Abstract
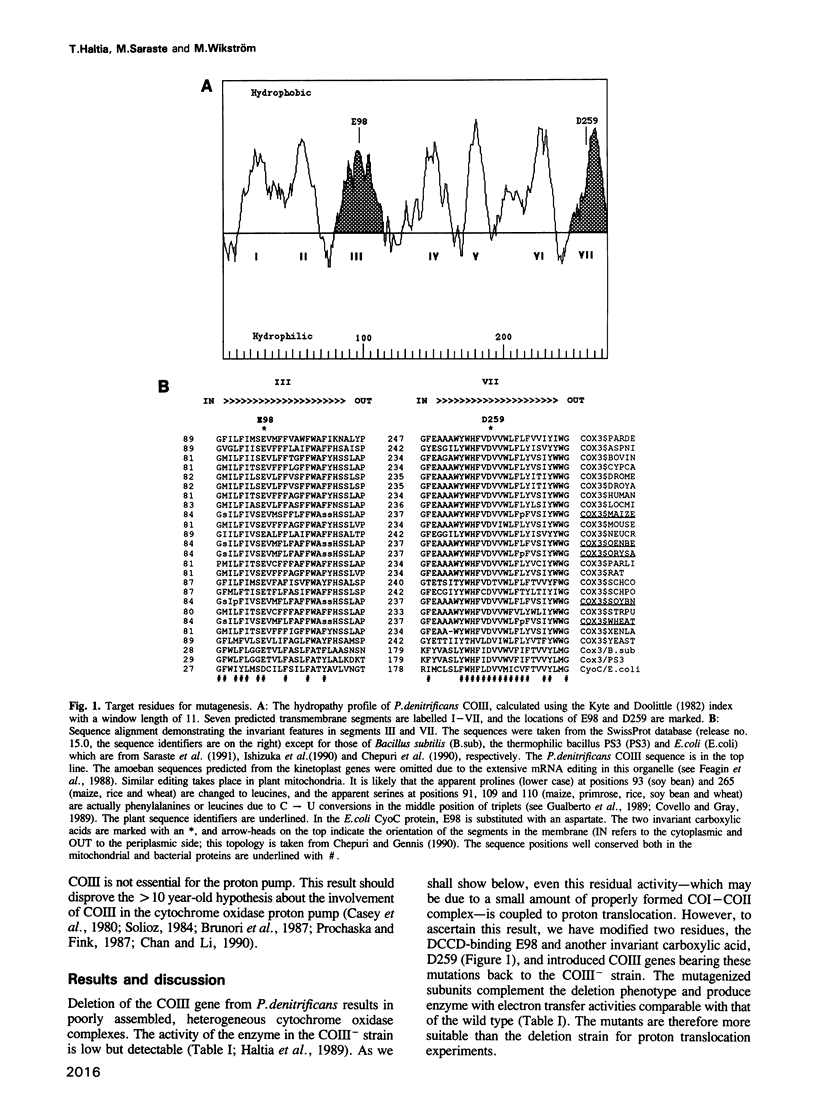
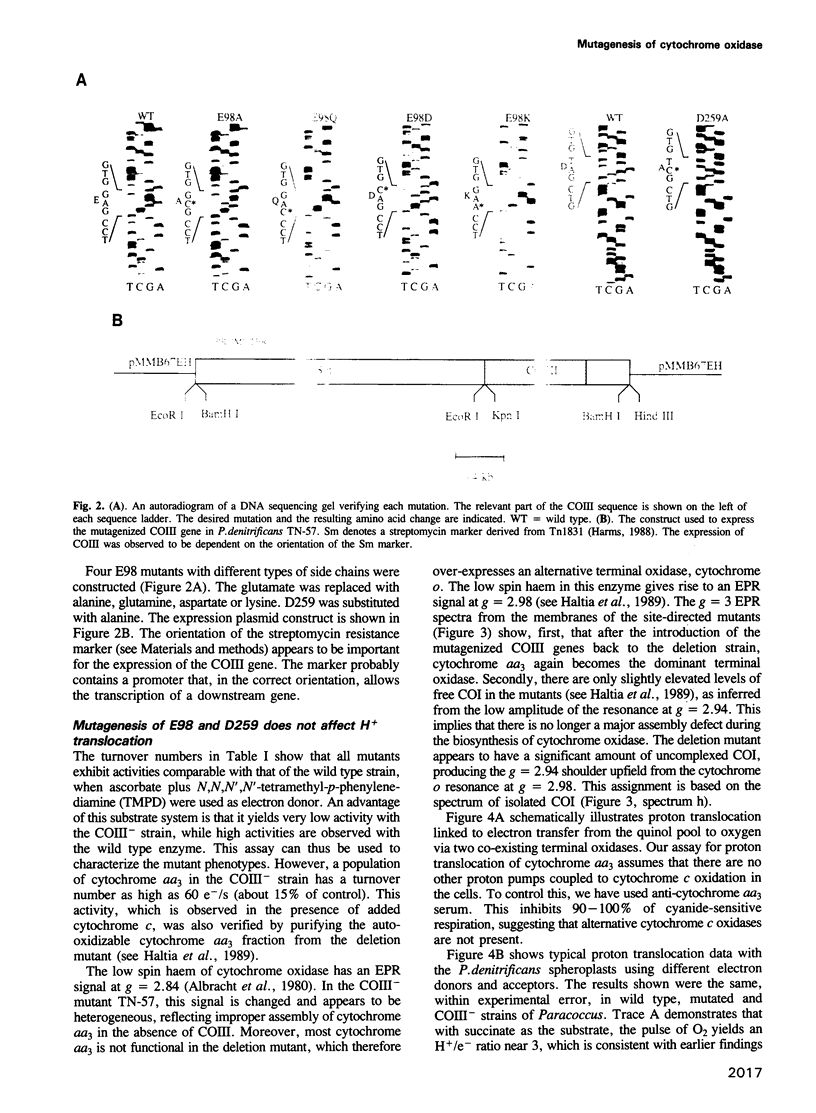
Subunit III (COIII) is one of the three core subunits of the aa3-type cytochrome c oxidase. COIII does not contain any of the redox centres and can be removed from the purified enzyme but has a function during biosynthesis of the enzyme. Dicyclohexyl carbodiimide (DCCD) modifies a conserved glutamic acid residue in COIII and abolishes the proton translocation activity of the enzyme. In this study, the invariant carboxylic acids E98 (the DCCD-binding glutamic acid) and D259 of COIII were changed by site-directed mutagenesis to study their role in proton pumping. Spectroscopy and activity measurements show that a structurally normal enzyme, which is active in electron transfer, is formed in the presence of the mutagenized COIII. Experiments with bacterial spheroplasts indicate that the mutant oxidases are fully competent in proton translocation. In the absence of the COIII gene, only a fraction of the oxidase is assembled into an enzyme with low but significant activity. This residual activity is also coupled to proton translocation. We conclude that, in contrast to numerous earlier suggestions, COIII is not an essential element of the proton pump.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albracht S. P., van Verseveld H. W., Hagen W. R., Kalkman M. L. A comparison of the respiratory chain in particles from Paracoccus denitrificans and bovine heart mitochondria by EPR spectroscopy. Biochim Biophys Acta. 1980 Dec 3;593(2):173–186. doi: 10.1016/0005-2728(80)90055-9. [DOI] [PubMed] [Google Scholar]
- Azzi A., Casey R. P., Nałecz M. J. The effect of N,N'-dicyclohexylcarbodiimide on enzymes of bioenergetic relevance. Biochim Biophys Acta. 1984 Dec 17;768(3-4):209–226. doi: 10.1016/0304-4173(84)90017-x. [DOI] [PubMed] [Google Scholar]
- Bolgiano B., Smith L., Davies H. C. Kinetics of the interaction of the cytochrome c oxidase of Paracoccus denitrificans with its own and bovine cytochrome c. Biochim Biophys Acta. 1988 Apr 22;933(2):341–350. doi: 10.1016/0005-2728(88)90041-2. [DOI] [PubMed] [Google Scholar]
- Brunori M., Antonini G., Malatesta F., Sarti P., Wilson M. T. Cytochrome-c oxidase. Subunit structure and proton pumping. Eur J Biochem. 1987 Nov 16;169(1):1–8. doi: 10.1111/j.1432-1033.1987.tb13572.x. [DOI] [PubMed] [Google Scholar]
- Buse G., Hensel S., Fee J. A. Evidence for cytochrome oxidase subunit I and a cytochrome c--subunit II fused protein in the cytochrome 'c1aa3' of Thermus thermophilus. How old is cytochrome oxidase? Eur J Biochem. 1989 Apr 15;181(1):261–268. doi: 10.1111/j.1432-1033.1989.tb14720.x. [DOI] [PubMed] [Google Scholar]
- Capitanio N., De Nitto E., Villani G., Capitanio G., Papa S. Protonmotive activity of cytochrome c oxidase: control of oxidoreduction of the heme centers by the protonmotive force in the reconstituted beef heart enzyme. Biochemistry. 1990 Mar 27;29(12):2939–2945. doi: 10.1021/bi00464a008. [DOI] [PubMed] [Google Scholar]
- Carroll R. C., Racker E. Preparation and characterization of cytochrome c oxidase vesicles with high respiratory control. J Biol Chem. 1977 Oct 25;252(20):6981–6990. [PubMed] [Google Scholar]
- Casey R. P., Thelen M., Azzi A. Dicyclohexylcarbodiimide binds specifically and covalently to cytochrome c oxidase while inhibiting its H+-translocating activity. J Biol Chem. 1980 May 10;255(9):3994–4000. [PubMed] [Google Scholar]
- Chan S. I., Li P. M. Cytochrome c oxidase: understanding nature's design of a proton pump. Biochemistry. 1990 Jan 9;29(1):1–12. doi: 10.1021/bi00453a001. [DOI] [PubMed] [Google Scholar]
- Chepuri V., Gennis R. B. The use of gene fusions to determine the topology of all of the subunits of the cytochrome o terminal oxidase complex of Escherichia coli. J Biol Chem. 1990 Aug 5;265(22):12978–12986. [PubMed] [Google Scholar]
- Chepuri V., Lemieux L., Au D. C., Gennis R. B. The sequence of the cyo operon indicates substantial structural similarities between the cytochrome o ubiquinol oxidase of Escherichia coli and the aa3-type family of cytochrome c oxidases. J Biol Chem. 1990 Jul 5;265(19):11185–11192. [PubMed] [Google Scholar]
- Covello P. S., Gray M. W. RNA editing in plant mitochondria. Nature. 1989 Oct 19;341(6243):662–666. doi: 10.1038/341662a0. [DOI] [PubMed] [Google Scholar]
- Feagin J. E., Abraham J. M., Stuart K. Extensive editing of the cytochrome c oxidase III transcript in Trypanosoma brucei. Cell. 1988 May 6;53(3):413–422. doi: 10.1016/0092-8674(88)90161-4. [DOI] [PubMed] [Google Scholar]
- Finel M., Wikström M. Studies on the role of the oligomeric state and subunit III of cytochrome oxidase in proton translocation. Biochim Biophys Acta. 1986 Aug 13;851(1):99–108. doi: 10.1016/0005-2728(86)90253-7. [DOI] [PubMed] [Google Scholar]
- Fürste J. P., Pansegrau W., Frank R., Blöcker H., Scholz P., Bagdasarian M., Lanka E. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene. 1986;48(1):119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]
- Gregory L. C., Ferguson-Miller S. Effect of subunit III removal on control of cytochrome c oxidase activity by pH. Biochemistry. 1988 Aug 23;27(17):6307–6314. doi: 10.1021/bi00417a016. [DOI] [PubMed] [Google Scholar]
- Gualberto J. M., Lamattina L., Bonnard G., Weil J. H., Grienenberger J. M. RNA editing in wheat mitochondria results in the conservation of protein sequences. Nature. 1989 Oct 19;341(6243):660–662. doi: 10.1038/341660a0. [DOI] [PubMed] [Google Scholar]
- Gyllensten U. B., Erlich H. A. Generation of single-stranded DNA by the polymerase chain reaction and its application to direct sequencing of the HLA-DQA locus. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7652–7656. doi: 10.1073/pnas.85.20.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haltia T., Finel M., Harms N., Nakari T., Raitio M., Wikström M., Saraste M. Deletion of the gene for subunit III leads to defective assembly of bacterial cytochrome oxidase. EMBO J. 1989 Dec 1;8(12):3571–3579. doi: 10.1002/j.1460-2075.1989.tb08529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haltia T., Puustinen A., Finel M. The Paracoccus denitrificans cytochrome aa3 has a third subunit. Eur J Biochem. 1988 Mar 15;172(3):543–546. doi: 10.1111/j.1432-1033.1988.tb13923.x. [DOI] [PubMed] [Google Scholar]
- Henderson R., Baldwin J. M., Ceska T. A., Zemlin F., Beckmann E., Downing K. H. Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy. J Mol Biol. 1990 Jun 20;213(4):899–929. doi: 10.1016/S0022-2836(05)80271-2. [DOI] [PubMed] [Google Scholar]
- Ishizuka M., Machida K., Shimada S., Mogi A., Tsuchiya T., Ohmori T., Souma Y., Gonda M., Sone N. Nucleotide sequence of the gene coding for four subunits of cytochrome c oxidase from the thermophilic bacterium PS3. J Biochem. 1990 Nov;108(5):866–873. doi: 10.1093/oxfordjournals.jbchem.a123294. [DOI] [PubMed] [Google Scholar]
- Khorana H. G. Bacteriorhodopsin, a membrane protein that uses light to translocate protons. J Biol Chem. 1988 Jun 5;263(16):7439–7442. [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Li P. M., Morgan J. E., Nilsson T., Ma M., Chan S. I. Heat treatment of cytochrome c oxidase perturbs the CuA site and affects proton pumping behavior. Biochemistry. 1988 Sep 20;27(19):7538–7546. doi: 10.1021/bi00419a054. [DOI] [PubMed] [Google Scholar]
- Malmström B. G. The mechanism of proton translocation in respiration and photosynthesis. FEBS Lett. 1989 Jun 19;250(1):9–21. doi: 10.1016/0014-5793(89)80675-1. [DOI] [PubMed] [Google Scholar]
- Morin P. E., Diggs D., Freire E. Thermal stability of membrane-reconstituted yeast cytochrome c oxidase. Biochemistry. 1990 Jan 23;29(3):781–788. doi: 10.1021/bi00455a028. [DOI] [PubMed] [Google Scholar]
- Nałecz M. J., Casey R. P., Azzi A. Use of N,N'-dicyclohexylcarbodiimide to study membrane-bound enzymes. Methods Enzymol. 1986;125:86–108. doi: 10.1016/s0076-6879(86)25009-0. [DOI] [PubMed] [Google Scholar]
- Nobrega M. P., Nobrega F. G., Tzagoloff A. COX10 codes for a protein homologous to the ORF1 product of Paracoccus denitrificans and is required for the synthesis of yeast cytochrome oxidase. J Biol Chem. 1990 Aug 25;265(24):14220–14226. [PubMed] [Google Scholar]
- Penttilä T. Properties and reconstitution of a cytochrome oxidase deficient in subunit III. Eur J Biochem. 1983 Jun 15;133(2):355–361. doi: 10.1111/j.1432-1033.1983.tb07470.x. [DOI] [PubMed] [Google Scholar]
- Prochaska L. J., Bisson R., Capaldi R. A., Steffens G. C., Buse G. Inhibition of cytochrome c oxidase function by dicyclohexylcarbodiimide. Biochim Biophys Acta. 1981 Sep 14;637(2):360–373. doi: 10.1016/0005-2728(81)90175-4. [DOI] [PubMed] [Google Scholar]
- Prochaska L. J., Fink P. S. On the role of subunit III in proton translocation in cytochrome c oxidase. J Bioenerg Biomembr. 1987 Apr;19(2):143–166. doi: 10.1007/BF00762722. [DOI] [PubMed] [Google Scholar]
- Puustinen A., Finel M., Virkki M., Wikström M. Cytochrome o (bo) is a proton pump in Paracoccus denitrificans and Escherichia coli. FEBS Lett. 1989 Jun 5;249(2):163–167. doi: 10.1016/0014-5793(89)80616-7. [DOI] [PubMed] [Google Scholar]
- Raitio M., Jalli T., Saraste M. Isolation and analysis of the genes for cytochrome c oxidase in Paracoccus denitrificans. EMBO J. 1987 Sep;6(9):2825–2833. doi: 10.1002/j.1460-2075.1987.tb02579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigell C. W., Freire E. Differential detergent solubility investigation of thermally induced transitions in cytochrome c oxidase. Biochemistry. 1987 Jul 14;26(14):4366–4371. doi: 10.1021/bi00388a027. [DOI] [PubMed] [Google Scholar]
- Rigell C. W., de Saussure C., Freire E. Protein and lipid structural transitions in cytochrome c oxidase-dimyristoylphosphatidylcholine reconstitutions. Biochemistry. 1985 Sep 24;24(20):5638–5646. doi: 10.1021/bi00341a053. [DOI] [PubMed] [Google Scholar]
- Saraste M., Metso T., Nakari T., Jalli T., Lauraeus M., Van der Oost J. The Bacillus subtilis cytochrome-c oxidase. Variations on a conserved protein theme. Eur J Biochem. 1991 Jan 30;195(2):517–525. doi: 10.1111/j.1432-1033.1991.tb15732.x. [DOI] [PubMed] [Google Scholar]
- Saraste M. Structural features of cytochrome oxidase. Q Rev Biophys. 1990 Nov;23(4):331–366. doi: 10.1017/s0033583500005588. [DOI] [PubMed] [Google Scholar]
- Sayers J. R., Schmidt W., Eckstein F. 5'-3' exonucleases in phosphorothioate-based oligonucleotide-directed mutagenesis. Nucleic Acids Res. 1988 Feb 11;16(3):791–802. doi: 10.1093/nar/16.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solioz M., Carafoli E., Ludwig B. The cytochrome c oxidase of Paracoccus denitrificans pumps protons in a reconstituted system. J Biol Chem. 1982 Feb 25;257(4):1579–1582. [PubMed] [Google Scholar]
- Tzagoloff A., Capitanio N., Nobrega M. P., Gatti D. Cytochrome oxidase assembly in yeast requires the product of COX11, a homolog of the P. denitrificans protein encoded by ORF3. EMBO J. 1990 Sep;9(9):2759–2764. doi: 10.1002/j.1460-2075.1990.tb07463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikström M., Casey R. P. What is the essential proton-translocating molecular machinery in cytochrome oxidase? J Inorg Biochem. 1985 Mar-Apr;23(3-4):327–334. doi: 10.1016/0162-0134(85)85042-x. [DOI] [PubMed] [Google Scholar]
- Wilson K. S., Prochaska L. J. Phospholipid vesicles containing bovine heart mitochondrial cytochrome c oxidase and subunit III-deficient enzyme: analysis of respiratory control and proton translocating activities. Arch Biochem Biophys. 1990 Nov 1;282(2):413–420. doi: 10.1016/0003-9861(90)90137-n. [DOI] [PubMed] [Google Scholar]




