Abstract
The CuH-catalyzed hydroamination of alkenes and alkynes using a silane and an amine transfer reagent represents a simple strategy to access chiral amine products. We have recently reported methods to prepare chiral amines with high efficiency and stereoselectivity using this approach. However, the current technology is limited to the synthesis of trialkylamines from dialkylamine transfer reagents (R2NOBz). When monoalkylamine transfer reagents [RN(H)OBz] were used for the synthesis of chiral secondary amines, competitive, nonproductive consumption of these reagents by the CuH species resulted in poor yields. In this paper, we report the design of a modified type of amine transfer reagent that addresses this limitation. This effort has enabled us to develop a CuH-catalyzed synthesis of chiral secondary amines using a variety of amine coupling partners, including those derived from amino acid esters, carbohydrates, and steroids. Mechanistic investigations indicated that the modified amine transfer reagents are less susceptible to direct reaction with CuH.
Introduction
Chiral amines are ubiquitous structural motifs found in many pharmaceutical agents, natural products, and catalysts for asymmetric synthesis (see Figure 1 for selected examples). As a result, general and selective methods for their synthesis have long been pursued.1 Methodologies using resolution2 or chiral auxiliaries3 have been established as reliable ways to procure these compounds. Significant progress has also been made in the development of catalytic, asymmetric methods for their preparation. Many of these reported catalytic methods are based on asymmetric transformations of imine, enamine, or enamide intermediates.4 Other strategies, such as asymmetric allylation of amine nucleophiles5 and direct C–H bond insertion by a nitrenoid species,6 are important alternatives.
Figure 1.

Representative natural products and pharmaceutical agents that feature a chiral amine motif.
Asymmetric hydroamination,7 the net stereoselective addition of a hydrogen atom and an amino group directly across a double bond, represents a particularly appealing strategy to prepare chiral amines. Typically, a hydroamination reaction entails the direct union of an alkene 1 with a primary or secondary amine nucleophile 2 in the presence of a catalyst (Figure 2a).7 Based on catalytic copper(I) hydride chemistry8 and recent developments in the copper-mediated amination of carbon-based nucleophiles,9 our group recently reported a mechanistically distinct approach toward asymmetric hydroamination10 (Figure 2b). In this technique, an olefin first undergoes asymmetric hydrocupration to provide an alkylcopper intermediate, which is then intercepted by a suitable electrophilic amine transfer reagent. Our laboratory has applied this hydroamination strategy to the synthesis of chiral tertiary alkylamines from styrenes,10a 1,1-disubstituted alkenes,10b vinylsilanes,10c and alkynes.10d Independently, Miura and co-workers have reported a similar approach for the hydroamination of styrenes11a and strained internal alkenes.11b
Figure 2.
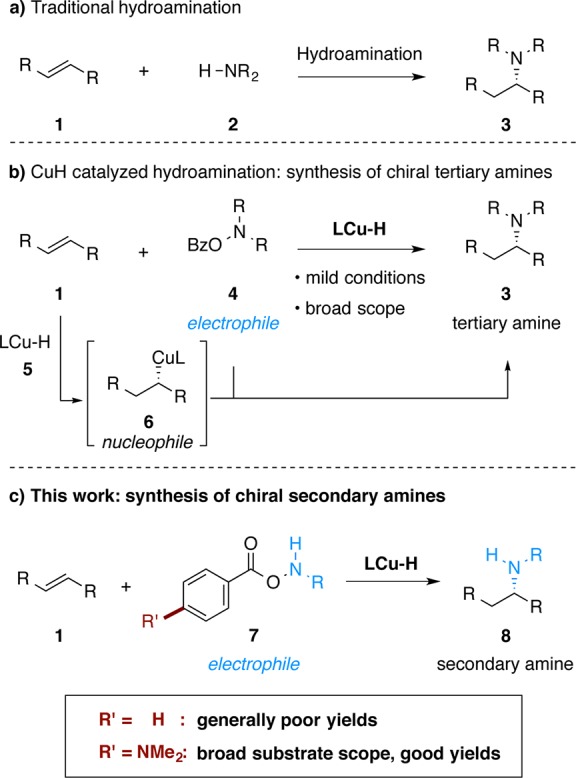
Hydroamination approaches to make α-chiral amines.
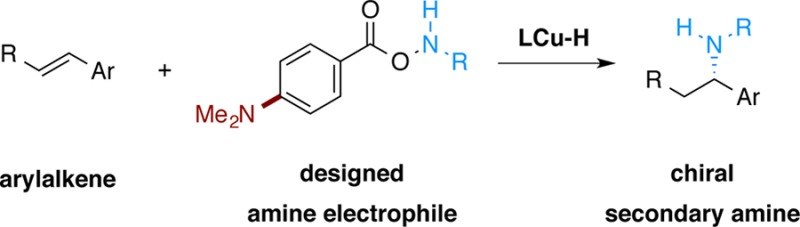
To date, this approach to hydroamination10,11 has been limited to the synthesis of tertiary alkylamines with O-benzoyl-N,N-dialkylhydroxylamines (R2NOBz, R = alkyl) as the dialkylamine transfer reagents. The expansion of this method to monoalkylamine transfer reagents to allow the direct preparation of chiral secondary amines would be of considerable interest. Herein we report the development of a copper-catalyzed hydroamination process to directly generate chiral, branched secondary amines 8 from styrenes (Figure 2c). One key factor in the development of this method is the design and use of a modified class of amine transfer reagents 7, which improved the efficiency and generality of the transformation. Mechanistic studies indicate that use of the modified amine transfer reagent suppresses nonproductive consumption of the reagent by the copper hydride intermediate.
Results and Discussion
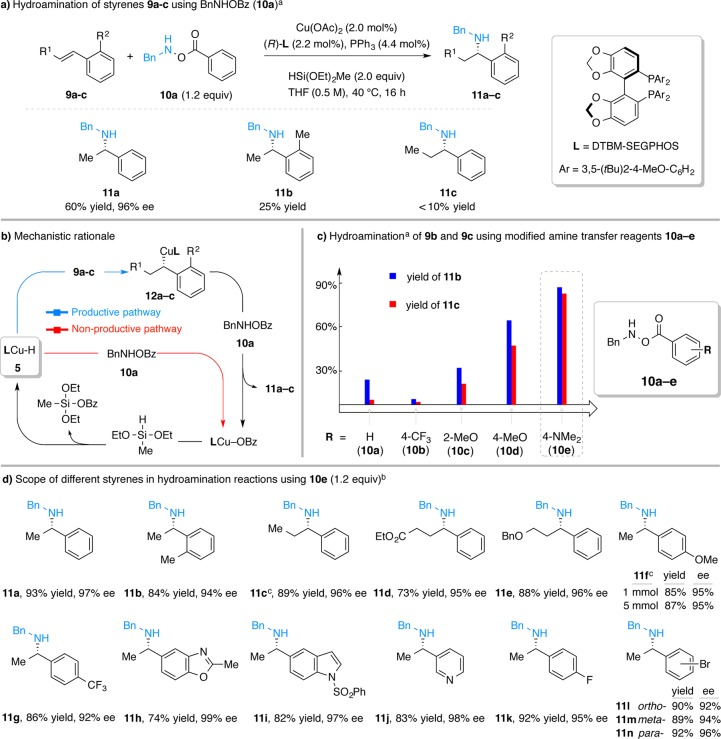
We began our work by studying the reaction between styrene (9a) and O-benzoyl-N-benzylhydroxylamine12 [BnN(H)OBz, 10a] utilizing our previously reported conditions10,13 (Figure 3a). It was found that the desired secondary amine 11a was produced in moderate yield (60%) and excellent enantioselectivity. This result suggested the compatibility between the copper hydride and alkylcopper species with the N–H bond contained in both the amine transfer reagent 10a and the secondary amine product 11a. However, we found that this transformation had a limited substrate scope: either ortho- or β-substitution on the styrene substrate led to a dramatic drop in reaction efficiency (11b and 11c, 25 and <10% yield, respectively). We reasoned that the poor yields of 11b and 11c might be caused by the more challenging hydrocupration of the substituted styrenes (9b and 9c). In these cases, the (relatively rapid) nonproductive consumption of amine transfer reagent 10a by LCuH (Figure 3b, red arrow) would diminish the overall yields of the respective secondary amine products.
Figure 3.
CuH-catalyzed hydroamination of styrenes for the formation of chiral secondary amines. aYields are determined using GC with dodecane as an internal standard; unless otherwise noted, CuH solution used in this study was prepared in a nitrogen-filled glovebox. bIsolated yields on 1 mmol scale (average of two runs); enantiomeric excesses (ee) were determined by chiral HPLC analysis; see Supporting Information for experimental details. cThree equivalents of HSi(OEt)2Me was used, and 10e was added over 1.5 h.
We postulated that the use of an amine transfer reagent that is less susceptible to direct reaction with LCuH 5 would change the relative rates of the desired (hydrocupration) versus the undesired [BnN(H)OBz decomposition] pathway, thus expanding the substrate scope for the synthesis of secondary amines. We hypothesized that perturbation of the electronic properties of the benzoyl group of the electrophilic amine source 10 might lead to such an effect.14 Thus, we prepared amine transfer reagents 10b–e (Figure 3c) by coupling commercially available N-benzyl hydroxylamine hydrochloride with various carboxylic acids and tested these new amine transfer reagents for the hydroamination of styrenes 9b and 9c under the same conditions as depicted in Figure 3a. As summarized in Figure 3c, we found that the use of more electron-rich amine transfer reagents 10c–e was beneficial to the reaction efficiency. Specifically, the use of 10e, an amine transfer reagent bearing a 4-(dimethylamino)benzoate group, provided the highest yields of 11b and 11c. In contrast, 10b, bearing the electron-deficient 4-(trifluoromethyl)benzoate group, gave poorer yields than the parent benzoate 10a.15
With 4-(dimethylamino)benzoate 10e as the amine transfer reagent, we found that a variety of styrene derivatives could be converted to the corresponding chiral secondary amines in good to excellent yield and with excellent enantioselectivity (Figure 3d). All these reactions proceeded to completion within 5 h at 40 °C or 16 h at room temperature under the reaction conditions shown in Figure 3a. For example, β-substituted styrenes and styrenes with ortho-substitution are suitable substrates (11b–e).16 Additionally, styrenes bearing both electron-donating and electron-withdrawing substituents are tolerated as well (11f–g), and the reaction efficiency is not reduced when performed on a 5 mmol scale (11f). Further, styrenes containing heterocyclic rings are effective reaction partners (11h–j). Use of 4-fluorostyrene yielded 11k, which resembles the core structure of PF-05105679 (Figure 1), in 92% yield and 95% ee. Lastly, this process also permits the preparation of 11l–n, which contain synthetically versatile aryl bromide. The successful formation of 11l was somewhat surprising since related alkylcopper intermediates had been shown to undergo rearrangement to afford the arylcopper species.17
We next investigated the scope of the amine transfer reagents that could be employed (Table 1). The amine transfer agents used in this study were prepared from the corresponding primary hydroxylamine 15 and 4-(dimethylamino)benzoic acid via condensation effected by 1,1′-carbonyldiimidazole (CDI).18 As summarized in Table 1, amine transfer reagents with secondary or tertiary alkyl group substituents are competent substrates, delivering products 17a–d in high yields and enantioselectivities. The use of chiral amine transfer agents afforded products with a high level of diastereoselectivity. The configuration of the newly generated stereocenter was determined by ligand employed (17c and 17d). When either racemic ligand or racemic electrophile was used, a near unity ratio of diastereomers was formed (see Supporting Information). These results are consistent with the diastereoselectivity of the hydroamination process being under catalyst control.
Table 1. Scope of Amine Transfer Reagents in Hydroamination Reactionsa.
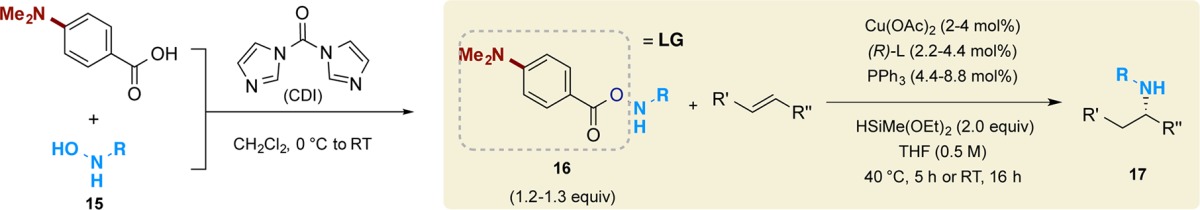

Reactions performed on 1 mmol scale for 17a–d and 0.5 mmol scale for 17e–l. Isolated yields are reported (average of two runs). Enantiomeric excesses (ee) were determined by chiral HPLC analysis or 1H NMR analysis. Diastereomeric ratios (dr) were determined by 1H NMR or gas chromatography analysis.
Toluene was used as the solvent, and amine transfer reagent with a pivolate leaving group was used as the substrate (see Supporting Information).
We found that amine transfer reagents prepared from α-amino esters19 were competent substrates as well, and gave the N-monoalkylated amino esters with high levels of stereocontrol (17e–l, Table 1). Amino esters spanning a range of steric and electronic properties could be utilized (17e–j). Importantly, the protecting groups on the amino esters could be methyl (17e,f and 17j), benzyl (17g), or tert-butyl (17h,i), allowing a variety of choices for the selection of downstream deprotection methods. No epimerization of the labile stereocenter adjacent to the carbonyl group was observed, reflecting the overall mildness of the reaction system. Not surprisingly, use of different enantiomers of the ligand led to the formation of different diastereomers (17h and 17i), again supporting a catalyst-controlled stereodetermining step. Furthermore, an amine transfer reagent derived from a quaternary α-amino ester could also be employed, giving 17j in excellent yield and enantioselectivity. This method could also transform a vinylsilane10c to the α-aminosilane (17k), a class of building blocks often employed for the synthesis of peptidomimetics.20 Finally, this hydroamination reaction can be integrated into a cascade sequence, delivering cyclic product 17l after in situ alkylation of the intermediate secondary amine.
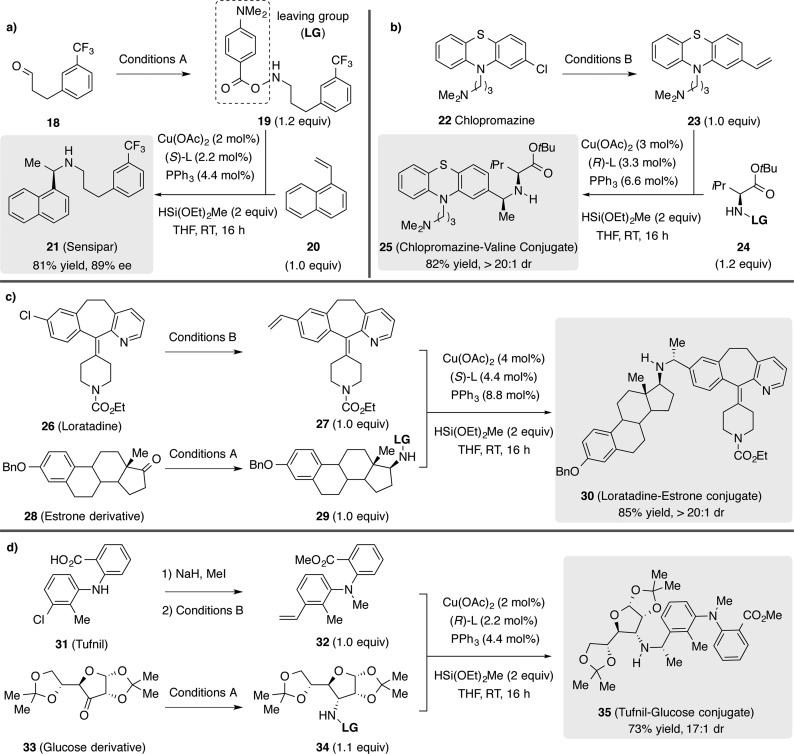
To further demonstrate the utility of this methodology, we applied it in the context of drug molecule synthesis (Scheme 1). For example, this method was applied to the synthesis of Sensipar (21), a drug used to treat secondary hyperparathyroidism. Commercially available aldehyde 18 was converted to amine transfer reagent 19 and then subjected to hydroamination conditions in the presence of 1-vinylnaphthalene (20) to give 21 in 81% yield and 89% ee (Scheme 1a). This methodology was also applied to the derivatization of commercial pharmaceuticals. For instance, chlorpromazine (22), an antipsychotic, could be converted to vinylarene 23,21 which then underwent hydroamination with l-valine-derived amine transfer reagent 24 to yield 25 (Scheme 1b). In a similar fashion, loratadine (26), an antihistamine drug, could be converted to 27 and then coupled with 29, an amine transfer reagent derived from an estrone derivative 28, to afford the conjugated product 30 in 85% yield and >20:1 dr (Scheme 1c). Lastly, vinylarene 32 made from tufnil (31), a nonsteroid anti-inflammatory drug, could successfully couple with 34, an amine transfer reagent prepared from a glucose derivative 33, to afford 35 in 73% yield and 17:1 dr (Scheme 1d).
Scheme 1. Hydroamination Reaction in the Synthesis and Derivatization of Drugs.
Reactions performed on 0.5 mmol scale. Isolated yields are reported (average of two runs). Enantioselectivities and diastereoselectivities were determined by chiral HPLC or 1H NMR analysis. Conditions A: (1) NH2OH·HCl, pyridine; (2) NaBH3CN, HCl in MeOH, MeOH/THF; (3) 4-(dimethylamino)benzoic acid, CDI, CH2Cl2. Conditions B: Pd(OAc)2, SPhos, potassium vinyltrifluoroborate, K2CO3, dioxane/H2O.
Mechanistic Studies
Competition experiments were performed to investigate the role of the modified amine transfer reagents used in this study. We had hypothesized that the narrow substrate scope of the CuH-catalyzed hydroamination reaction using monoalkylamine transfer agents [e.g., BnN(H)OBz] was due to the susceptibility of these reagents toward direct, nonproductive reduction by LCuH. To address this, we conducted a competition experiment by exposing a 1:1 mixture of a pair of mono- and dialkylamine transfer reagents, 10a and 36, to HSi(OEt)2Me and copper catalyst in THF-d8in the absence of styrene and monitored the consumption of these two reagents by 1H NMR spectroscopy (Figure 4a). We found that LCuH was capable of directly reacting with the amine transfer reagents, and over 80% of the monoalkylamine transfer agent 10a was consumed within 1 h to give BnNH2 and the corresponding silylated benzoyl ester (37).22 In contrast, only a trace (<5%) of the dialkylamine transfer agent 36 was consumed during the same period of time.23 We then subjected a 1:1 mixture of a pair of monoalkylamine transfer reagents, 10a (parent benzoate) and 10e [4-(dimethylamino)benzoate], to identical conditions as described above (Figure 4b). In this case, both 10a and 10e were gradually consumed in the reaction system, giving the corresponding silylated esters (37 and 38) as products. Importantly, we found that the modified amine transfer reagent 10e was consumed at a considerably slower rate than was 10a. The higher stability of the modified monoalkylamine transfer reagents toward direct reaction with LCuH reaction is consistent with the increased substrate scope seen using the 4-(dimethylamino)benzoate-derived amine transfer reagents.
Figure 4.
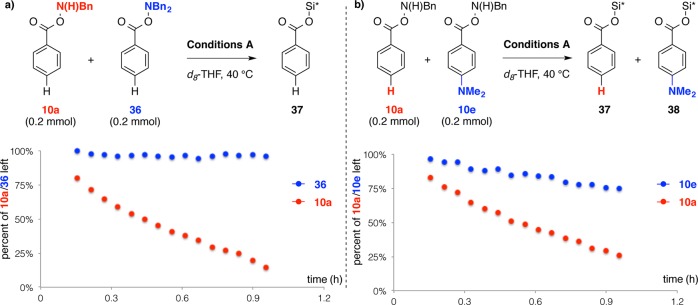
Relative rates of the reactions between LCuH and different amine transfer agents. Si* = Si(OEt)2Me. Conditions A: a 0.6 mL of a stock solution made from Cu(OAc)2 (3.6 mg), (R)-DTBM-SEGPHOS (26 mg), PPh3 (11.6 mg), HSi(OEt)2Me (0.32 mL, 2.0 mmol), and THF-d8 (1.0 mL) is used. The progress of these experiments was monitored by 1H NMR.
Conclusion
In conclusion, we have designed a new type of amine transfer reagent that possesses a 4-(dimethylamino)benzoate group. The use of these reagents enabled the development of a general method to directly convert styrenes to chiral secondary amines. This process was applicable to mono- and disubstituted styrenes and allowed the use of a variety of functionalized, structurally diverse amine transfer reagents, including those derived from carbohydrates, steroids, and amino acid esters. The utility of this reaction was highlighted by its application to the synthesis of pharmaceutically important drugs as well as the conjugation of other ones. Competition experiments have revealed that, relative to the corresponding O-benzoyl-N-alkyl hydroxylamines, the modified amine transfer reagents (O-[4-(dimethylamino)benzoyl]-N-alkyl hydroxylamines) are less susceptible to direct reaction with LCuH. The information gained from this study should prove useful in the design and development of other CuH-catalyzed processes.24
Acknowledgments
The authors acknowledge the National Institutes of Health for financial support (GM58160). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Dr. M.T. Pirnot and Dr. Y. Wang for assistance in the preparation of this manuscript.
Supporting Information Available
Experimental procedures and characterization data for all compounds. The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.5b05446.
The authors declare no competing financial interest.
Supplementary Material
References
- Nugent T. C., Ed. Chiral Amine Synthesis: Methods, Developments and Applications; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Turner N. J.; Carr R.. Biocatalytic Routes to Nonracemic Chiral Amines. In Biocatalysis in the Pharmaceutical and Biotechnology Industries; Patel R. N., Ed.; CRC Press LLC: Boca Raton, FL, 2006; pp 743–755. [Google Scholar]
- Robak M. T.; Herbage M. A.; Ellman J. A. Chem. Rev. 2010, 110, 3600. 10.1021/cr900382t. [DOI] [PubMed] [Google Scholar]
- a Nugent T. C.; El-Shazly M. Adv. Synth. Catal. 2010, 352, 753. 10.1002/adsc.200900719. [DOI] [Google Scholar]; b Xie J.; Zhu S.; Zhou Q. Chem. Rev. 2011, 111, 1713. 10.1021/cr100218m. [DOI] [PubMed] [Google Scholar]; N Tufvesson P.; Lima-Ramos J.; Jensen J. S.; Al-Haque N.; Neto W.; Woodley J. M. Biotechnol. Bioeng. 2011, 108, 1479. 10.1002/bit.23154. [DOI] [PubMed] [Google Scholar]; c Gopalaiah K. Chem. Rev. 2013, 113, 3248. 10.1021/cr300236r. [DOI] [PubMed] [Google Scholar]; d Feng X.; Du H. Tetrahedron Lett. 2014, 55, 6959. 10.1016/j.tetlet.2014.10.138. [DOI] [Google Scholar]; e Bloch R. Chem. Rev. 1998, 98, 1407. 10.1021/cr940474e. [DOI] [PubMed] [Google Scholar]; f Kobayashi S.; Ishitani H. Chem. Rev. 1999, 99, 1069. 10.1021/cr980414z. [DOI] [PubMed] [Google Scholar]; g Friestad G. K.; Mathies A. K. Tetrahedron 2007, 63, 2541. 10.1016/j.tet.2006.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Yamada K.; Tomioka K. Chem. Rev. 2008, 108, 2874. 10.1021/cr078370u. [DOI] [PubMed] [Google Scholar]; i Kobayashi S.; Mori Y.; Fossey J. S.; Salter M. M. Chem. Rev. 2011, 111, 2626. 10.1021/cr100204f. [DOI] [PubMed] [Google Scholar]; j Yus M.; González-Gómez J. C.; Foubelo F. Chem. Rev. 2011, 111, 7774. 10.1021/cr1004474. [DOI] [PubMed] [Google Scholar]; k Parmar D.; Sugiono E.; Raja S.; Rueping M. Chem. Rev. 2014, 114, 9047. 10.1021/cr5001496. [DOI] [PubMed] [Google Scholar]
- a Johannsen M.; Jørgensen K. A. Chem. Rev. 1998, 98, 1689. 10.1021/cr970343o. [DOI] [PubMed] [Google Scholar]; b Helmchen G.Iridium-Catalyzed Asymmetric Allylic Substitutions. In Iridium Complexes in Organic Synthesis; Oro L. A., Claver C., Eds.; Wiley-VCH: Weinheim, Germany, 2009; pp 211–250. [Google Scholar]; c Hartwig J. F.; Stanley L. M. Acc. Chem. Res. 2010, 43, 1461. 10.1021/ar100047x. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Liu W.-B.; Xia J.-B.; You S.-L. Top. Organomet. Chem. 2012, 38, 155. 10.1007/3418_2011_10. [DOI] [Google Scholar]; e Tosatti P.; Nelson A.; Marsden S. P. Org. Biomol. Chem. 2012, 10, 3147. 10.1039/c2ob07086c. [DOI] [PubMed] [Google Scholar]
- a Roizen J. L.; Harvey M. E.; Du Bois J. Acc. Chem. Res. 2012, 45, 911. 10.1021/ar200318q. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Davies H. M. L.; Manning J. R. Nature 2008, 451, 417. 10.1038/nature06485. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Müller P.; Fruit C. Chem. Rev. 2003, 103, 2905. 10.1021/cr020043t. [DOI] [PubMed] [Google Scholar]
- a Muller T. E.; Beller M. Chem. Rev. 1998, 98, 675. 10.1021/cr960433d. [DOI] [PubMed] [Google Scholar]; b Hartwig J. F. Pure Appl. Chem. 2004, 76, 507. 10.1351/pac200476030507. [DOI] [Google Scholar]; c Hong S.; Marks T. J. Acc. Chem. Res. 2004, 37, 673. 10.1021/ar040051r. [DOI] [PubMed] [Google Scholar]; d Beller M.; Seayad J.; Tillack A.; Jiao H. Angew. Chem., Int. Ed. 2004, 43, 3368. 10.1002/anie.200300616. [DOI] [PubMed] [Google Scholar]; e Hultzsch K. C. Org. Biomol. Chem. 2005, 3, 1819. 10.1039/b418521h. [DOI] [PubMed] [Google Scholar]; f Muller T. E.; Hultzsch K. C.; Yus M.; Foubelo F.; Tada M. Chem. Rev. 2008, 108, 3795. 10.1021/cr0306788. [DOI] [PubMed] [Google Scholar]; g Hultzsch K. C. Adv. Synth. Catal. 2005, 347, 367. 10.1002/adsc.200404261. [DOI] [Google Scholar]; h Huang L.; Arndt M.; Gooβen K.; Heydt H.; Gooβen L. J. Chem. Rev. 2015, 115, 2596. 10.1021/cr300389u. [DOI] [PubMed] [Google Scholar]
- a Appella D. H.; Moritani Y.; Shintani R.; Ferreira E. M.; Buchwald S. L. J. Am. Chem. Soc. 1999, 121, 9473. 10.1021/ja992366l. [DOI] [Google Scholar]; b Hughes G.; Kimura M.; Buchwald S. L. J. Am. Chem. Soc. 2003, 125, 11253. 10.1021/ja0351692. [DOI] [PubMed] [Google Scholar]; c Rainka M. P.; Aye Y.; Buchwald S. L. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 5821. 10.1073/pnas.0307764101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Berman A. M.; Johnson J. S. J. Am. Chem. Soc. 2004, 126, 5680. 10.1021/ja049474e. [DOI] [PubMed] [Google Scholar]; b Berman A. M.; Johnson J. S. J. Org. Chem. 2006, 71, 219. 10.1021/jo051999h. [DOI] [PubMed] [Google Scholar]; c Campbell M. J.; Johnson J. S. Org. Lett. 2007, 9, 1521. 10.1021/ol0702829. [DOI] [PubMed] [Google Scholar]; d Rucker R. P.; Whittaker A. M.; Dang H.; Lalic G. Angew. Chem., Int. Ed. 2012, 51, 3953. 10.1002/anie.201200480. [DOI] [PubMed] [Google Scholar]; e Yan X.; Yang X.; Xi C. Catal. Sci. Technol. 2014, 4, 4169. 10.1039/C4CY00773E. [DOI] [Google Scholar]; f Erdik E.; Ay M. Chem. Rev. 1989, 89, 1947. 10.1021/cr00098a014. [DOI] [Google Scholar]; g Barker T. J.; Jarvo E. R. Synthesis 2011, 24, 3954. 10.1055/s-0031-1289581. [DOI] [Google Scholar]
- a Zhu S.; Niljianskul N.; Buchwald S. L. J. Am. Chem. Soc. 2013, 135, 15746. 10.1021/ja4092819. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Zhu S.; Buchwald S. L. J. Am. Chem. Soc. 2014, 136, 15913. 10.1021/ja509786v. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Niljianskul N.; Zhu S.; Buchwald S. L. Angew. Chem., Int. Ed. 2015, 54, 1638. 10.1002/anie.201410326. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Shi S.; Buchwald S. L. Nat. Chem. 2015, 7, 38. 10.1038/nchem.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Miki Y.; Hirano K.; Satoh T.; Miura M. Angew. Chem., Int. Ed. 2013, 52, 10830. 10.1002/anie.201304365. [DOI] [PubMed] [Google Scholar]; b Miki Y.; Hirano K.; Satoh T.; Miura M. Org. Lett. 2014, 16, 1498. 10.1021/ol5003219. [DOI] [PubMed] [Google Scholar]
- In contrast to the large number of successful examples using dialkylamine transfer reagents (R2NOBz, R ≠ H) in copper-mediated amination reactions, use of the analogous monoalkylamine transfer reagents [RN(H)OBz] remains underdeveloped. For representative examples, see ref (9b) and; a Yotphan S.; Beukeaw D.; Reutrakul V. Tetrahedron 2013, 69, 6627. 10.1016/j.tet.2013.05.127. [DOI] [Google Scholar]; b McDonald S. L.; Wang Q. Angew. Chem., Int. Ed. 2014, 53, 1867. 10.1002/anie.201308890. [DOI] [PubMed] [Google Scholar]; c Matsuda N.; Hirano K.; Satoh T.; Miura M. Org. Lett. 2011, 13, 2860. 10.1021/ol200855t. [DOI] [PubMed] [Google Scholar]
- a Ascic E.; Buchwald S. L. J. Am. Chem. Soc. 2015, 137, 4666–4669 10.1021/jacs.5b02316. [DOI] [PMC free article] [PubMed] [Google Scholar]; b PPh3 is used as a secondary ligand due to the observed beneficial effects it has on CuH-catalyzed reactions. This concept was developed by Lipshutz:Lipshutz B. H.; Noson K.; Chrisman W.; Lower A. J. Am. Chem. Soc. 2003, 125, 8779. 10.1021/ja021391f. [DOI] [PubMed] [Google Scholar]
- Attempts to modify the properties of dialkylamine transfer reagents by adjusting the benzoate group have been reported by Johnson.9c
-
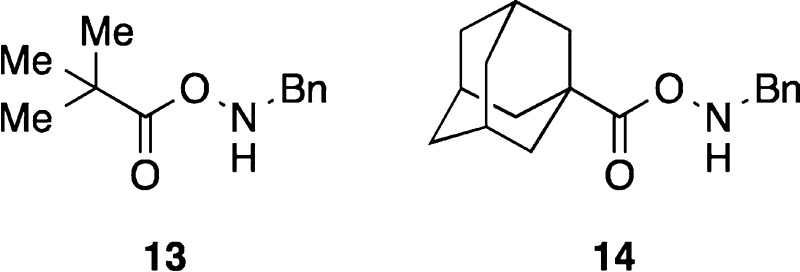
a Alkyl carboxylate-based amine transfer reagents 13 and 14 were also tested. Reagent 14 containing
a bulky adamantyl group provided yields of 11b and 11c similar to that of 4-(dimethylamino)benzoate 10e. Due to the availability of the 4-(dimethylamino)benzoic acid and
its ease of removal after reaction,15b 4-(dimethylamino)benzoates
were used for most of this work; b 4-(Dimethylamino)benzoic acid
is sparingly soluble in Et2O, EtOAc, or CH2Cl2 but readily soluble in 2 M aqueous K2CO3.
- Attempts to use 10e in the hydroamination of β,β-disubstituted styrenes or terminal unactivated alkenes gave low yields of secondary amine products.
- a Grigg R. D.; Van Hoveln R.; Schomaker J. M. J. Am. Chem. Soc. 2012, 134, 16131. 10.1021/ja306446m. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Van Hoveln R. J.; Schmid S. C.; Schomaker J. M. Org. Biomol. Chem. 2014, 12, 7655. 10.1039/C4OB01294A. [DOI] [PubMed] [Google Scholar]
- Smithen D. A.; Mathews C. J.; Tomkinson N. C. O. Org. Biomol. Chem. 2012, 10, 3756. 10.1039/c2ob25293g. [DOI] [PubMed] [Google Scholar]
- Several methodologies have been developed to convert α-amino acid esters into the corresponding primary N-hydroxylamines. For references, see:; a Tokuyama H.; Kuboyama T.; Fukuyama T. Org. Synth. 2003, 80, 207. 10.15227/orgsyn.080.0207. [DOI] [Google Scholar]; b Wittman M. D.; Halcomb R. L.; Danishefsky S. J. J. Org. Chem. 1990, 55, 1981. 10.1021/jo00294a005. [DOI] [Google Scholar]; c Fukuzumi T.; Bode J. W. J. Am. Chem. Soc. 2009, 131, 3864. 10.1021/ja900601c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H.; Martin C.; Kesselring D.; Keller R.; Moeller K. D. J. Am. Chem. Soc. 2006, 128, 13761. 10.1021/ja064737l. [DOI] [PubMed] [Google Scholar]
- Molander G. A.; Brown A. R. J. Org. Chem. 2006, 71, 9681. 10.1021/jo0617013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formation of BnNH2 and the silylated ester 37 could be detected by GC/MS and 1H NMR analysis.
- Reduction of the dialkylamine transfer agent 36 is much slower. Approximately, 60% of 36 was consumed after 20 h under described conditions.
- A manuscript detailing the use of related modified amine transfer reagents to effect hydroamination of unactivated internal alkenes is in press.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.