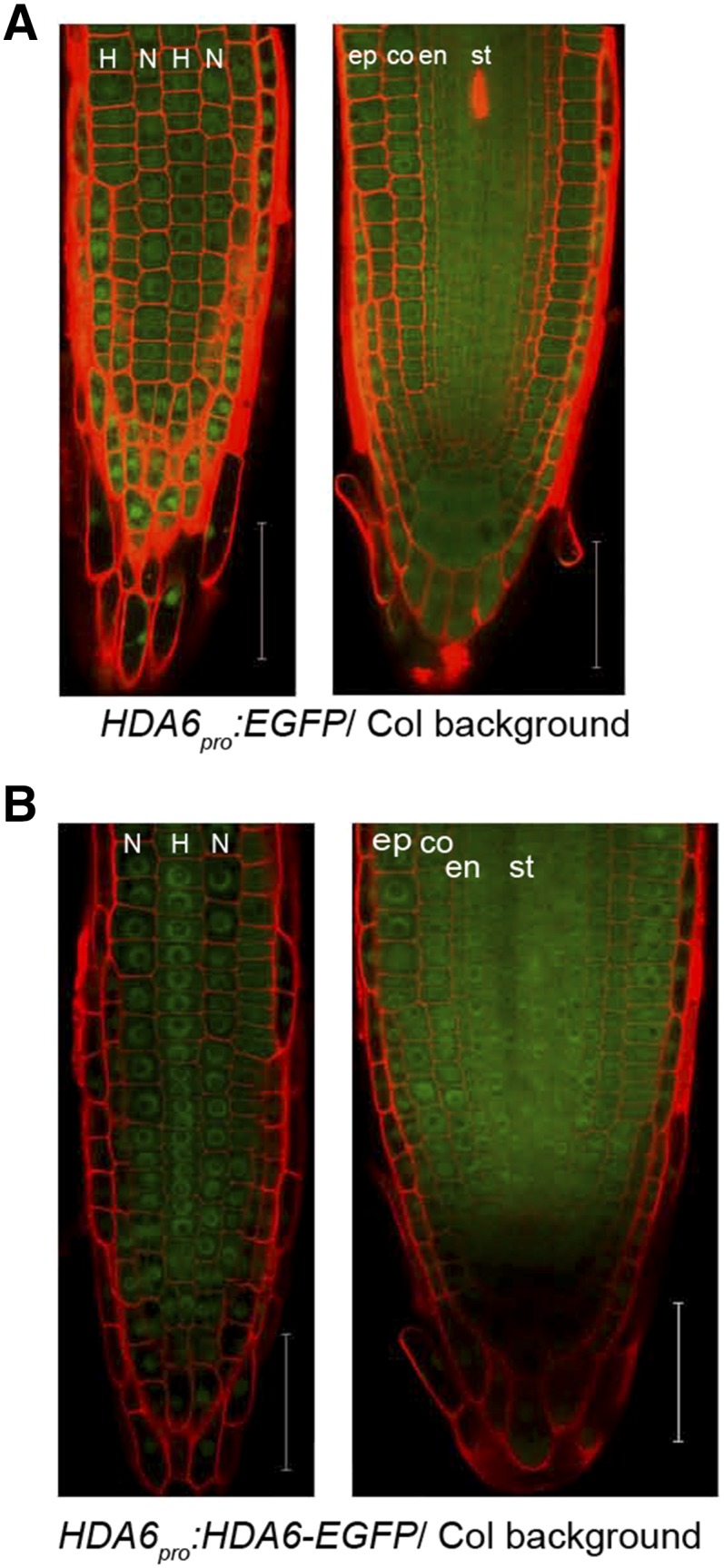
HDA6 localizes ubiquitously in Arabidopsis root tip. A, Confocal laser scanning microscope (CLSM) images of 7-d-old root tip of HDA6pro:EGFP reporting the location of HDA6 expression: epidermal view (left) and median view (right). Bars = 50 μm. B, CLSM images of 7-d-old root tip of HDA6pro:HDA6-EGFP showing HDA6 protein localization: epidermal view (left) and median view (right). Bars = 50 μm. In all images, N indicates nonhair cell position and H indicates hair cell position. co, Cortex; en, endodermis; ep, epidermis; st, stele. Red areas are propidium iodide (PI) signals and green areas are GFP signals.
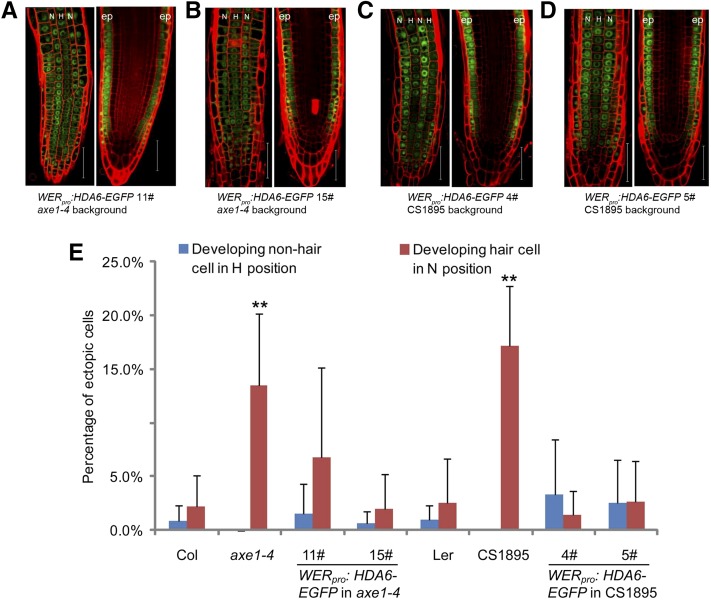
To clarify whether the effects of HDA6 on the cellular patterning is epidermis specific, we expressed HDA6 protein exclusively in root epidermis using a 2.5-kb WER promoter (Lee and Schiefelbein, 1999) in the axe1-4 and CS1895 backgrounds (Supplemental Fig. S5). As shown in Figure 3, A to D, HDA6 was specifically expressed in root epidermis in the WERpro:HDA6-EGFP axe1-4 and CS1895 lines. The altered cellular patterning of the hda6 mutants was successfully rescued in these transgenic lines (Fig. 3E), indicating that HDA6’s effects on cellular patterning are epidermis specific.
Figure 3.
Epidermis-specific expression of HDA6 can restore the altered root epidermal cellular pattern of the hda6 mutant. A to D, Seven-day-old roots of WERpro:HDA6-EGFP transgenic plants were analyzed for HDA6-EGFP localization in the root tip. Note that A and B are axe1-4 background and C and D are CS1895 background. Epidermal view is on the left and median view is on the right. N indicates nonhair cell position and H indicates hair cell position. ep, Epidermis. Bars = 50 μm. E, Quantification of the root epidermal cellular pattern of transgenic plants, showing frequencies of ectopically developing nonhair cells (blue bars) and ectopically developing hair cells (red bars). At least 12 root tips were analyzed for each line. The mean and sd are indicated for each line. **, P < 0.01, Student’s t test.
HDA6 Affects Both the Levels and Patterns of ETC1 and GL2 Expression
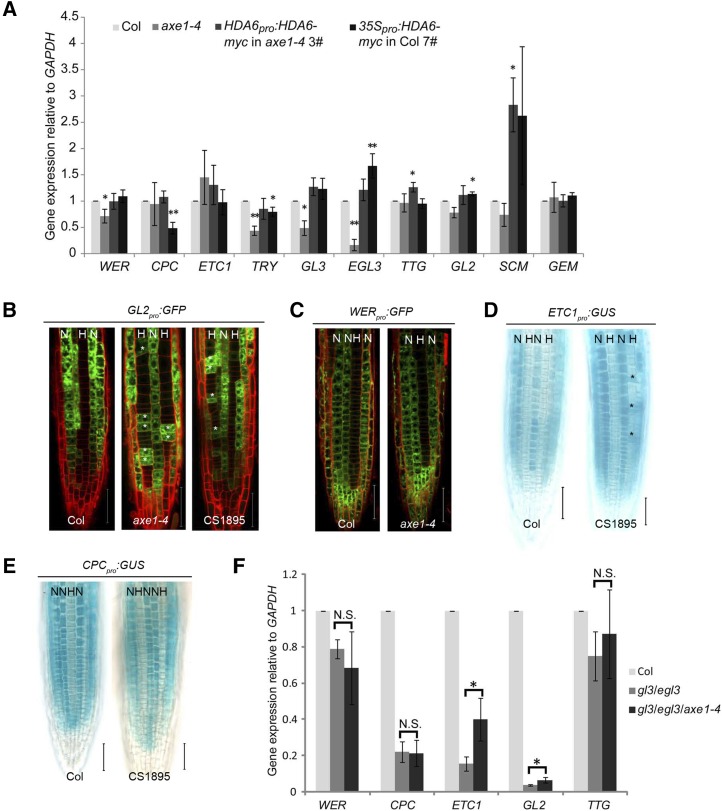
Next, we asked whether HDA6 influences cellular patterning through affecting pattern genes. We checked the expression of known pattern genes in the root tip of hda6 mutants (axe1-4 and CS1895), of the above-mentioned complementation line, HDA6pro:HDA6-myc/axe1-4 3#, and of overexpression line 35Spro:HDA6-myc/Col 7#. As shown in Figure 4A, expression levels of WER, TRY, GL3, and EGL3 were decreased in axe1-4 but were rescued in the complementation line. Expression of CPC, ETC1, TTG, GL2, SCM, and GEM did not show obvious changes in axe1-4. However, the expression of TTG and SCM increased unexpectedly in the complementation line (Fig. 4A). In CS1895, the expression levels of only TRY and GL2 were slightly reduced (Supplemental Fig. S6). In the overexpression line, the expression levels of CPC and TRY were decreased while those of EGL3 and GL2 were slightly increased (Fig. 4A). These results indicate that altered expression of HDA6 indeed affects the expression of pattern genes.
Figure 4.
HDA6 affects the expression level and pattern of ETC1 and GL2. A, Expression levels of pattern genes were analyzed in root tips of axe1-4, HDA6 complementation line, and HDA6 overexpression line compared with Col. Error bars represent sd values from at least three biological replicates each consisting of three technical replicates. **, P < 0.01 and *, P < 0.05, Student’s t test. B, Root epidermal view of 7-d-old seedlings bearing the GL2pro:GFP reporter gene. Bars = 50 μm. C, Root epidermal view of 7-d-old seedlings bearing the WERpro:GFP reporter gene. Bars = 50 μm. D, Seven-day-old seedlings bearing the ETC1pro:GUS reporter gene were stained for GUS activity. Bars = 50 μm. E, Seven-day-old seedlings bearing the CPCpro:GUS reporter gene were stained for GUS activity. Bars = 50 μm. In B to E, N indicates nonhair cell position, H indicates hair cell position, and asterisks indicate ectopic reporter gene-expressing cells. F, ETC1 and GL2 expression levels are significantly increased in the root tip of gl3/egl3/axe1-4 compared with gl3/egl3. Error bars represent sd values from at least three biological replicates each consisting of three technical replicates. *, P < 0.05, Student’s t test; N.S., no significance.
To identify the potential targets of HDA6, we analyzed HDA6’s effect on the location of the expression of pattern genes such as GL2, WER, CPC, and ETC1 by introducing GL2pro:GFP, WERpro:GFP, CPCpro:GUS, and ETC1pro:GUS into hda6 mutants. We found that GL2pro:GFP was ectopically expressed in cells at the H position in the root epidermis of hda6 mutants (Fig. 4B). ETC1pro:GUS was also ectopically expressed in a few H-position cells in root epidermis of hda6 (Fig. 4D). By contrast, there were no alterations in the signals detected for WERpro:GFP and CPCpro:GUS in hda6 (Fig. 4, C and E). These results suggested that GL2 and ETC1 might be targets of HDA6.
As mentioned above, we did not detect any changes in the expression level of GL2 and ETC1 in hda6 (Fig. 4A; Supplemental Fig. S6). It is possible that GL2 and ETC1 were ectopically expressed in only a few cells of the hda6 mutant (Fig. 4, B and D) and that any changes in GL2 and ETC1 expression were lost in the overall expression analyzed using the entire root tip. We reasoned that if we could reduce the expression of GL2 and ETC1 in the root tip, we might uncover an effect of HDA6 on the expression level of GL2 and ETC1 in the epidermis, for instance. According to the current model (Supplemental Fig. S1), ETC1 and GL2 are positively regulated by the WER-GL3/EGL3-TTG complex (Bernhardt et al., 2003, 2005; Morohashi et al., 2007). Accordingly, we crossed axe1-4 into a gl3/egl3 double mutant, which has low expression of GL2 and ETC1 (Fig. 4F). Compared with gl3/egl3, the expression levels of GL2 and ETC1 in the root of the gl3/egl3/axe1-4 triple mutant were significantly increased, while the expression of WER, CPC, and TTG remained unchanged (Fig. 4F). These results indicate that HDA6 can affect the transcript levels of GL2 and ETC1 and may be antagonistic to GL3/EGL3.
Taken together, the above data suggest that GL2 and ETC1 could be target genes of HDA6 to regulate the cellular patterning of Arabidopsis root epidermis.
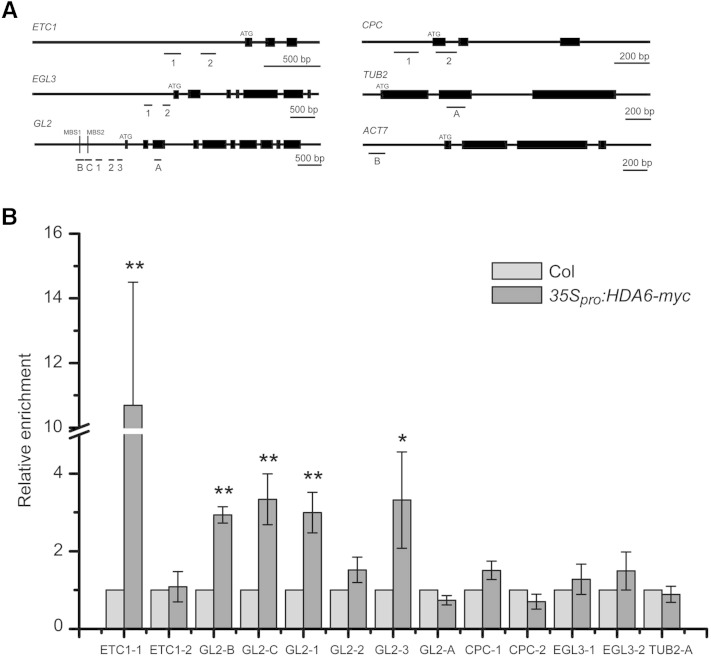
HDA6 Binds to Promoters of ETC1 and GL2 and Affects the Histone Acetylation Status of the Bound Regions
To examine whether HDA6 directly affects GL2 and ETC1, we performed chromatin immunoprecipitation (ChIP) assays using 35Spro:HDA6-myc transgenic plants with commercial Myc antibody (Supplemental Fig. S7). Regions on ETC1 and GL2 promoters for ChIP-PCR were selected randomly (Fig. 5A). Enrichments of the selected sequences by Myc antibody (Fig. 5B) demonstrated that HDA6 directly binds to some of the selected regions in the ETC1 and GL2 promoters, and not to those in EGL3, CPC, and TUBULIN promoters, consistent with the effects of the HDA6 gene on the respective pattern genes described above.
Figure 5.
HDA6 binds to the promoter regions of ETC1 and GL2. A, Schematic representation of selected regions for ChIP-PCR analyses. B, Enrichment of HDA6 at the promoter regions of ETC1 and GL2 was tested by ChIP-PCR. Transgenic plants bearing the 35Spro:HDA6-myc construct were subjected to ChIP assays with an anti-myc antibody. Col plants were used as negative controls. ACTIN7 and TUBULIN2 (TUB2) were used as an internal control for normalization. Error bars represent sd values from at least three biological replicates each consisting of three technical replicates. **, P < 0.01 and *, P < 0.05, Student’s t test.
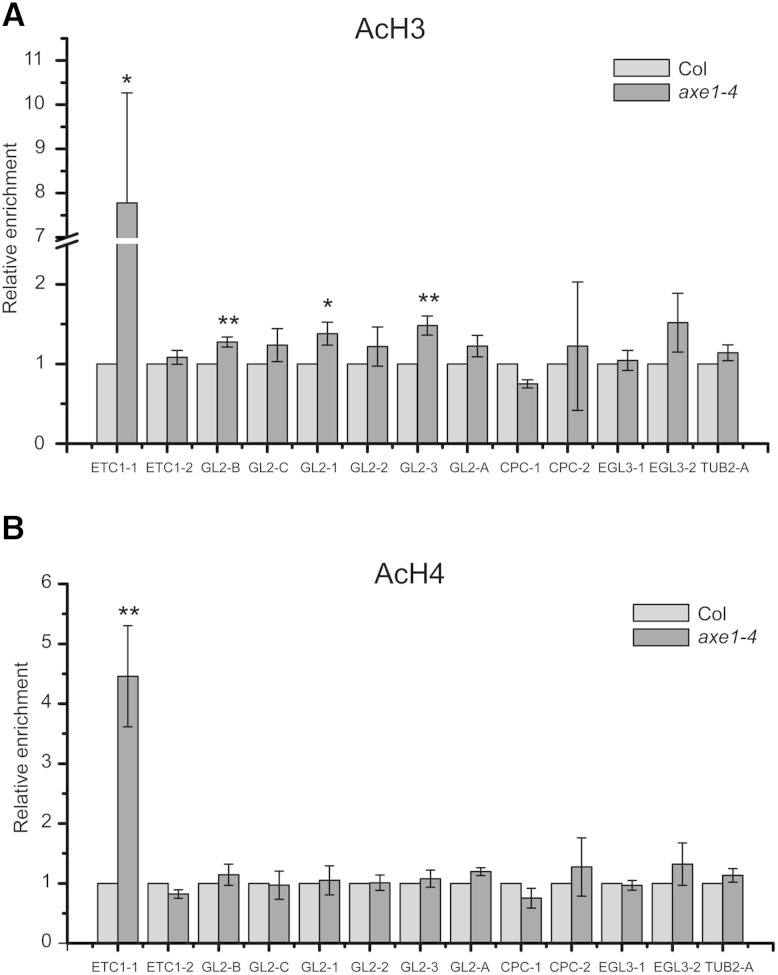
Since HDA6 has been reported to have histone deacetylase enzyme activity (Earley et al., 2006), we further monitored whether histone acetylation status at the HDA6-binding regions was affected by the hda6 mutation by performing a ChIP assay with acetylated histone antibodies. Both H3 and H4 acetylation levels at the ETC1 promoter regions bound by HDA6 were significantly increased in axe1-4 (Figs. 5B and 6). However, while H3 acetylation levels at three of the four HDA6-binding sites of GL2 promoter regions were increased in axe1-4 (Figs. 5B and 6), no H4 acetylation increase was detected at the HDA6-binding sites of the GL2 promoter in axe1-4 (Figs. 5B and 6). These data indicate that HDA6 likely affects the expression of ETC1 and GL2 through affecting the histone acetylation status of their promoters, but the detailed mechanism regarding histone types and binding sites is different for each gene.
Figure 6.
HDA6 affects histone acetylation of the ETC1 and GL2 promoter regions. A, Histone H3 acetylation status at selected promoter regions of pattern genes in axe1-4 was analyzed by ChIP assay using antibodies specific to acetylated histone H3K9K14 followed by quantitative PCR. ACTIN7 was used as an internal control for normalization. Error bars represent sd values from at least three biological replicates each consisting of three technical replicates. **, P < 0.01 and *, P < 0.05, Student’s t test. B, Histone H4 acetylation status at selected promoter regions of pattern genes in axe1-4 was analyzed by ChIP assay using antibodies specific to acetylated histone H4K5K8K12K16. ACTIN7 was used as an internal control for normalization. Error bars represent sd values from at least three biological replicates each consisting of three technical replicates. **, P < 0.01, Student’s t test. TUB2, TUBULIN2.
HDA6 Is Genetically Upstream of ETC1 in the Cellular Patterning of Root Epidermis
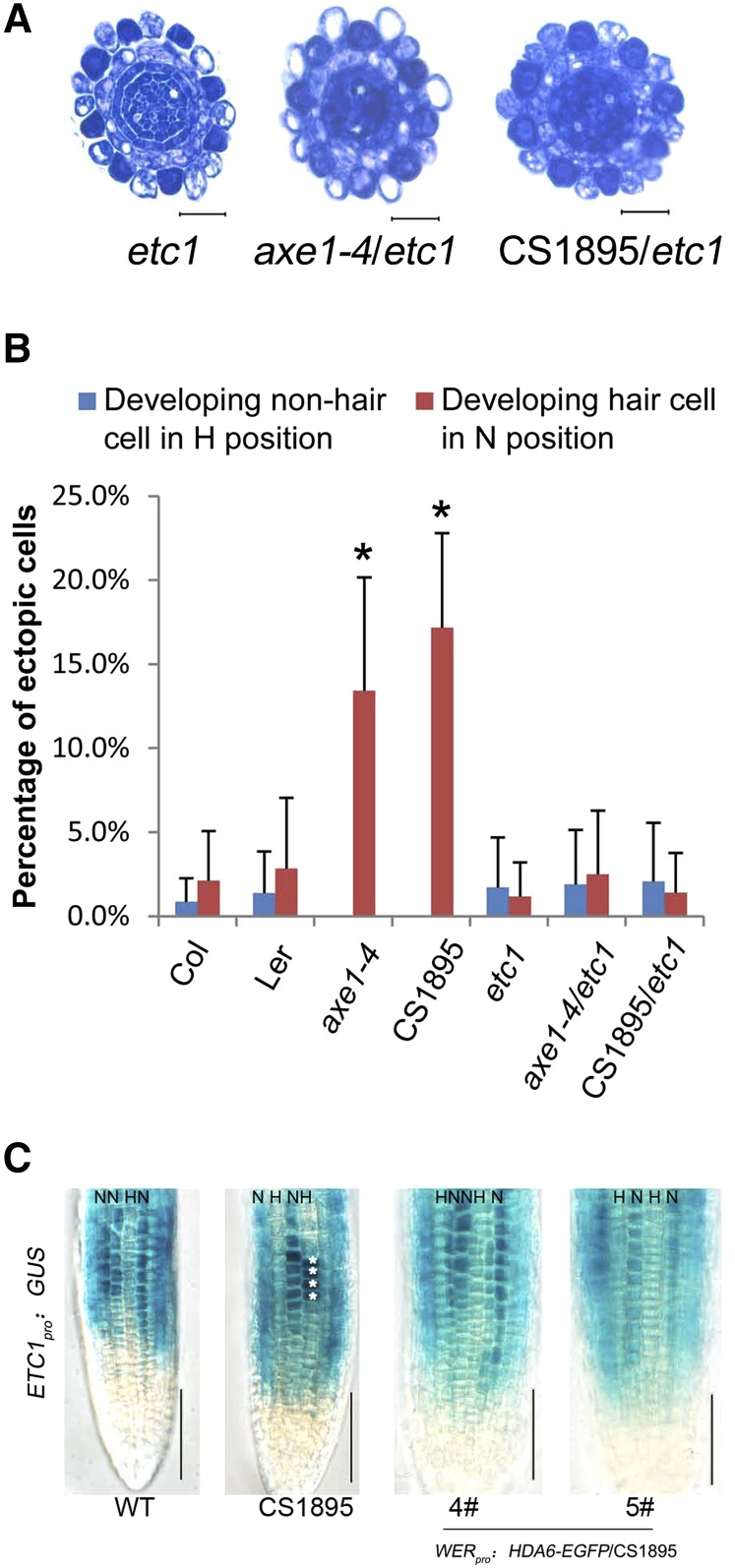
It has been reported that ETC1 overexpression causes excessive root hair production, although the etc1 single mutant has no significant root hair phenotype (Kirik et al., 2004). Consistent with that report, we found that etc1 had normal differential staining of epidermal cells (Fig. 7A), while ETC1 overexpression caused almost all of the N-position epidermal cells to be densely stained like the H-position epidermal cells, indicating that the N-position cells were transformed into developing hair cells (Supplemental Fig. S8). To further clarify the genetically regulatory relationship between HDA6 and ETC1, we constructed hda6/etc1 double mutants. Similar to the phenotype of etc1, both axe1-4/etc1 and CS1895/etc1 double mutants had normal differential staining of epidermal cells (Fig. 7, A and B). This suggests that ETC1 is genetically epistatic to HDA6 in affecting the cellular pattern of Arabidopsis root epidermis, consistent with the idea that HDA6 affects the cellular pattern phenotype through directly binding and acetylating histones at ETC1 promoter regions.
Figure 7.
Phenotype analysis of the hda6/etc1 double mutant. A, Cross sections of root tips of etc1 and hda6/etc1. Bars = 20 μm. B, Quantification of the root epidermal cellular pattern of the hda6/etc1 double mutant, showing frequencies of ectopic developing nonhair cells (blue bars) and ectopic developing hair cells (red bars). At least 12 root tips were analyzed for each line. The mean and sd are indicated for each line. *, P < 0.05, Student’s t test. C, Seven-day-old seedlings bearing the ETC1pro:GUS reporter gene were stained for GUS activity. WT, Wild type. Bars = 50 μm.
To further demonstrate that HDA6 is upstream of ETC1, we introduced WER promoter-driven HDA6 into the hda6 mutant (CS1895) containing the ETC1pro:GUS marker (Fig. 7C) and found that epidermis-specific expression of HDA6 could rescue the altered expression pattern of ETC1 in the hda6 mutant.
HDA6 Can Directly Affect GL2 Expression Independently of Its Effect on ETC1
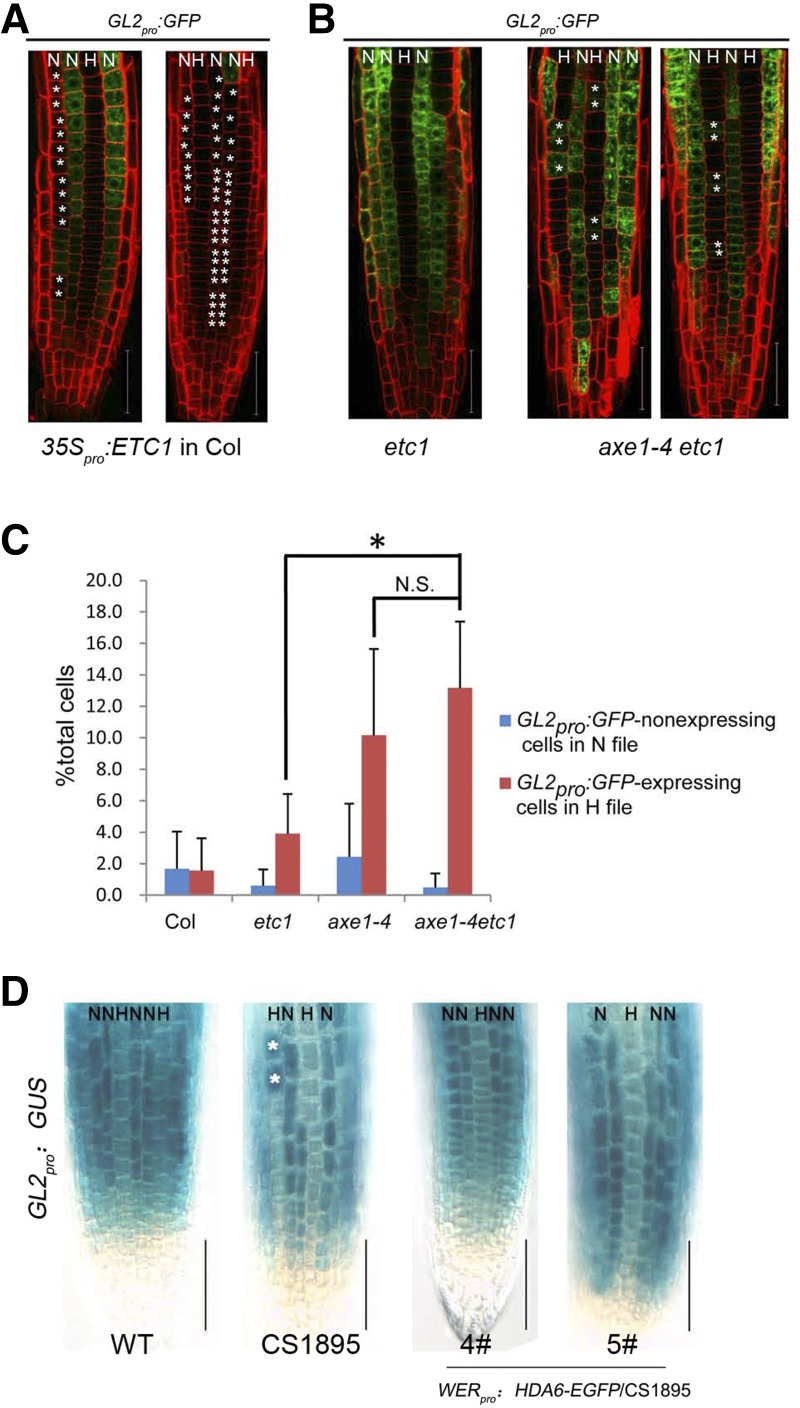
According to the current model, ETC1 can function redundantly with CPC and TRY to repress GL2 expression (Grebe, 2012; Schiefelbein et al., 2014). Our data showed that while HDA6 directly affects ETC1 by binding its promoter and affecting the histone acetylation status at the binding region, HDA6 can also directly bind the GL2 promoter and affect H3 acetylation at its binding sites (Fig. 6). This suggested that HDA6 may be able to influence GL2 expression independently of ETC1. We began by introducing a GL2 promoter reporter into the ETC1 overexpression line. As predicted by the model described above, the GL2 promoter activity was severely suppressed by overexpression of ETC1 (Fig. 8A), while GL2 expression was a little increased in the axe1-4 root, in which ETC1 expression was increased (Fig. 4). The observation that GL2 expression in axe1-4 is not suppressed by increased ETC1 expression in the same background suggests that the effect of HDA6 on GL2 may be independent of ETC1. To test this, we examined GL2 promoter activity in both etc1 single mutant and axe1-4/etc1 double mutant backgrounds. We found that while the GL2 expression pattern was not affected in the etc1 single mutant compared with the wild type (Fig. 4B), it was significantly altered in the axe1-4/etc1 double mutant (Fig. 8, B and C), similar to that in the axe1-4 single mutant (Fig. 4B). These genetic data suggest that while overexpression of ETC1 indeed affects GL2 expression, HDA6 can directly affect GL2 expression independent of its effect on ETC1.
Figure 8.
Analysis of HDA6’s effects on GL2. A, CLSM images of root tip of ETC1 overexpression lines bearing the GL2pro:GFP reporter gene. The asterisks indicate ectopic GL2pro:GFP-nonexpressing cells in the N position. Bars = 50 μm. B, CLSM images of root tip of the hda6/etc1 double mutant bearing the GL2pro:GFP reporter gene. The asterisks indicate ectopic GL2pro:GFP-expressing cells in the H positon. Bars = 50 μm. C, Quantification of the epidermal cell type pattern, showing percentages of ectopic GL2pro:GFP-nonexpressing cells in the N position (blue bars) and ectopic GL2pro:GFP-expressing cells in the H positon (red bars) for each line. *, P < 0.05, Student’s t test; N.S., no significance. D, Seven-day-old seedlings bearing the GL2pro:GUS reporter gene were stained for GUS activity. WT, Wild type. Bars = 50 μm.
To further demonstrate the effects of HDA6 on GL2 expression, we also introduced WER promoter-driven HDA6 into the hda6 mutant (CS1895) containing the GL2pro:GUS marker (Fig. 8D), showing that the epidermis-specific expression of HDA6 could rescue the altered expression pattern of GL2 in the hda6 mutant.
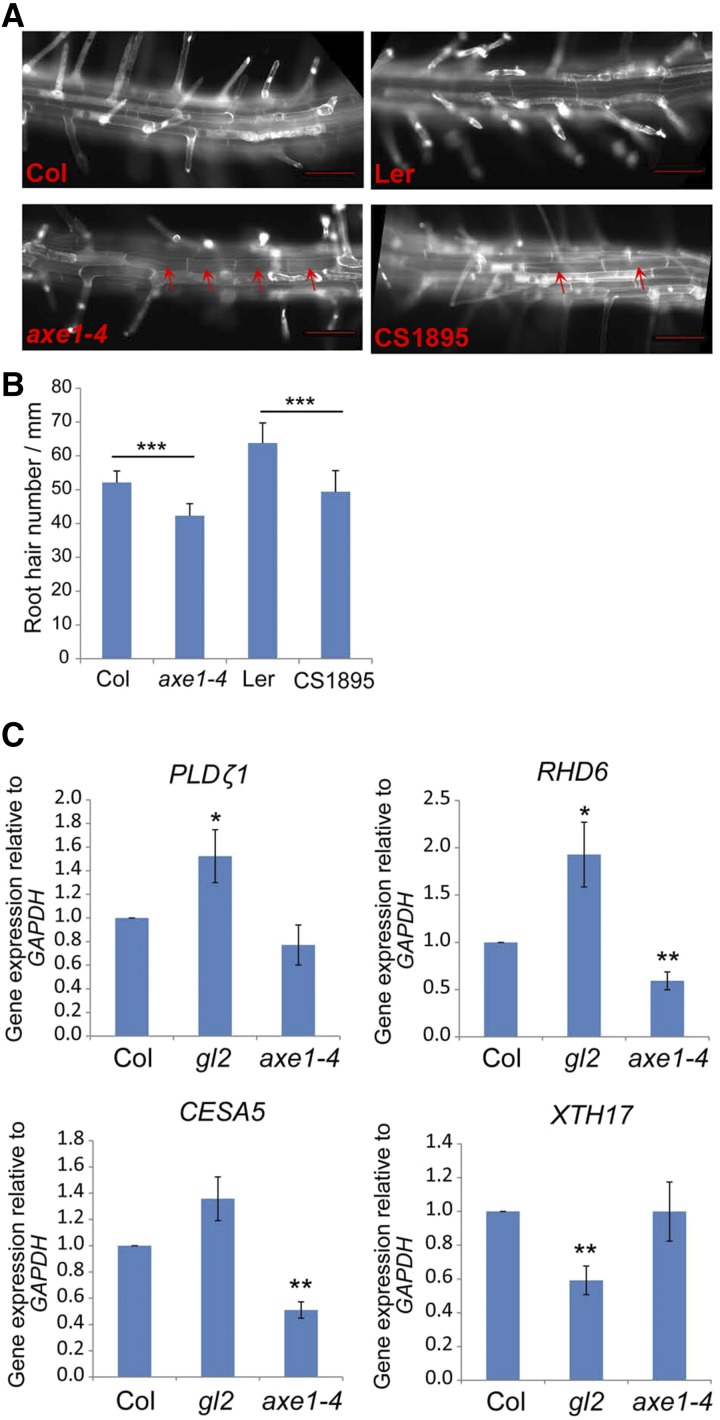
The effect of HDA6 upon GL2 could further be supported by observation of the inhibition of root hair initiation in the hda6 mutant. GL2 plays a key role in root hair initiation and elongation (Di Cristina et al., 1996; Masucci et al., 1996). In the hda6 mutant, we found that while more developing hair cells, based on deep staining, were found in the N position by sections of root tips (Fig. 1), fewer root hairs were observed (Fig. 9, A and B). It is not clear how GL2 regulates root hair initiation and elongation. However, some genes related to root hair initiation, such as PHOSPHOLIPASE D ZETA1 (PLDζ1), CELLULOSE SYNTHASE5 (CESA5), XYLOGLUCAN ENDOTRANSGLUCOSYLASE17, and ROOT HAIR DEFECT6 (RHD6), are known to be regulated by GL2 (Ohashi et al., 2003; Tominaga-Wada et al., 2009; Bruex et al., 2012; Schiefelbein et al., 2014). Therefore, we examined the expression of these genes downstream of GL2. We found that, consistent with the finding that HDA6 represses GL2 expression (Fig. 4, B and F), GL2-repressed genes such as PLDζ1, CESA5, and RHD6 were up-regulated in the gl2 mutant and repressed in the hda6 mutant, in which GL2 expression is increased (Fig. 9C).
Figure 9.
Root hair number was reduced in hda6 roots. A, Ectopic nonroot hairs at the H position were observed in the mature zone of hda6 root. Bars = 50 μm. B, Root hair density was reduced in hda6 root. At least 20 roots were analyzed for each line. ***, P < 0.001, Student’s t test. C, The expression of GL2 downstream genes was detected in hda6. **, P < 0.01 and *, P < 0.05, Student’s t test.
DISCUSSION
Previously, mainly based on the effects of trichostatin A, an inhibitor of histone deacetylase, we proposed that histone acetylation is involved in the regulation of cellular pattern formation in Arabidopsis root epidermis (Xu et al., 2005). Functional analysis of HDA18 showed that it directly binds and regulates kinase genes involved in cellular patterning (Liu et al., 2013a). Here, we report another mechanism by which HDAC genes affect cellular patterning; in this case, HDA6 affects the expression of ETC1 and GL2, two transcription factors that are components of the network determining the cell fates at the N and H positions in root epidermis. Taken together with the previously reported role of GEM in cell fate determination through histone modification (Caro et al., 2007), it is clear that, while the involvement of HDACs in the cellular patterning of Arabidopsis root epidermis is confirmed, the detailed mechanisms are highly diversified for each member of the HDAC family. Considering the key roles of the GL2-centered transcription factor network in cellular patterning in Arabidopsis root epidermis, it seems that histone acetylation plays an important role in maintaining the robustness of the GL2-centered transcription factor network, through directly maintaining the proper expression levels and expression patterns of transcription factors such as ETC1 and GL2, as for HDA6, and/or adjusting the transmission of positional information from cortical cells, as for HDA18.
HDA6 has been demonstrated to be involved in the regulation of many physiological processes in plants. It has been reported that HDA6 and HDA19 interact with various transcription factors and chromatin-remolding factors and play different roles, with a range of target genes in various developmental processes (Liu et al., 2014). Here, we demonstrated that, in the involvement in cellular patterning, HDA6 selectively affects the expression of ETC1 and GL2 but not other known pattern genes. Our results suggest that, even in this particular process, HDA6 plays different roles through different target genes. These characteristics bring up the question of how particular HDAC family members evolved to regulate the expression of some genes but not others. From this perspective, the cellular patterning of Arabidopsis root epidermis can serve as a good system in which to investigate how particular members of the HDAC family function upon various target genes and how these effects are integrated together.
It is worth noting that, compared with the phenotypes of the mutants of the pattern genes, such as wer, cpc, and gl2, the phenotypes of hda6 in this work and hda18 in previous work (Liu et al., 2013a) were not as prominent. One explanation could be that, in cellular patterning, the regulatory network of transcriptional factors are the key players and the histone modification of the transcription of the components comprising the network plays a role in enhancing the robustness of the regulatory network.
MATERIALS AND METHODS
Plant Material and Growth Conditions
The Arabidopsis (Arabidopsis thaliana) hda6 point mutants axe1-4 and sil1 (CS1894 and CS1895) were purchased from the Arabidopsis Biological Resource Center (http://www.arabidopsis.org/). The following mutants and reporter lines were previously described: etc1 and ETC1pro:GUS (Kirik et al., 2004), WERpro:GFP (Lee and Schiefelbein, 1999), GL2pro:GFP (Lin and Schiefelbein, 2001), EGL3pro:GUS (Zhang et al., 2003), and CPCpro:GUS (Wada et al., 2002). Double and triple mutants were constructed by crossing. Reporter lines were introduced into mutants or transgenic plants by crossing. Homozygous lines were verified by molecular genotyping, phenotype analysis, and reporter gene expression. Seeds were sterilized and planted on 0.5× Murashige and Skoog medium solidified with 0.8% (m/v) agar. Plates were placed in the dark at 4°C for 2 d and then moved to 22°C with a period of 16 h of light/8 h of dark.
Construction of Plasmids and Transgenic Plants
For the 35Spro:HDA6-myc construct, the coding sequence of HDA6 (without the stop codon) was first fused in frame to the 5′ end of nine tandem repeats encoding the MYC epitope, and then the fusion was cloned into the pDR vector containing the CaMV35S promoter. For the HDA6pro:HDA6-myc construct, a 2,918-bp genomic fragment of HDA6 was first fused in frame to the 5′ end of nine tandem repeats encoding the MYC epitope, and then the fusion was cloned into a modified pDR vector containing the 3′ untranslated region and intergenic sequence of HDA6. For the HDA6pro:EGFP construct, a 524-bp region upstream of the HDA6 start codon was introduced into the pEGAD-EGFP vector to replace the CaMV35S promoter. For the HDA6pro:HDA6-EGFP construct, the same fragment as used for the HDA6pro:HDA6-myc construct was introduced into the pEGAD-EGFP vector to replace the CaMV35S promoter and fused in frame to the 5′ end of the sequence encoding the EGFP epitope at the same time. The 3′ untranslated region and intergenic sequence of HDA6 were inserted at the 3′ end of the sequence encoding the EGFP epitope. For epidermis-specific and hair cell-specific expression constructs, a 2,500-bp fragment upstream of the WER start codon (Lee and Schiefelbein, 1999) and a 2,500-bp fragment upstream of the GL3 start codon (Zhang et al., 2003) were separately introduced into pEGAD-EGFP vector to replace the CaMV35S promoter. Then, a genomic HDA6 fragment (from the start codon to the stop codon) was introduced into the above-modified pEGAD-EGFP vector and fused in frame to the 5′ end of the sequence encoding the EGFP epitope to generate WERpro:HDA6-EGFP. For 35Spro:ETC1, the coding sequence of ETC1 was cloned into the pDR vector containing the CaMV35S promoter. All the resultant constructs were verified by sequencing. Plasmid DNA was transformed into Agrobacterium tumefaciens strain GV3101. Plant transformation was undertaken according to an A. tumefaciens-mediated floral dip transformation protocol (Clough and Bent, 1998).
RNA Isolation and qRT-PCR
Total RNA was extracted from root tips of 7-d-old seedlings using the RNeasy Plant Mini kit (Qiagen) according to the manufacturer’s instructions. After treatment with DNase I (Promega), complementary DNA was synthesized from 2 μg of total RNA using the SuperScript III Reverse Transcriptase kit (Invitrogen). The qRT-PCR was performed with the Applied Biosystems 7500 Real-Time PCR System using TaKaRa SYBR Premix Ex Taq (Tli RNaseH Plus). Gene-specific primers were used for qRT-PCR (Supplemental Table S1). Relative gene expression was calculated by the ΔΔCT method (Livak and Schmittgen, 2001) using GAPDH as an internal control.
The primers used for RNA level detection are listed in Supplemental Table S1.
ChIP Assays
ChIP for acetylated histones H3 and H4 was carried out as described previously (Saleh et al., 2008). Root tips were cut from 7-d-old seedlings and treated with formaldehyde. Extracted chromatin was sheared to an average length of 500 bp by sonication and immunoprecipitated with specific antibodies including anti-acetylated histone H3K9K14 (catalog no. 06-599; Millipore) and anti-acetylated histone H4K5K8K12K16 (catalog no. 06-866; Millipore). The ChIP DNA was analyzed by quantitative PCR.
ChIP for HDA6 protein was carried out with some changes according to Liu et al. (2013b). Seven-day-old seedlings of the 35Spro:HDA6-myc transgenic line (Col background) or Col were harvested and kept in liquid nitrogen. After the samples were ground into powder in liquid nitrogen, the slurry was resuspended with 1× phosphate buffer containing 100 mm Suc, 10 mm dimethyl adipimidate (Sigma; Kurdistani and Grunstein, 2003), and 1% (v/v) formaldehyde. After incubation for 20 min at 4°C, the cross-linking was stopped by incubation with 150 mm Gly for 5 min at 4°C. Chromatin was then extracted and sonicated to produce DNA fragments of about 500 bp. Rabbit antibody anti-c-myc (C3956; Sigma) was used for immunoprecipitation. The ChIP DNA was analyzed by quantitative PCR with Col as a negative control. The commercial myc antibody (Sigma) was confirmed by ChIP-western before use (Supplemental Fig. S7).
The primers used for ChIP assays are listed in Supplemental Table S2.
Microscopy
Transverse serial sections from Arabidopsis root tips were stained with Toluidine Blue to analyze the epidermal cellular pattern (Galway et al., 1994; Xu et al., 2005). For all GUS reporter lines, histochemical analysis was performed as described previously (Masucci et al., 1996). Photographs were taken with a microscope.
Observation of ectopic nonhairs in the mature zone of root was performed as described previously (Caro et al., 2007). Root hair number per millimeter was measured as described previously (Galway et al., 1994).
All EGFP or GFP expression lines were counterstained with 20 μg mL−1 PI in water for 1 to 2 min and examined with a Zeiss LSM 710 NLO & DuoScan System. GFP and PI signals were observed sequentially on separate channels, with excitation at 488 nm and detection between bandwidths 493 and 542 nm for GFP and with excitation at 561 nm and detection between bandwidths 566 and 628 nm for PI. For quantitative analysis of root epidermis cellular patterning using reporter-expressing cells, at least 10 roots of 7-d-old seedlings were examined for each line (Wieckowski and Schiefelbein, 2012).
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL data libraries under the following accession numbers: CPC (At2g46410), EGL3 (At1g63650), GL2 (At1g79840), GL3 (At5g41315), WER (At5g14750), TTG (At5g24520), TRY (At5g53200), ETC1 (At1g01380), SCM (At1g11130), GEM (At2g22475), HDA6 (At5g63110), and HDA18 (At5g61070).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. The regulation network of cell fate specification in the Arabidopsis root epidermis, with minor modifications from Schiefelbein et al. (2014).
Supplemental Figure S2. Detection of HDA6 expression in the root tip of hda6 mutants and the overexpression line.
Supplemental Figure S3. Complementation of the hda6 mutant with genomic HDA6.
Supplemental Figure S4. HDA6 expression information retrieved from AREX LITE: The Arabidopsis Gene Expression Database (http://www.arexdb.org/).
Supplemental Figure S5. Schematic representation of the WERpro:HDA6-EGFP construct.
Supplemental Figure S6. Detection of expression levels of pattern genes in root tips of CS1895.
Supplemental Figure S7. ChIP-western with myc antibody using total protein extract from Arabidopsis seedlings.
Supplemental Figure S8. Analysis of ETC1 overexpression lines.
Supplemental Table S1. List of primers used for RNA level detection.
Supplemental Table S2. List of primers used for ChIP assays.
Supplementary Material
Acknowledgments
We thank John Schiefelbein (University of Michigan) and Philip Benfey (Duke University) for providing marker lines used in this study and Martin Hülskamp (University of Koeln) for providing seeds of etc1 and ETC1pro:GUS.
Glossary
- Col
Columbia
- Ler
Landsberg erecta
- ChIP
chromatin immunoprecipitation
- qRT
quantitative reverse transcription
- PI
propidium iodide
- CLSM
confocal laser scanning microscope
Footnotes
This work was supported by the National Natural Science Foundation (grant nos. 30393114 and 30570901) and the Ministry of Science and Technology of the People’s Republic of China (grant no. 2003CB715906 to S.-N.B.).
References
- An L, Zhou Z, Sun L, Yan A, Xi W, Yu N, Cai W, Chen X, Yu H, Schiefelbein J, et al. (2012) A zinc finger protein gene ZFP5 integrates phytohormone signaling to control root hair development in Arabidopsis. Plant J 72: 474–490 [DOI] [PubMed] [Google Scholar]
- Aufsatz W, Mette MF, van der Winden J, Matzke M, Matzke AJ (2002) HDA6, a putative histone deacetylase needed to enhance DNA methylation induced by double-stranded RNA. EMBO J 21: 6832–6841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aufsatz W, Stoiber T, Rakic B, Naumann K (2007) Arabidopsis histone deacetylase 6: a green link to RNA silencing. Oncogene 26: 5477–5488 [DOI] [PubMed] [Google Scholar]
- Berger F, Hung CY, Dolan L, Schiefelbein J (1998) Control of cell division in the root epidermis of Arabidopsis thaliana. Dev Biol 194: 235–245 [DOI] [PubMed] [Google Scholar]
- Bernhardt C, Lee MM, Gonzalez A, Zhang F, Lloyd A, Schiefelbein J (2003) The bHLH genes GLABRA3 (GL3) and ENHANCER OF GLABRA3 (EGL3) specify epidermal cell fate in the Arabidopsis root. Development 130: 6431–6439 [DOI] [PubMed] [Google Scholar]
- Bernhardt C, Zhao M, Gonzalez A, Lloyd A, Schiefelbein J (2005) The bHLH genes GL3 and EGL3 participate in an intercellular regulatory circuit that controls cell patterning in the Arabidopsis root epidermis. Development 132: 291–298 [DOI] [PubMed] [Google Scholar]
- Brady SM, Orlando DA, Lee JY, Wang JY, Koch J, Dinneny JR, Mace D, Ohler U, Benfey PN (2007) A high-resolution root spatiotemporal map reveals dominant expression patterns. Science 318: 801–806 [DOI] [PubMed] [Google Scholar]
- Bruex A, Kainkaryam RM, Wieckowski Y, Kang YH, Bernhardt C, Xia Y, Zheng X, Wang JY, Lee MM, Benfey P, et al. (2012) A gene regulatory network for root epidermis cell differentiation in Arabidopsis. PLoS Genet 8: e1002446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro E, Castellano MM, Gutierrez C (2007) A chromatin link that couples cell division to root epidermis patterning in Arabidopsis. Nature 447: 213–217 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Devoto A, Nieto-Rostro M, Xie D, Ellis C, Harmston R, Patrick E, Davis J, Sherratt L, Coleman M, Turner JG (2002) COI1 links jasmonate signalling and fertility to the SCF ubiquitin-ligase complex in Arabidopsis. Plant J 32: 457–466 [DOI] [PubMed] [Google Scholar]
- Di Cristina M, Sessa G, Dolan L, Linstead P, Baima S, Ruberti I, Morelli G (1996) The Arabidopsis Athb-10 (GLABRA2) is an HD-Zip protein required for regulation of root hair development. Plant J 10: 393–402 [DOI] [PubMed] [Google Scholar]
- Dolan L, Janmaat K, Willemsen V, Linstead P, Poethig S, Roberts K, Scheres B (1993) Cellular organisation of the Arabidopsis thaliana root. Development 119: 71–84 [DOI] [PubMed] [Google Scholar]
- Earley K, Lawrence RJ, Pontes O, Reuther R, Enciso AJ, Silva M, Neves N, Gross M, Viegas W, Pikaard CS (2006) Erasure of histone acetylation by Arabidopsis HDA6 mediates large-scale gene silencing in nucleolar dominance. Genes Dev 20: 1283–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley KW, Pontvianne F, Wierzbicki AT, Blevins T, Tucker S, Costa-Nunes P, Pontes O, Pikaard CS (2010) Mechanisms of HDA6-mediated rRNA gene silencing: suppression of intergenic Pol II transcription and differential effects on maintenance versus siRNA-directed cytosine methylation. Genes Dev 24: 1119–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galway ME, Masucci JD, Lloyd AM, Walbot V, Davis RW, Schiefelbein JW (1994) The TTG gene is required to specify epidermal cell fate and cell patterning in the Arabidopsis root. Dev Biol 166: 740–754 [DOI] [PubMed] [Google Scholar]
- Grebe M. (2012) The patterning of epidermal hairs in Arabidopsis: updated. Curr Opin Plant Biol 15: 31–37 [DOI] [PubMed] [Google Scholar]
- Hassan H, Scheres B, Blilou I (2010) JACKDAW controls epidermal patterning in the Arabidopsis root meristem through a non-cell-autonomous mechanism. Development 137: 1523–1529 [DOI] [PubMed] [Google Scholar]
- Kang YH, Kirik V, Hulskamp M, Nam KH, Hagely K, Lee MM, Schiefelbein J (2009) The MYB23 gene provides a positive feedback loop for cell fate specification in the Arabidopsis root epidermis. Plant Cell 21: 1080–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirik V, Simon M, Huelskamp M, Schiefelbein J (2004) The ENHANCER OF TRY AND CPC1 gene acts redundantly with TRIPTYCHON and CAPRICE in trichome and root hair cell patterning in Arabidopsis. Dev Biol 268: 506–513 [DOI] [PubMed] [Google Scholar]
- Kuppusamy KT, Chen AY, Nemhauser JL (2009) Steroids are required for epidermal cell fate establishment in Arabidopsis roots. Proc Natl Acad Sci USA 106: 8073–8076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurdistani SK, Grunstein M (2003) In vivo protein-protein and protein-DNA crosslinking for genomewide binding microarray. Methods 31: 90–95 [DOI] [PubMed] [Google Scholar]
- Kwak SH, Schiefelbein J (2007) The role of the SCRAMBLED receptor-like kinase in patterning the Arabidopsis root epidermis. Dev Biol 302: 118–131 [DOI] [PubMed] [Google Scholar]
- Kwak SH, Schiefelbein J (2008) A feedback mechanism controlling SCRAMBLED receptor accumulation and cell-type pattern in Arabidopsis. Curr Biol 18: 1949–1954 [DOI] [PubMed] [Google Scholar]
- Kwak SH, Shen R, Schiefelbein J (2005) Positional signaling mediated by a receptor-like kinase in Arabidopsis. Science 307: 1111–1113 [DOI] [PubMed] [Google Scholar]
- Lee MM, Schiefelbein J (1999) WEREWOLF, a MYB-related protein in Arabidopsis, is a position-dependent regulator of epidermal cell patterning. Cell 99: 473–483 [DOI] [PubMed] [Google Scholar]
- Lin Y, Schiefelbein J (2001) Embryonic control of epidermal cell patterning in the root and hypocotyl of Arabidopsis. Development 128: 3697–3705 [DOI] [PubMed] [Google Scholar]
- Liu C, Li LC, Chen WQ, Chen X, Xu ZH, Bai SN (2013a) HDA18 affects cell fate in Arabidopsis root epidermis via histone acetylation at four kinase genes. Plant Cell 25: 257–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Teo ZW, Bi Y, Song S, Xi W, Yang X, Yin Z, Yu H (2013b) A conserved genetic pathway determines inflorescence architecture in Arabidopsis and rice. Dev Cell 24: 612–622 [DOI] [PubMed] [Google Scholar]
- Liu X, Yang S, Zhao M, Luo M, Yu CW, Chen CY, Tai R, Wu K (2014) Transcriptional repression by histone deacetylases in plants. Mol Plant 7: 764–772 [DOI] [PubMed] [Google Scholar]
- Liu X, Yu CW, Duan J, Luo M, Wang K, Tian G, Cui Y, Wu K (2012) HDA6 directly interacts with DNA methyltransferase MET1 and maintains transposable element silencing in Arabidopsis. Plant Physiol 158: 119–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Masucci JD, Rerie WG, Foreman DR, Zhang M, Galway ME, Marks MD, Schiefelbein JW (1996) The homeobox gene GLABRA2 is required for position-dependent cell differentiation in the root epidermis of Arabidopsis thaliana. Development 122: 1253–1260 [DOI] [PubMed] [Google Scholar]
- Morohashi K, Zhao M, Yang M, Read B, Lloyd A, Lamb R, Grotewold E (2007) Participation of the Arabidopsis bHLH factor GL3 in trichome initiation regulatory events. Plant Physiol 145: 736–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murfett J, Wang XJ, Hagen G, Guilfoyle TJ (2001) Identification of Arabidopsis histone deacetylase HDA6 mutants that affect transgene expression. Plant Cell 13: 1047–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi Y, Oka A, Rodrigues-Pousada R, Possenti M, Ruberti I, Morelli G, Aoyama T (2003) Modulation of phospholipid signaling by GLABRA2 in root-hair pattern formation. Science 300: 1427–1430 [DOI] [PubMed] [Google Scholar]
- Pandey R, Müller A, Napoli CA, Selinger DA, Pikaard CS, Richards EJ, Bender J, Mount DW, Jorgensen RA (2002) Analysis of histone acetyltransferase and histone deacetylase families of Arabidopsis thaliana suggests functional diversification of chromatin modification among multicellular eukaryotes. Nucleic Acids Res 30: 5036–5055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontvianne F, Blevins T, Chandrasekhara C, Mozgová I, Hassel C, Pontes OM, Tucker S, Mokros P, Muchová V, Fajkus J, et al. (2013) Subnuclear partitioning of rRNA genes between the nucleolus and nucleoplasm reflects alternative epiallelic states. Genes Dev 27: 1545–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst AV, Fagard M, Proux F, Mourrain P, Boutet S, Earley K, Lawrence RJ, Pikaard CS, Murfett J, Furner I, et al. (2004) Arabidopsis histone deacetylase HDA6 is required for maintenance of transcriptional gene silencing and determines nuclear organization of rDNA repeats. Plant Cell 16: 1021–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh A, Alvarez-Venegas R, Avramova Z (2008) An efficient chromatin immunoprecipitation (ChIP) protocol for studying histone modifications in Arabidopsis plants. Nat Protoc 3: 1018–1025 [DOI] [PubMed] [Google Scholar]
- Schellmann S, Schnittger A, Kirik V, Wada T, Okada K, Beermann A, Thumfahrt J, Jürgens G, Hülskamp M (2002) TRIPTYCHON and CAPRICE mediate lateral inhibition during trichome and root hair patterning in Arabidopsis. EMBO J 21: 5036–5046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefelbein J. (2003) Cell-fate specification in the epidermis: a common patterning mechanism in the root and shoot. Curr Opin Plant Biol 6: 74–78 [DOI] [PubMed] [Google Scholar]
- Schiefelbein J, Huang L, Zheng X (2014) Regulation of epidermal cell fate in Arabidopsis roots: the importance of multiple feedback loops. Front Plant Sci 5: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefelbein J, Kwak SH, Wieckowski Y, Barron C, Bruex A (2009) The gene regulatory network for root epidermal cell-type pattern formation in Arabidopsis. J Exp Bot 60: 1515–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga-Wada R, Iwata M, Sugiyama J, Kotake T, Ishida T, Yokoyama R, Nishitani K, Okada K, Wada T (2009) The GLABRA2 homeodomain protein directly regulates CESA5 and XTH17 gene expression in Arabidopsis roots. Plant J 60: 564–574 [DOI] [PubMed] [Google Scholar]
- Wada T, Kurata T, Tominaga R, Koshino-Kimura Y, Tachibana T, Goto K, Marks MD, Shimura Y, Okada K (2002) Role of a positive regulator of root hair development, CAPRICE, in Arabidopsis root epidermal cell differentiation. Development 129: 5409–5419 [DOI] [PubMed] [Google Scholar]
- Wada T, Tachibana T, Shimura Y, Okada K (1997) Epidermal cell differentiation in Arabidopsis determined by a Myb homolog, CPC. Science 277: 1113–1116 [DOI] [PubMed] [Google Scholar]
- Walker AR, Davison PA, Bolognesi-Winfield AC, James CM, Srinivasan N, Blundell TL, Esch JJ, Marks MD, Gray JC (1999) The TRANSPARENT TESTA GLABRA1 locus, which regulates trichome differentiation and anthocyanin biosynthesis in Arabidopsis, encodes a WD40 repeat protein. Plant Cell 11: 1337–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieckowski Y, Schiefelbein J (2012) Nuclear ribosome biogenesis mediated by the DIM1A rRNA dimethylase is required for organized root growth and epidermal patterning in Arabidopsis. Plant Cell 24: 2839–2856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K, Zhang L, Zhou C, Yu CW, Chaikam V (2008) HDA6 is required for jasmonate response, senescence and flowering in Arabidopsis. J Exp Bot 59: 225–234 [DOI] [PubMed] [Google Scholar]
- Xu CR, Liu C, Wang YL, Li LC, Chen WQ, Xu ZH, Bai SN (2005) Histone acetylation affects expression of cellular patterning genes in the Arabidopsis root epidermis. Proc Natl Acad Sci USA 102: 14469–14474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CW, Liu X, Luo M, Chen C, Lin X, Tian G, Lu Q, Cui Y, Wu K (2011) HISTONE DEACETYLASE6 interacts with FLOWERING LOCUS D and regulates flowering in Arabidopsis. Plant Physiol 156: 173–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Gonzalez A, Zhao M, Payne CT, Lloyd A (2003) A network of redundant bHLH proteins functions in all TTG1-dependent pathways of Arabidopsis. Development 130: 4859–4869 [DOI] [PubMed] [Google Scholar]
- Zhu Z, An F, Feng Y, Li P, Xue L, A M, Jiang Z, Kim JM, To TK, Li W, et al. (2011) Derepression of ethylene-stabilized transcription factors (EIN3/EIL1) mediates jasmonate and ethylene signaling synergy in Arabidopsis. Proc Natl Acad Sci USA 108: 12539–12544 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.