Abstract
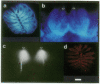
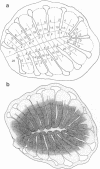
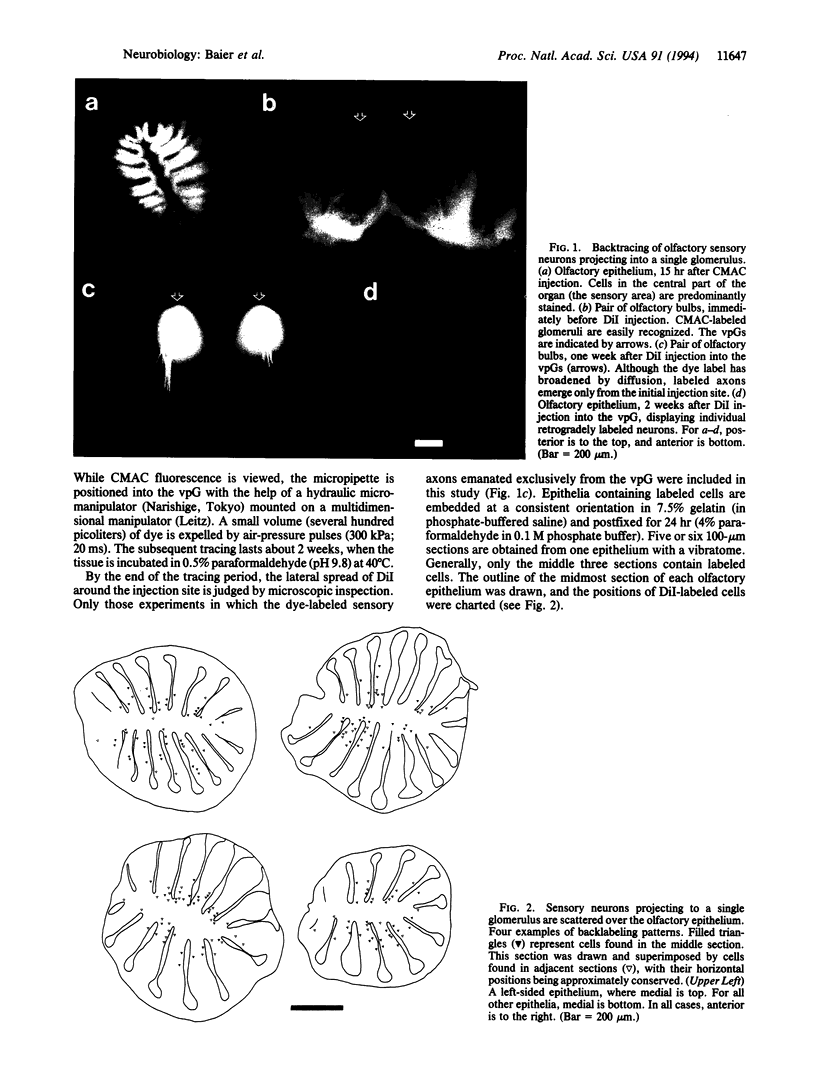
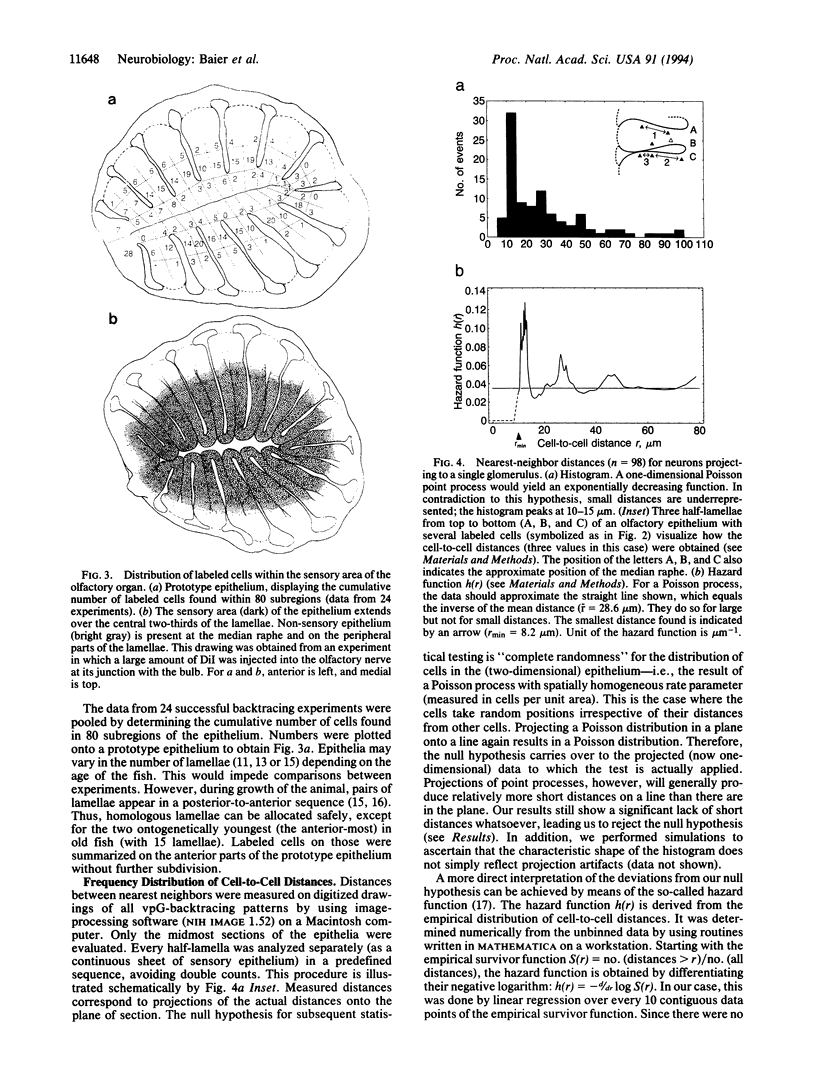
It is unknown how neuronal connections are specified in the olfactory system. To define rules of connectivity in this system, we investigated whether the projection of sensory neurons from the olfactory epithelium to the olfactory bulb is topographically ordered. By backtracking with 1,1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate (DiI), we find that neurons projecting into a single identified glomerulus are widely dispersed over the olfactory epithelium. Their positions in the sensory surface do not predict their glomerulus specificity and are probably random. A statistical analysis reveals that neurons connected to the same glomerulus are spaced at distances of several cell diameters from each other. The convergence of projections to one point in the target area from neurons that are widely and evenly distributed in the sensory surface constitutes an unusual type of connectional topography that contrasts with the precise topological (neighborhood-preserving) maps found in other sensory systems. It may maximize the probability to detect odorants that activate a single glomerular unit.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astic L., Saucier D., Holley A. Topographical relationships between olfactory receptor cells and glomerular foci in the rat olfactory bulb. Brain Res. 1987 Oct 20;424(1):144–152. doi: 10.1016/0006-8993(87)91204-2. [DOI] [PubMed] [Google Scholar]
- Baier H., Korsching S. Olfactory glomeruli in the zebrafish form an invariant pattern and are identifiable across animals. J Neurosci. 1994 Jan;14(1):219–230. doi: 10.1523/JNEUROSCI.14-01-00219.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg H. C., Purcell E. M. Physics of chemoreception. Biophys J. 1977 Nov;20(2):193–219. doi: 10.1016/S0006-3495(77)85544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck L., Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991 Apr 5;65(1):175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- Buonviso N., Chaput M. A. Response similarity to odors in olfactory bulb output cells presumed to be connected to the same glomerulus: electrophysiological study using simultaneous single-unit recordings. J Neurophysiol. 1990 Mar;63(3):447–454. doi: 10.1152/jn.1990.63.3.447. [DOI] [PubMed] [Google Scholar]
- Duncan H. J., Nickell W. T., Shipley M. T., Gesteland R. C. Organization of projections from olfactory epithelium to olfactory bulb in the frog, Rana pipiens. J Comp Neurol. 1990 Sep 15;299(3):299–311. doi: 10.1002/cne.902990304. [DOI] [PubMed] [Google Scholar]
- Fraser S. E. Patterning of retinotectal connections in the vertebrate visual system. Curr Opin Neurobiol. 1992 Feb;2(1):83–87. doi: 10.1016/0959-4388(92)90167-j. [DOI] [PubMed] [Google Scholar]
- Godement P., Vanselow J., Thanos S., Bonhoeffer F. A study in developing visual systems with a new method of staining neurones and their processes in fixed tissue. Development. 1987 Dec;101(4):697–713. doi: 10.1242/dev.101.4.697. [DOI] [PubMed] [Google Scholar]
- Greenwald I., Rubin G. M. Making a difference: the role of cell-cell interactions in establishing separate identities for equivalent cells. Cell. 1992 Jan 24;68(2):271–281. doi: 10.1016/0092-8674(92)90470-w. [DOI] [PubMed] [Google Scholar]
- Imamura K., Mataga N., Mori K. Coding of odor molecules by mitral/tufted cells in rabbit olfactory bulb. I. Aliphatic compounds. J Neurophysiol. 1992 Dec;68(6):1986–2002. doi: 10.1152/jn.1992.68.6.1986. [DOI] [PubMed] [Google Scholar]
- Jastreboff P. J., Pedersen P. E., Greer C. A., Stewart W. B., Kauer J. S., Benson T. E., Shepherd G. M. Specific olfactory receptor populations projecting to identified glomeruli in the rat olfactory bulb. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5250–5254. doi: 10.1073/pnas.81.16.5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancet D., Greer C. A., Kauer J. S., Shepherd G. M. Mapping of odor-related neuronal activity in the olfactory bulb by high-resolution 2-deoxyglucose autoradiography. Proc Natl Acad Sci U S A. 1982 Jan;79(2):670–674. doi: 10.1073/pnas.79.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay-Sim A., Nathan M. H. The projection from the olfactory epithelium to the olfactory bulb in the salamander, Ambystoma tigrinum. Anat Embryol (Berl) 1984;170(1):93–97. doi: 10.1007/BF00319463. [DOI] [PubMed] [Google Scholar]
- Ngai J., Chess A., Dowling M. M., Necles N., Macagno E. R., Axel R. Coding of olfactory information: topography of odorant receptor expression in the catfish olfactory epithelium. Cell. 1993 Mar 12;72(5):667–680. doi: 10.1016/0092-8674(93)90396-8. [DOI] [PubMed] [Google Scholar]
- Riddle D. R., Oakley B. Evaluation of projection patterns in the primary olfactory system of rainbow trout. J Neurosci. 1991 Dec;11(12):3752–3762. doi: 10.1523/JNEUROSCI.11-12-03752.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd G. M. Neurobiology. Modules for molecules. Nature. 1992 Aug 6;358(6386):457–458. doi: 10.1038/358457a0. [DOI] [PubMed] [Google Scholar]
- Udin S. B., Fawcett J. W. Formation of topographic maps. Annu Rev Neurosci. 1988;11:289–327. doi: 10.1146/annurev.ne.11.030188.001445. [DOI] [PubMed] [Google Scholar]