Abstract
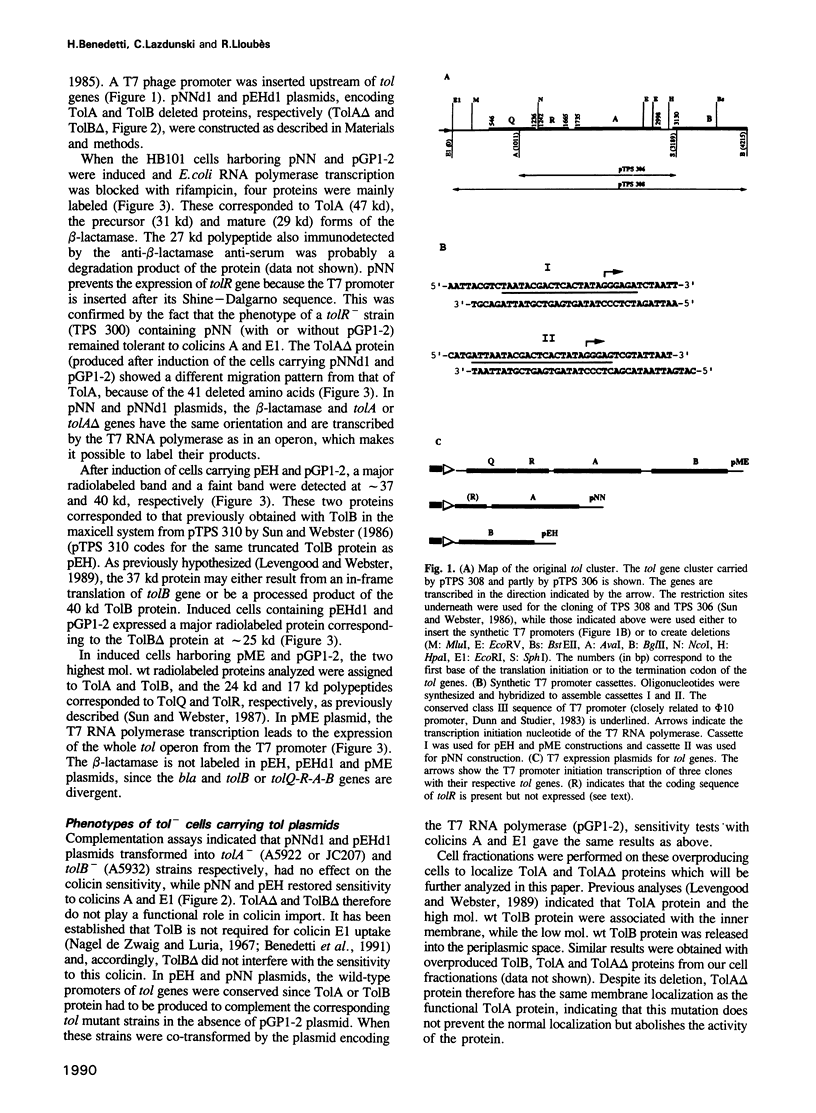
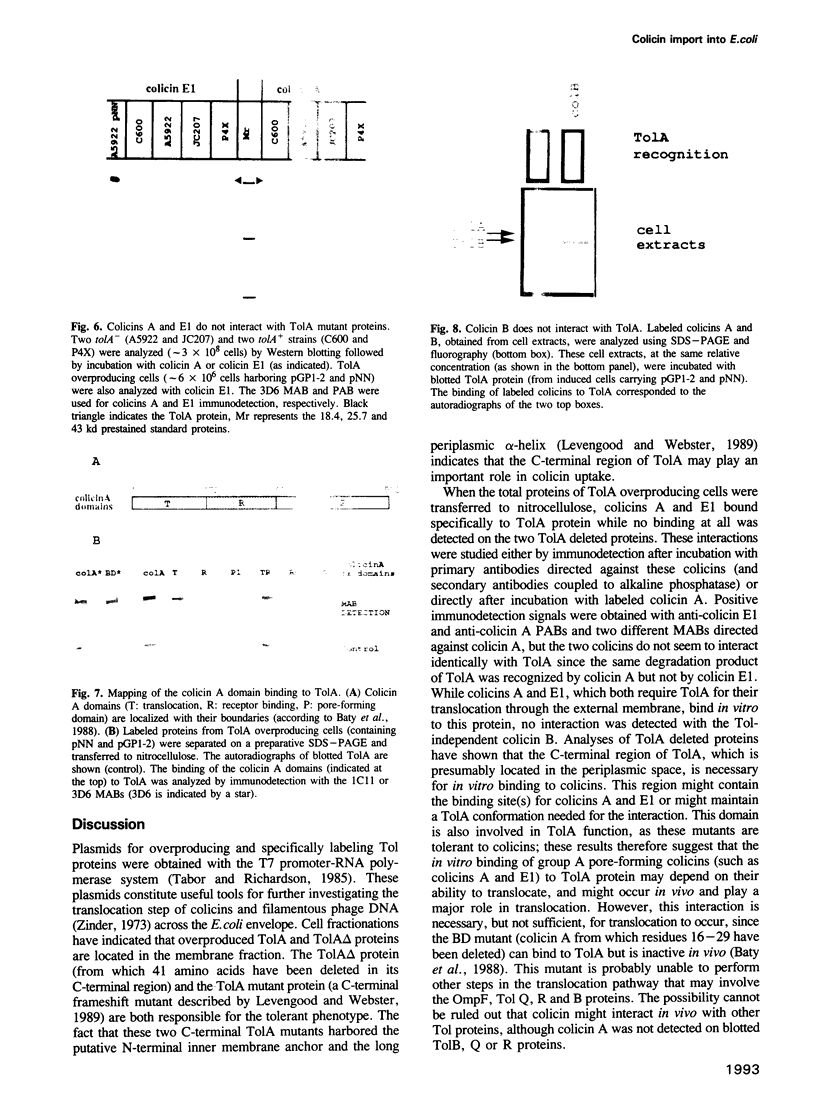
Colicins are antibiotic proteins that kill sensitive Escherichia coli cells. Their mode of action involves three steps: binding to specific receptors located in the outer membrane, translocation across this membrane, and action on their targets. A specific colicin domain can be assigned to each of these steps. Colicins have been subdivided into two groups (A and B) depending on the proteins required for them to cross the external membrane. Plasmids were constructed which led to an overproduction of the Tol proteins involved in the import of group A colicins. In vitro binding of overexpressed Tol proteins to either Tol-dependent (group A) or TonB-dependent (group B) colicins was analyzed. The Tol dependent colicins A and E1 were able to interact with TolA but the TonB dependent colicin B was not. The C-terminal region of TolA, which is necessary for colicin uptake, was also found to be necessary for colicin A and E1 binding to occur. Furthermore, only the isolated N-terminal domain of colicin A, which is involved in the translocation step, was found to bind to TolA. These results demonstrate the existence of a correlation between the ability of group A colicins to translocate and their in vitro binding to TolA protein, suggesting that these interactions might be part of the colicin import process.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baty D., Frenette M., Lloubès R., Geli V., Howard S. P., Pattus F., Lazdunski C. Functional domains of colicin A. Mol Microbiol. 1988 Nov;2(6):807–811. doi: 10.1111/j.1365-2958.1988.tb00092.x. [DOI] [PubMed] [Google Scholar]
- Baty D., Knibiehler M., Verheij H., Pattus F., Shire D., Bernadac A., Lazdunski C. Site-directed mutagenesis of the COOH-terminal region of colicin A: effect on secretion and voltage-dependent channel activity. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1152–1156. doi: 10.1073/pnas.84.5.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baty D., Lloubès R., Geli V., Lazdunski C., Howard S. P. Extracellular release of colicin A is non-specific. EMBO J. 1987 Aug;6(8):2463–2468. doi: 10.1002/j.1460-2075.1987.tb02526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baty D., Pattus F., Parker M., Benedetti H., Frenette M., Bourdineaud J. P., Cavard D., Knibiehler M., Lazdunski C. Uptake across the cell envelope and insertion into the inner membrane of ion channel-forming colicins in E coli. Biochimie. 1990 Feb-Mar;72(2-3):123–130. doi: 10.1016/0300-9084(90)90137-6. [DOI] [PubMed] [Google Scholar]
- Benedetti H., Frenette M., Baty D., Knibiehler M., Pattus F., Lazdunski C. Individual domains of colicins confer specificity in colicin uptake, in pore-properties and in immunity requirement. J Mol Biol. 1991 Feb 5;217(3):429–439. doi: 10.1016/0022-2836(91)90747-t. [DOI] [PubMed] [Google Scholar]
- Bourdineaud J. P., Boulanger P., Lazdunski C., Letellier L. In vivo properties of colicin A: channel activity is voltage dependent but translocation may be voltage independent. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1037–1041. doi: 10.1073/pnas.87.3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavard D., Crozel V., Gorvel J. P., Pattus F., Baty D., Lazdunski C. A molecular, genetic and immunological approach to the functioning of colicin A, a pore-forming protein. J Mol Biol. 1986 Feb 5;187(3):449–459. doi: 10.1016/0022-2836(86)90445-6. [DOI] [PubMed] [Google Scholar]
- Chan P. T., Ohmori H., Tomizawa J., Lebowitz J. Nucleotide sequence and gene organization of ColE1 DNA. J Biol Chem. 1985 Jul 25;260(15):8925–8935. [PubMed] [Google Scholar]
- Cramer W. A., Cohen F. S., Merrill A. R., Song H. Y. Structure and dynamics of the colicin E1 channel. Mol Microbiol. 1990 Apr;4(4):519–526. doi: 10.1111/j.1365-2958.1990.tb00619.x. [DOI] [PubMed] [Google Scholar]
- Davies J. K., Reeves P. Genetics of resistance to colicins in Escherichia coli K-12: cross-resistance among colicins of group A. J Bacteriol. 1975 Jul;123(1):102–117. doi: 10.1128/jb.123.1.102-117.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. K., Reeves P. Genetics of resistance to colicins in Escherichia coli K-12: cross-resistance among colicins of group B. J Bacteriol. 1975 Jul;123(1):96–101. doi: 10.1128/jb.123.1.96-101.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. Complete nucleotide sequence of bacteriophage T7 DNA and the locations of T7 genetic elements. J Mol Biol. 1983 Jun 5;166(4):477–535. doi: 10.1016/s0022-2836(83)80282-4. [DOI] [PubMed] [Google Scholar]
- Fischer E., Günter K., Braun V. Involvement of ExbB and TonB in transport across the outer membrane of Escherichia coli: phenotypic complementation of exb mutants by overexpressed tonB and physical stabilization of TonB by ExbB. J Bacteriol. 1989 Sep;171(9):5127–5134. doi: 10.1128/jb.171.9.5127-5134.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fognini-Lefebvre N., Lazzaroni J. C., Portalier R. tolA, tolB and excC, three cistrons involved in the control of pleiotropic release of periplasmic proteins by Escherichia coli K12. Mol Gen Genet. 1987 Sep;209(2):391–395. doi: 10.1007/BF00329670. [DOI] [PubMed] [Google Scholar]
- Frenette M., Benedetti H., Bernadac A., Baty D., Lazdunski C. Construction, expression and release of hybrid colicins. J Mol Biol. 1991 Feb 5;217(3):421–428. doi: 10.1016/0022-2836(91)90746-s. [DOI] [PubMed] [Google Scholar]
- Geli V., Baty D., Lazdunski C. Use of a foreign epitope as a "tag" for the localization of minor proteins within a cell: the case of the immunity protein to colicin A. Proc Natl Acad Sci U S A. 1988 Feb;85(3):689–693. doi: 10.1073/pnas.85.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger-Schnarr M., Lloubes R., de Murcia G., Schnarr M. Specific protein-DNA complexes: immunodetection of the protein component after gel electrophoresis and Western blotting. Anal Biochem. 1988 Oct;174(1):235–238. doi: 10.1016/0003-2697(88)90540-4. [DOI] [PubMed] [Google Scholar]
- Laffan J., Firshein W. Membrane protein binding to the origin region of Bacillus subtilis. J Bacteriol. 1987 Sep;169(9):4135–4140. doi: 10.1128/jb.169.9.4135-4140.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazdunski C. J., Baty D., Geli V., Cavard D., Morlon J., Lloubes R., Howard S. P., Knibiehler M., Chartier M., Varenne S. The membrane channel-forming colicin A: synthesis, secretion, structure, action and immunity. Biochim Biophys Acta. 1988 Oct 11;947(3):445–464. doi: 10.1016/0304-4157(88)90003-2. [DOI] [PubMed] [Google Scholar]
- Lazdunski C. J., Benedetti H. Insertion and translocation of proteins into and through membranes. FEBS Lett. 1990 Aug 1;268(2):408–414. doi: 10.1016/0014-5793(90)81295-y. [DOI] [PubMed] [Google Scholar]
- Lazzaroni J. C., Fognini-Lefebvre N., Portalier R. Cloning of the excC and excD genes involved in the release of periplasmic proteins by Escherichia coli K12. Mol Gen Genet. 1989 Sep;218(3):460–464. doi: 10.1007/BF00332410. [DOI] [PubMed] [Google Scholar]
- Levengood S. K., Webster R. E. Nucleotide sequences of the tolA and tolB genes and localization of their products, components of a multistep translocation system in Escherichia coli. J Bacteriol. 1989 Dec;171(12):6600–6609. doi: 10.1128/jb.171.12.6600-6609.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloubes R., Baty D., Lazdunski C. The promoters of the genes for colicin production, release and immunity in the ColA plasmid: effects of convergent transcription and Lex A protein. Nucleic Acids Res. 1986 Mar 25;14(6):2621–2636. doi: 10.1093/nar/14.6.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez M. C., Lazdunski C., Pattus F. Isolation, molecular and functional properties of the C-terminal domain of colicin A. EMBO J. 1983;2(9):1501–1507. doi: 10.1002/j.1460-2075.1983.tb01614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel de Zwaig R., Luria S. E. Genetics and physiology of colicin-tolerant mutants of Escherichia coli. J Bacteriol. 1967 Oct;94(4):1112–1123. doi: 10.1128/jb.94.4.1112-1123.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattus F., Massotte D., Wilmsen H. U., Lakey J., Tsernoglou D., Tucker A., Parker M. W. Colicins: prokaryotic killer-pores. Experientia. 1990 Feb 15;46(2):180–192. [PubMed] [Google Scholar]
- Pressler U., Braun V., Wittmann-Liebold B., Benz R. Structural and functional properties of colicin B. J Biol Chem. 1986 Feb 25;261(6):2654–2659. [PubMed] [Google Scholar]
- Schramm E., Mende J., Braun V., Kamp R. M. Nucleotide sequence of the colicin B activity gene cba: consensus pentapeptide among TonB-dependent colicins and receptors. J Bacteriol. 1987 Jul;169(7):3350–3357. doi: 10.1128/jb.169.7.3350-3357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonin F., Ménissier-de Murcia J., Poch O., Muller S., Gradwohl G., Molinete M., Penning C., Keith G., de Murcia G. Expression and site-directed mutagenesis of the catalytic domain of human poly(ADP-ribose)polymerase in Escherichia coli. Lysine 893 is critical for activity. J Biol Chem. 1990 Nov 5;265(31):19249–19256. [PubMed] [Google Scholar]
- Sun T. P., Webster R. E. Nucleotide sequence of a gene cluster involved in entry of E colicins and single-stranded DNA of infecting filamentous bacteriophages into Escherichia coli. J Bacteriol. 1987 Jun;169(6):2667–2674. doi: 10.1128/jb.169.6.2667-2674.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T. P., Webster R. E. fii, a bacterial locus required for filamentous phage infection and its relation to colicin-tolerant tolA and tolB. J Bacteriol. 1986 Jan;165(1):107–115. doi: 10.1128/jb.165.1.107-115.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinder N. D. Resistance to colicins E3 and K induced by infection with bacteriophage f1. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3160–3164. doi: 10.1073/pnas.70.11.3160. [DOI] [PMC free article] [PubMed] [Google Scholar]








