Abstract
The way in which emotion is represented and processed in the human brain is an expanding area of research and has key implications for how we understand and potentially treat affective disorders such as depression. Characterizing the effects of pharmacological manipulations of key neurotransmitter systems can also help reveal the neurochemical underpinnings of emotional processing and how common antidepressant drugs may work in the treatment of depression and anxiety. This approach has revealed that depression is associated with both neural and behavioural biases towards negative over positive stimuli. Evidence from pharmacological challenge studies suggests that antidepressant treatment acts to normalize these biases early on in treatment, resulting in patients experiencing the world in a more positive way, improving their mood over time. This model is supported by evidence from both pharmacological and non-pharmacological interventions. The unique perspective on antidepressant treatment offered by this approach provides some insights into individual response to treatment, as well as novel approaches to drug development.
Keywords: depression, emotional processing, neuroimaging
1. Introduction
The field of affective neuroscience considers how the brain represents and processes emotion. This usually involves employing tasks designed to tap into the processing of emotional information, including aspects of cognition such as memory, attention and perception, as well as more elaborative processes. These tasks will often be paired with neuroimaging methods, such as functional magnetic resonance imaging (fMRI), in order to ascertain how regions and networks of the brain represent this information.
In the absence of any intervention, these studies can be valuable in understanding how the brain processes emotional information. However, interfering with neural functioning through the use of pharmacological interventions can provide additional insight. Researchers can examine the effects of drugs that are known to generate or reduce a given emotion and relate these to behavioural and neural changes. For example, the increased amygdala response to fearful faces after administration of anxiogenic drugs like amphetamines [1] and decreased response after anxiolytic drugs like benzodiazepines [2] reinforce the central role of this structure for attending to negative, particularly fear-related, information.
With an increased understanding of how pharmacological interventions affect the behavioural and neural processing of emotional information, we can also gain a unique perspective into how clinically useful drugs might exert their effects. Rather than being secondary to the clinical effects of these drugs, the neuropsychological changes may in fact produce these clinical effects. To take the example above, perhaps the reduction of amygdala response to fearful faces after benzodiazepine administration is in fact instrumental in decreasing anxiety. This is the basis for the neurocognitive model for understanding treatment action in depression.
Models of treatment action in depression have generally focused on the molecular and cellular changes thought to underlie the clinical response. Because improvement in depressive symptoms is traditionally thought to take several weeks to emerge [3], these models often concern slow, adaptive processes in the brain. One of the more common forms of antidepressant, the selective serotonin reuptake inhibitor (SSRI), works by blocking the serotonin reuptake transporter, increasing availability of serotonin in the synapse. However, one popular theory is that clinical effects are not seen immediately owing to the existence of negative feedback from autoreceptors, and it is not until these are desensitized after chronic treatment that improvements in mood emerge [4]. More recently, hippocampal neurogenesis has been suggested to be fundamental to the clinical effects of antidepressant drugs. In animal models, neurogenesis is stimulated by antidepressant treatment, and some of the behavioural effects of these treatments are blocked by ablating neurogenesis [5]. The maturation of new cells takes several weeks, in line with the delay in treatment response [6].
One of the challenges for these models is to explain exactly how molecular- and cellular-level changes produce improvements in mood. The neurocognitive model provides an alternative approach to understanding treatment action, which places more of an emphasis on how clinical effects emerge. There is growing evidence that antidepressant interventions produce relatively immediate neural and behavioural changes in relation to emotional processing. Specifically, antidepressants appear to bias emotional processing in favour of more positive stimuli and away from negative stimuli [7,8].
Patients suffering from depression display baseline negative biases in emotional processing, which may serve to produce and maintain lowered mood [9]. The effects of antidepressants on emotional processing thus serve to remediate these biases. After commencing antidepressant treatment, a patient begins to see the world around them in a more positive way, for example attending less to negative information, or becoming better at remembering positive events. With more and more experience of their environment in this new, more positive way, the patient feels increasingly better. Thus cognitive responses to affective situations and experiences will be altered straightaway and will culminate in symptomatic improvement that becomes evident over time, consistent with recent studies into the time course of clinical effects [10].
In this review, we describe the neurocognitive model in more detail, examining first the kind of changes in emotional processing that antidepressant drugs cause, and then converging evidence from studies looking at antidepressant drugs with atypical mechanisms of action, novel putative antidepressant treatments and directional effects in the model following treatment with drugs that may cause lowering of mood. We also examine the value of these early neurocognitive changes in producing later improvements in mood. Finally, we discuss the implications of the model for understanding individual response to antidepressants and for future drug development.
2. Cognitive biases in depression
The presence of emotional biases among patients suffering from depression is well established [11]. Behaviourally, depressed patients show increased processing of negative versus positive emotional information. These biases are apparent in a range of tasks measuring attention, perception and memory for emotional stimuli: for example, compared with healthy controls, depressed patients are slower at categorizing positive self-referent personality words, and later worse at remembering these [9] (figure 1a). By contrast, they are better at recalling negative words [12]. They are also worse at recognizing happy facial expressions, and interpret ambiguous expressions as more sad than healthy controls [9,13].
Figure 1.
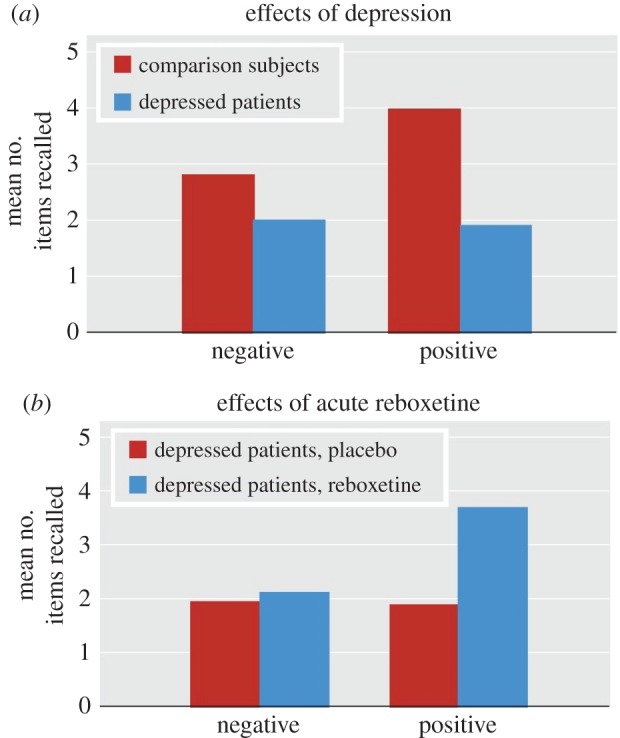
(a) Number of words correctly recalled for depressed patients and healthy volunteers given a placebo. Depressed patients show significantly reduced recall for positive words, p < 0.05; (b) Effect of an acute dose of reboxetine on word recall. Patients given reboxetine remember more positive words than those taking placebo, p < 0.01. Adapted from [9], with permission from the American Journal of Psychiatry (Copyright © 2009 American Psychiatric Association). (Online version in colour.)
These differences are mirrored at the neural level. In functional imaging studies, depressed patients show greater blood oxygenation level-dependent (BOLD) response to negative stimuli in a network of areas thought to be involved in detecting and responding to salient emotional information, including the amygdala, insula and anterior cingulate cortex (ACC) [14,15]. In parallel with this hyperactivity in limbic areas, there is also reduced activity in dorsolateral prefrontal cortex (DLPFC) to both positive and negative stimuli, as well as lower resting blood flow [14,16]. Thus, the model to emerge from neuroimaging literature involves a hyperactive limbic system that biases emotional processing towards negative stimuli at an early stage, while a hypoactive DLPFC is in turn less able to provide top–down regulation of the limbic system [15,17].
These neuropsychological biases are thought to play a fundamental role in producing a depressed mood. Processing of information about the self and the surrounding world in a more negative fashion produces depressive symptoms, through increased attention and memory for negative information, as well as through more elaborative processes like rumination and negative interpretations [15,18]. A number of studies provide evidence for an explicitly causative role of emotional processing biases on depressed mood: heightened processing of negative facial expressions has been shown to predict relapse among remitted depressed patients [19], while generating or reducing bias with cognitive bias modification techniques can affect responses to emotional information and markers of depressive relapse [20,21]. Once established, the symptoms of depression then reinforce the original negative biases, serving to maintain the depressed mood [13].
3. Antidepressant drug treatment produces changes in cognitive biases
The normalization of biases in emotional processing is central to the neurocognitive model of antidepressant treatment. By producing a more positive way of processing emotional information, antidepressant treatments effectively break this cycle and relieve symptoms of depression. The following sections examine evidence for such changes after antidepressant treatment.
(a). Healthy volunteer studies
Healthy volunteer studies have been the most important in establishing the neurocognitive effects of antidepressant treatments. Examining the effects of these drugs in healthy volunteers demonstrates that any neurocognitive changes cannot be attributed to early improvements in depressive symptoms. Unless specified, the results described below (and throughout the rest of the review) compare drug effects to a placebo control group. Further information about doses given can be found in tables 1 and 2; these tended to be at the lower end of the normal clinical range.
Table 1.
Summary table of the effects of pharmacological interventions on measures of emotional processing in healthy volunteers, depressed patients and high neurotic volunteers. Blank cells indicate that the task was not conducted; non-significant findings are noted. Doses were take orally unless otherwise stated. RT, reaction time.
| dose | face recognition | word categorization (RTs) | emotional word recall | other | ||
|---|---|---|---|---|---|---|
| short-term (7 days unless stated) | ||||||
| healthy volunteers | ||||||
| citalopram | 20 mg | ↓ fear, anger, disgust, surprise | ↓ pos vs neg (trend) | ↑ pos vs neg | ↓ startle response during neg pictures | [22] |
| citalopram | 20 mg | ↓ fear | — | — | — | [23] |
| citalopram | 20 mg | — | — | — | ↓ attentional vigilance to fearful faces | [24] |
| reboxetine | 4 mg | ↓ fear, anger | ↓ pos vs neg | ↑ pos vs neg | no effect on startle response | [22] |
| reboxetine | 4 mg | — | — | — | no effect on attentional vigilance to fearful faces | [24] |
| tryptophan | 3 g (14 days) | ↑ happy, ↓ disgust | no sig effects | no sig effects | ↓ attentional vigilance to neg words; ↓ startle response | [25] |
| agomelatine | 25 mg | ↓ sad | no sig effects | ↑ pos vs neg | ↓ startle response during neg pictures, ↑ during pos pictures | [26] |
| depressed patients | ||||||
| citalopram | 10 mg | ↑ happy | — | — | — | [27] |
| citalopram | 20 mg (14 days) | ↑ happy, disgust, surprise | — | — | — | [28] |
| reboxetine | 4 mg (14 days) | ↑ happy, disgust, surprise | — | — | — | [28] |
| high neurotics | ||||||
| citalopram | 20 mg | ↑ pos vs neg | — | — | ↑ gaze at facial expressions | [29] |
| acute dose | ||||||
| healthy volunteers | ||||||
| citalopram | 10 mg (i.v.) | ↑ happy, fear | — | — | — | [7] |
| citalopram | 20 mg | ↑ happy | — | — | — | [30] |
| citalopram | 20 mg | ↑ fear | no sig effects | no sig effects | ↑ attentional bias to pos words; ↑ baseline startle response | [31] |
| reboxetine | 4 mg | ↑ happy | ↓ pos vs neg | ↓ negative | — | [8] |
| reboxetine | 4 mg | ↑ happy | ↓ pos vs neg | no sig effects | — | [9] |
| reboxetine | 4 mg | — | no sig effects | — | ↓ RT in pos word recognition | [32] |
| duloxetine | 60 mg | ↑ happy, disgust | no sig effects | ↑ false positives | no effects on startle | [33] |
| mirtazapine | 15 mg | ↓ fear | ↓ pos and neg | ↑ pos versus neg | ↓ startle response | [34] |
| depressed patients | ||||||
| reboxetine | 4 mg | ↑ happy | ↓ pos vs neg | ↑ pos vs neg | — | [9] |
Table 2.
Effects of acute and short-term antidepressant administration on BOLD response while viewing emotional facial expressions. Arrows indicate increases or decreases in BOLD response for participants given drug compared with placebo. ACC, anterior cingulate cortex; OFC, orbitofrontal cortex; PFC, prefrontal cortex.
| dose | amygdala | other areas | ||
|---|---|---|---|---|
| short-term dose (7–10 days) | ||||
| healthy volunteers | ||||
| citalopram | 20 mg | ↓ fear | amygdala–hippocampal area, medial frontal gyrus: ↓ fear | [23] |
| citalopram | 20 mg | ↑ happy | [35] | |
| reboxetine | 4 mg | ↓ fear | R fusiform gyrus: ↑ happy | [36] |
| depressed patients | ||||
| escitalopram | 10 mg | ↓ fear | [37] | |
| high neurotics | ||||
| citalopram | 20 mg | ↑ happy, fear, neutral | PFC: ↑ fear versus happy | [38] |
| acute dose | ||||
| healthy volunteers | ||||
| citalopram | 20 mg | ↓ fear | [30] | |
| citalopram | 7.5 mg (i.v.) | ↓ fear | L ACC: ↑ happy R posterior insula, R lateral OFC: ↓sad |
[39] |
| citalopram | 50 mg over approx. 3 h | ↓ fear | fusiform gyrus, posterior occipital cortex, R superior temporal sulcus, ventral striatum, medial PFC: ↓ fear faces | [40] |
| citalopram | 7.5 mg (i.v.) | ↓ disgust, fear | posterior insula: ↑ disgust | [41] |
| citalopram | 7.5 mg (i.v.) | ↓ aversive (anger/disgust/fear) | lateral OFC: ↓aversive fusiform gyrus, thamlus: ↑ aversive |
[42] |
| citalopram | 20 mg over 30 min (i.v.) | ↑ general emotional (anger/fear/surprise) | [43] | |
| mirtazapine | 15 mg | ↓ fear; ↑ happy | fusiform gyrus: ↓ fear | [44] |
(i). Short-term administration
Short-term antidepressant treatment produces biases in the processing of emotional facial expressions (see table 1 for a summary of behavioural results). For example, in response to 7 days' administration of the noradrenaline reuptake inhibitor (NRI) reboxetine, recognition of both fearful and angry faces decreased [22]. Seven days of the SSRI citalopram produced broader changes, reducing recognition of fearful and angry faces but also disgusted and surprised faces (see also [23]).
These effects are reflected in changes in neural processing. Seven days' administration of both citalopram [23] and reboxetine [36] reduced BOLD response in the amygdala in response to fearful faces. Increases in neural response to happy faces have also been seen, both in the amygdala [35] and the right fusiform [36] (see table 2 for a summary of neural effects relating to face processing). These patterns of neural activity, restricted to areas involved in relatively low-level processing of emotional stimuli, suggest that early effects of antidepressants may be working in a ‘bottom-up’ fashion, affecting the automatic evaluation of emotional stimuli [45]. Indeed, a recent meta-analysis found that across a range of different cognitive tasks, short-term antidepressant treatment increased activation to positive emotional information, and decreased activation to negative information, across a network including the amygdala, putamen, ACC, parahippocampal gyrus and medial prefrontal cortex [46]. These limbic and paralimbic structures are involved in detecting and responding to salient emotional information, supporting this ‘bottom-up’ interpretation of early antidepressant effects.
Seven days' administration of antidepressants also affects processing of self-referent words and subsequent memory for these. Seven days of reboxetine reduced the reaction time to classify positive self-referent words, and both reboxetine and citalopram increased later recall of positive versus negative words [22]. At the neural level, 7 days of reboxetine increased activity to positive relative to negative words in the inferior frontal gyrus and precuneus during categorization, while decreasing activity in medial frontal gyrus and precuneus during correct recognition of positive words [47]. Increased activity to positive words during categorization may reflect heightened attentional processing of these words. Conversely, reduced activity during the subsequent memory task may be attributed to decreased retrieval effort for positive words.
It should be noted here that doubts have been raised about the antidepressant efficacy of reboxetine in recent meta-analyses [48]. However, it has been shown that these meta-analyses have produced biased accounts of the drug's efficacy, by classing participants who dropped out as non-responders or by using an individual's earlier data in place of later missing data [49]. This is a problem as reboxetine is less well tolerated than many other antidepressants, and so tends to result in more drop-outs. However, when non-adherence is factored into the analysis, there is no difference between the efficacy of reboxetine and citalopram. Therefore, the neurocognitive effects discussed here appear consistent with the drug's clinical profile.
Finally, citalopram but not reboxetine reduced attentional vigilance to fearful faces [24] and reduced the eyeblink startle response to a short, loud burst of sound when viewing negative pictures [22]. These effects may be related specifically to citalopram's anxiolytic effects, and both heightened startle response and negative attentional biases have been related more consistently to anxiety than depression [50,51] (see §4b for more details on distinguishing between the anxiolytic and antidepressant effects of SSRIs).
(ii). Acute effects
Acute doses of antidepressants also alter the processing of emotional facial expressions. After acute doses of citalopram [7,30], reboxetine [8,9] and duloxetine [33], healthy participants were better at recognizing happy faces. The noradrenergic and specific serotonergic antidepressant (NaSSA) mirtazapine has also been shown to decrease recognition of fearful faces [34]. Interestingly, SSRIs such as citalopram have sometimes been found to increase fear recognition at acute doses [7,31]. However, SSRIs are known to produce anxiety early on in treatment, which resolves over time [52], and so this effect may reflect the acute anxiogenic potential of these drugs. Conversely, reboxetine, which does not have the same acute anxiogenic effect as the SSRIs, does not produce early increases in fear recognition [53].
These early effects of antidepressants on facial expression processing are related to neural changes in fMRI studies (table 2). Again, a network of limbic and paralimbic structures shows altered activation after acute doses, with a key role for the amygdala. For example, participants who took a dose of mirtazapine showed decreased response in the amygdala to fearful faces presented for 100 ms, and an increased amygdala response to happy faces [44]. Contrary to the behavioural results, several studies of acute SSRI administration also found decreased amygdala response to fearful faces [30,39,41,42]. However, other studies have found increased amygdala response [43], and a recent meta-analysis showed that the direction of effects in this network of structures varies between studies [46]. Future work should attempt to establish the reason behind these discrepancies, including a focus on dose–response relationships and the basal characteristics of the volunteers included in the studies.
At acute doses, noradrenergic drugs appear to have particular effects on the categorization of personality characteristic words, as well as later memory for these words. Reboxetine decreased the time taken to categorize self-referent personality words as positive [8,9], and also increased recognition of positive words [32] and decreased recall of negative words [8]. These effects are not as consistently seen in acute SSRI treatment, although they are seen later, after short-term SSRI treatment [22], so it may be that potentiation of noradrenaline has earlier effects on memory than serotonin. Indeed, while acute studies using citalopram have failed to find acute effects on word categorization or memory [31], duloxetine, a serotonin and norepinephrine reuptake inhibitor (SNRI), increased the number of positive, but not negative, words falsely recalled in a memory task [33].
At the neural level, an acute dose of reboxetine decreased activation in a right fronto-parietal network including the medial frontal gyrus when recognizing previously seen positive words [32]. This again appears consistent with reduced retrieval effort for positive, but not negative, words.
(b). Depressed patient studies
One of the limitations of studying depressed populations is that they display cognitive deficits such as impairment in working memory, executive dysfunction and psychomotor problems [54,55]. This makes it hard to separate the effects of antidepressants on emotional processing per se from these possible confounding factors. In addition, early effects of the drugs on patients' mood could influence the results.
Nevertheless, it is important to confirm that the findings from healthy volunteer studies are replicated in depressed populations. A number of studies have examined changes in emotional processing after long-term treatment. After chronic SSRI administration, depressed patients show reduced response in the amygdala, ventral striatum and frontal–parietal cortex to negative faces [56,57], as well as increased response in extra-striate cortex to happy faces [58]. However, because these changes were only examined after several weeks of treatment, it is possible that they were the result, rather than cause, of improvements in mood.
A number of studies have examined emotional processing in patients after short-term or acute doses. These changes occur in the absence of any significant effects on mood, and so cannot be attributed to individuals feeling happier. One study found that 7 days of citalopram treatment improved recognition of happy facial expressions compared with baseline, although the lack of a placebo control condition means that this could be the result of practice effects [27]. However, another study found that 7 days of escitalopram reduced right amygdala response to fearful faces, compared with those given a placebo [37].
After an acute dose of reboxetine, patients showed an increase in ability to recognize happy facial expressions [9]. They also showed a decrease in time to categorize words as positive, and increased memory for positive words at a later recall test (figure 1b). The effect of acute antidepressant treatment on neural activity in depressed participants remains to be explored. In particular, it would be interesting to see whether the neural correlates of the acute anxiogenic effects of SSRIs might be seen more clearly in this population than in healthy volunteers, as has been suggested [38].
(c). Studies with high neurotic volunteers
An alternative approach is to select participants from the population who score highly on a measure of neuroticism (high-Ns). Neuroticism has long been considered a risk factor for depression (e.g. [59,60]), and people with a high N score also show similar cognitive and neural biases to depressed subjects [61,62]. However, these participants typically do not display the deficits in memory or executive functioning that may be present in a clinical population, nor do they present with the confounding factor of clinical levels of depressed mood.
Studies of short-term antidepressant administration in high-Ns have supported the neurocognitive model of antidepressant action. Seven days of citalopram treatment increased recognition of positive facial expressions in a sample of high-Ns [29]. The same study showed that those in the citalopram group maintained their gaze at facial expressions longer than those in the placebo group, suggesting that the drug was reducing avoidance of emotional expressions. Interestingly, other studies have found that acute doses of citalopram reduce time fixated on faces, particularly the eye region, which appears consistent with acute anxiogenic effects of SSRIs [63].
Another study found that 7 days of citalopram increased amygdala response to fearful faces, as well as to happy and neutral faces, in high-Ns [38]. This increased amygdala response to fearful faces is in contrast to both unselected healthy populations [23] and depressed patients [37]. It may reflect the reversal of the avoidance of emotional expressions in high-N participants, but further research is needed to compare short-term effects on amygdala activation between these different populations.
Short-term antidepressant treatment in high-Ns also affects neural activation to self-referent words: 7 days' citalopram treatment resulted in decreased activation in ventromedial prefrontal cortex while categorizing negative words [64]. Again, this may reflect decreased allocation of attentional resources to negative self-referent words.
4. Converging evidence for the neurocognitive model
The above studies provide strong evidence that conventional antidepressants (mainly SSRIs and NRIs) produce early neurocognitive changes. The neurocognitive model predicts that early effects are common across effective antidepressant treatments with diverse pharmacological (or non-pharmacological) mechanisms of action. It is therefore necessary to show that other antidepressant treatments produce similar effects—and that these effects are not caused by drugs that do not act as antidepressants. And perhaps most importantly, these early changes must be associated with later improvements in depressive symptoms. The following sections examine each of these lines of evidence.
(a). Other antidepressant treatments produce similar neurocognitive changes
An increasing body of research suggests that early neurocognitive changes do occur as a result of other interventions useful in depression. The serotonin precursor l-tryptophan appears to have antidepressant effects, although it is not widely used as there is limited data on the safety of the drug [65]. However, 14 days of 3 g d−1 tryptophan induced emotional processing changes in healthy female participants similar to those of other antidepressants [25]. Participants were worse at recognizing facial expressions of disgust, and better at recognizing happy faces than those who took a placebo, and also showed reduced attentional vigilance to negative words on a dot-probe task.
Agomelatine is a novel antidepressant that acts on the melatonin system, as an agonist at M1 and M2 receptors, and on the serotonin system, as a 5-HT2C antagonist. The drug is thought to exert antidepressant effects in part via the correction of disturbances in circadian rhythms [66]. Despite these novel mechanisms, the drug appears to have similar psychological effects to the conventional antidepressants. Seven days of 25 mg d−1 agomelatine reduced recognition of sad facial expressions and increased recall of positive versus negative self-referent words [26]. It also reduced the acoustic startle response when viewing negative pictures, similar to the SSRIs [22].
In recent years, a number of non-pharmacological interventions for mood disorders have been examined. There is preliminary evidence that high-density negative ion (HDNI) treatment over several weeks could be an effective treatment for seasonal affective disorder (SAD; [67]). Compared to a sham condition, a single session of HDNI treatment increased recognition of happy facial expressions and reduced recognition of disgust, and also increased vigilance to positive words in a dot-probe task, in both healthy volunteers and patients suffering from SAD [68]. In the SAD group, treatment also increased recognition of positive words.
Transcranial direct current stimulation (tDCS) also has antidepressant effects. tDCS is a form of non-invasive brain stimulation that involves applying an electric current to the head in order to increase or decrease neuronal excitability. A recent meta-analysis found that anodal stimulation (that is, positively charged stimulation that increases neuronal excitability) of the DLPFC over the course of several days was better than sham tDCS at treating depression [69].
Acute stimulation with tDCS affects emotional processing. Active versus sham tDCS reduced reaction time to identify emotional, but not neutral faces [70]. This was true for both angry and happy faces, though the effect was stronger for the happy faces. Importantly, similar stimulation had no effect on participants' mood. A single session of tDCS over the DLPFC also reduced attentional vigilance to fearful faces [71]. Similar results have been found in depressed populations: depressed patients were slower to name the colours of negative words than positive words in an emotional Stroop task; however, a single session of tDCS abolished this effect, reducing response times to negative words [72]. Thus, participants appeared to be better able to suppress the emotional content of negative words after stimulation.
Finally, deep brain stimulation (DBS) also has effects on emotional processing. A recent study found that after one and six months of DBS for 7 patients with treatment-resistant depression, categorization of negative words as self-descriptive was reduced compared with baseline [73]. After six months of DBS, this change was strongly correlated with change in depression severity. The authors also found that event-related potential components, measured using EEG, were affected: after one month, there was a reduced P1 amplitude, corresponding to reduced attentional bias to negative words, while after six months, a reduced P3 component likely corresponded to reduction in more elaborative processing of negative stimuli.
(b). Drugs that are not antidepressants do not produce the same neurocognitive changes
It is also important to show that the production of positive biases in emotional processing is limited to drugs that actually act as antidepressants. One of the most important distinctions is that between anxiolytic and antidepressant drugs. Comorbidity rates of depression and anxiety are very high [74] and, as in the case of depression, cognitive models suggest that anxiety disorders are underpinned by negative biases in emotional processing. These biases tend to be characterized by a narrower focus on threat-related stimuli than in depression, and may particularly involve the initial orienting towards these stimuli [53,75]. Nevertheless, SSRIs, which are effective anxiolytics as well as antidepressants, reduce these biases in anxiety disorders [76]. It is therefore important to demonstrate that the antidepressant effects of SSRIs per se can account for the changes they produce in emotional processing, and that these cannot simply be attributed to the drugs' anxiolytic effects.
This can be done by comparing the action of SSRIs with that of solely anxiolytic drugs, which do not have an antidepressant effect. These show a different profile from the antidepressants, suggesting that at least some of the early cognitive changes produced by SSRIs must be specifically related to their antidepressant mechanism. Acute benzodiazepine treatment reduces ability to recognize angry and fearful faces [77,78] as well as amygdala activation to fearful faces [2]. Yet, unlike antidepressants, a dose of a benzodiazepine does not reduce processing of other negative facial expressions, such as sad faces, or increase processing of positive faces, and there is no evidence for changes in the processing of, or memory for, positive or negative self-referent words [79]. Thus, the cognitive effects of anxiolytic drugs appear to be restricted to threat processing.
It has also been possible to demonstrate that the model is directionally sensitive. Rimonabant, an inverse agonist of the cannabinoid receptor CB1, was originally marketed as an antiobesity drug, but was withdrawn after being associated with an increased incidence of mood disorders [80]. An acute dose of rimonabant was found to impair the recall of positive but not negative words [81], opposite to the effect seen in antidepressants. A short-term dose of 7 days also affected word recall, reducing the number of false recollections of positive words [82]. These studies raise the intriguing possibility that healthy volunteer models of emotional processing may be useful in detecting adverse psychiatric effects.
Moreover, neurocognitive changes following an intervention appear to differentiate between effective and non-effective treatments. Memantine, an NMDA antagonist, has been proposed as a novel antidepressant. While animal models and an open-label trial held promise, randomized controlled trials have failed to find antidepressant effects with the drug [83]. In line with the lack of clinical effects, one study found that a single dose of memantine had very limited effects on emotional processing when compared with other antidepressants [84]. The drug increased startle response and produced a non-significant trend towards a reduction in false recollections of negative words in a memory task. However, it had no effects on any other measures of emotional memory, face recognition or attentional vigilance to emotional words.
Likewise, antagonists of the neurokinin 1 (NK1) receptor have received attention as possible novel antidepressants, but results from clinical trials have shown mixed success [85,86]. Consistent with this, the effects of these drugs on emotional processing were more restricted than those typically seen with antidepressant drug treatment [87–90]. For example, the NK1 antagonist aprepitant increased ACC and amygdala activity to happy faces, but unlike other antidepressant studies had no effect on neural processing of fearful faces [87]. Similarly, the drug increased recognition of happy faces and vigilance towards emotional words, regardless of valence, but had no effect on categorization or memory of emotional words, nor on recognition of fearful or other negative faces [88].
These studies illustrate an important question that future research will need to answer: what is the extent of change in emotional processing that is needed to produce clinical effects? Both memantine and aprepitant appeared to produce some changes in emotional processing, but these clearly fell short of the changes seen in the case of citalopram or reboxetine. It will be vital to quantify the necessary changes in order to use the neurocognitive model as a tool for measuring antidepressant efficacy. However, it is clear that healthy volunteer models of emotional processing can be useful assays of efficacy, and may be more sensitive than conventional animal models.
(c). Early neurocognitive changes predict later treatment response
One of the most important findings in support of the neurocognitive model is that the early production of a more positive bias in emotional processing is actually predictive of ultimate improvement in symptoms in depressed patients.
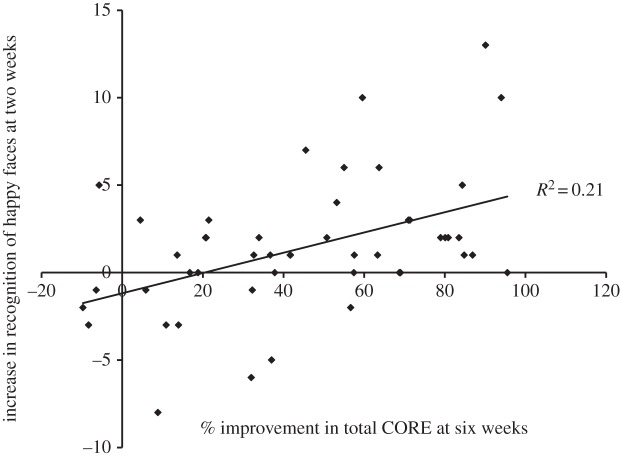
In one study, a group of patients suffering from depression were assigned to either 20 mg d−1 citalopram or 4 mg d−1 reboxetine for six weeks [28]. Both antidepressants increased recognition of happy faces at two weeks compared with baseline, as well as recognition of disgust and surprise. But importantly, the increase in recognition of happy faces at two weeks was significantly positively associated with improvement in clinical outcomes (measured by the Clinical Outcomes in Routine Evaluation; CORE) at six weeks (R2 = 0.21; figure 2).
Figure 2.
Change in recognition of happy faces from baseline to two weeks of citalopram/reboxetine treatment against percentage improvement in clinical outcome (CORE score) from baseline to six weeks of treatment. Adapted from with permission from Tranter et al. [28] (Copyright © 2009 Elsevier).
This finding is important, as it demonstrates that early changes towards more positive emotional processing are directly associated with later symptom improvement—a fundamental assumption of the neurocognitive model. On the other hand, given evidence that improvement in mood occurs during the first weeks of antidepressant treatment [10], changes in emotional processing after two weeks could be the product of symptom change. Research has yet to examine whether similar changes in emotional processing after an acute dose predict clinical response. However, two recent papers have examined changes in emotional processing at an earlier time point during short-term administration.
In one study, 27 older patients suffering from depression were given open-label citalopram treatment for eight weeks [27]. Before beginning treatment and after 7 days of treatment, participants were presented with neutral faces and happy faces of different intensities and had to indicate whether or not a face was happy. In line with the neurocognitive model, recognition of happy faces improved between baseline and day 7, and the extent of this improvement was a predictor of improvement on depression scores on the Hamilton Depression Rating Scale at eight weeks.
Early neural changes can also predict later treatment response. A recent study found that the change from baseline in neural activity in a network of brain regions following 7 days of escitalopram treatment can be used to differentiate responders and non-responders to the treatment six weeks later. Specifically, responders showed more of a reduction than non-responders in BOLD activity to fearful versus happy faces in a network that included the ACC, insula, thalamus and amygdala [91].
5. Future directions
The neurocognitive model has a number of implications for our approach to the treatment of depression. In particular, it may be useful in understanding why participants do or do not respond to a particular treatment, and could be a valuable tool for drug discovery. However, there are also challenges to the model that must be addressed in future research.
(a). Explaining individual differences in treatment response
Given that more than half of patients suffering from depression may not benefit from the first treatment they are prescribed [92], it is important to understand the factors determining whether people respond. The neurocognitive model provides a unique tool with which to examine these individual differences. If, as the above studies suggest, early changes in emotional processing predict the extent to which a patient will respond to treatment, then individual factors that influence these early changes could help to explain why people respond differently to antidepressant treatment. These individual factors could take a range of forms, but one category that has received recent attention is genetic polymorphisms.
For example, the polymorphism 5-HTTLPR occurs in the gene SLC6A4 that codes for the serotonin transporter, and there is some evidence that carriers of the long (l) allele are more likely to respond to SSRI treatment than carriers of the short (s) allele [93]. In a cohort of healthy female participants, those with more l alleles showed more of an increase in left amygdala response after a single dose of escitalopram when viewing positive or neutral pictures, and more of a decrease in left amygdala response when viewing negative pictures [94]. The effects of the polymorphism on response to escitalopram treatment may therefore be mediated through its effects on early neuropsychological changes.
The above study demonstrates that a gene known to influence response to SSRI treatment might act via its effects on emotional processing. But equally, the neurocognitive model could be used to identify individual factors that were previously unknown to affect clinical response to antidepressant treatment. The ADRA2B gene encodes the α2b-adrenergic receptor, and a deletion variant of this gene has been associated with enhanced memory for emotional information [95]. A single 4 mg dose of reboxetine was found to reduce recall of negative pictures in healthy male volunteers, but only for those who did not have the deletion variant of this gene [96]. ADRA2B deletions may therefore decrease antidepressant efficacy, by making patients resistant to early neuropsychological changes, and further investigation into this is warranted.
In this way, the cognitive neuropsychological model could be used to build up a picture of individual factors—genetic or otherwise—which might influence response to treatment. Because any differences will be seen at an early stage after commencing treatment and will be detectable in healthy volunteer populations, the model allows for relatively fast identification of these factors that does not rely on the complexities of testing a patient population or several weeks or months of drug administration.
(b). Drug development
The neurocognitive model may also be a useful human assay of novel, putative treatments. A large proportion of patients fail to respond to current antidepressant drugs, even after switching treatments [97]. Moreover, drugs which show promise in animal studies often fail to prove effective in patient samples. If the production of early changes in emotional processing is a marker of antidepressant efficacy, then investigating the presence of these changes in novel substances could allow quick identification of a potentially useful drug.
A series of studies has investigated the antidepressant potential of Erythropoietin (Epo). Epo plays a key role in regulating red blood cells, but also has neurotrophic effects [98]. Seven days after a single intravenous dose of Epo, healthy volunteers showed reduced recognition of fearful faces, and reduced BOLD response in the fusiform gyrus and visual–parietal areas in response to fearful faces [99]. Another study found that 3 days after infusion, Epo increased activation for both happy and fearful faces in the amygdala [100], while a patient study found decreased activation to negative scenes in the hippocampus and ventral PFC, as well as increased activation to positive scenes in the latter structure [101].
These studies suggest that Epo could be a useful antidepressant, as it appears to affect emotional processing in a similar way to other antidepressants. Recently, the first phase II trial of the drug has shown that weekly infusions of Epo does indeed improve scores on a number of measures of depression as well as cognitive function up to 14 weeks [102].
(c). Future challenges
This review has highlighted the strong evidence in support of the neurocognitive model of antidepressant treatment. However, there are still some questions that future research will need to address.
One issue emerging from recent research is that certain drugs produce very fast-acting antidepressant effects. In particular, the NMDA antagonist ketamine can produce an antidepressant effect after a single dose [103,104]. These fast clinical actions are difficult to reconcile with the key hypothesis of the model that changes in emotional processing need time and interaction with the social environment to generate an eventual improvement in mood. Nevertheless, neurocognitive changes are seen both acutely and 24 h after a single infusion of ketamine [105,106], and one possible solution comes from suggestions that ketamine could produce greater shifts in emotional bias than the traditional antidepressants, which could result in faster relearning [53]. Another potential answer comes from a recent rodent study demonstrating that ketamine reduced the retrieval of negative affective memories, suggesting that the psychological actions of the drug may not need any relearning or further exposure to social and emotional cues [107]. Future research is needed to address whether a similar process applies in human models and whether it predicts clinical response.
It will also be important to establish the underlying mechanism of the early cognitive and neural changes produced by antidepressant treatment. Cellular and molecular accounts of antidepressant action have tended to focus on relatively slow-acting effects; however, it is clear from the neurocognitive model that more immediate effects on brain chemistry or structure must also be important. There is some evidence that short-term antidepressant treatment may increase expression of neurotrophins and produce synaptic remodelling in the hippocampus [108,109], suggesting one possible molecular locus for early behavioural and neuroimaging changes. However, it remains to be seen whether these or any other early molecular effects do in fact underlie changes in emotional processing.
Finally, another challenge is whether we can harness early change in emotional processing as a measure of response that is useful for clinical decision-making. A marker of response after a single dose or after 7 days' treatment would be faster than standard clinical practice and may speed up finding the right treatment for a particular patient. Whether making this information available to prescribers improves speed to remission will need further empirical testing.
6. Conclusion
Although the role of cognitive biases in producing and maintaining depression has long been acknowledged, it is only in the past decade that we have really begun to recognize that the normalization of these biases is a key action of antidepressant treatment. Over this time, a large body of evidence has built up that supports this cognitive neuropsychological account. But perhaps the most exciting ideas to come out of the literature are the numerous potential practical applications afforded by an early neurocognitive marker of antidepressant efficacy. In a time when depression is a major burden to health worldwide, the cognitive neuropsychological model may help not just to refine treatment strategies for patients, but also provide a novel paradigm with which to develop new treatments. The challenge for the field is to now translate encouraging findings from the laboratory into the real world.
Authors' contributions
M.B.W. provided the first draft of the article. C.J.H. and A.P. provided additional content and all contributors edited and revised the article. All contributors approved the final version.
Competing interests
C.J.H. has received consultancy income from Servier, Lundbeck and P1vital. She is a director of Oxford Psychologists Ltd and holds shares in the company. M.B.W. and A.P. have no competing interests.
Funding
M.B.W. is funded by the University of Oxford Clarendon Fund.
References
- 1.Hariri AR, Mattay VS, Tessitore A, Fera F, Smith WG, Weinberger DR. 2002. Dextroamphetamine modulates the response of the human amygdala. Neuropsychopharmacology 27, 1036–1040. ( 10.1016/s0893-133x(02)00373-1) [DOI] [PubMed] [Google Scholar]
- 2.Del-Ben CM, Ferreira CA, Sanchez TA, Alves-Neto WC, Guapo VG, de Araujo DB, Graeff FG. 2012. Effects of diazepam on BOLD activation during the processing of aversive faces. J. Psychopharmacol. 26, 443–451. ( 10.1177/0269881110389092) [DOI] [PubMed] [Google Scholar]
- 3.Frazer A, Benmansour S. 2002. Delayed pharmacological effects of antidepressants. Mol. Psychiatry 7(Suppl. 1), S23–S28. ( 10.1038/sj.mp.4001015) [DOI] [PubMed] [Google Scholar]
- 4.Stahl SM. 1998. Mechanism of action of serotonin selective reuptake inhibitors. J. Affect. Disord. 51, 215–235. ( 10.1016/S0165-0327(98)00221-3) [DOI] [PubMed] [Google Scholar]
- 5.Perera TD, et al. 2011. Necessity of hippocampal neurogenesis for the therapeutic action of antidepressants in adult nonhuman primates. PLoS ONE 6, e17600 ( 10.1371/journal.pone.0017600) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becker S, Wojtowicz JM. 2007. A model of hippocampal neurogenesis in memory and mood disorders. Trends Cogn. Sci. 11, 70–76. ( 10.1016/j.tics.2006.10.013) [DOI] [PubMed] [Google Scholar]
- 7.Harmer CJ, Bhagwagar Z, Perrett DI, Vollm BA, Cowen PJ, Goodwin GM. 2003. Acute SSRI administration affects the processing of social cues in healthy volunteers. Neuropsychopharmacology 28, 148–152. ( 10.1038/sj.npp.1300004) [DOI] [PubMed] [Google Scholar]
- 8.Harmer CJ, Hill SA, Taylor MJ, Cowen PJ, Goodwin GM. 2003. Toward a neuropsychological theory of antidepressant drug action: increase in positive emotional bias after potentiation of norepinephrine activity. Am. J. Psychiatry 160, 990–992. ( 10.1176/appi.ajp.160.5.990) [DOI] [PubMed] [Google Scholar]
- 9.Harmer CJ, O'Sullivan U, Favaron E, Massey-Chase R, Ayres R, Reinecke A, Goodwin GM, Cowen PJ. 2009. Effect of acute antidepressant administration on negative affective bias in depressed patients. Am. J. Psychiatry 166, 1178–1184. ( 10.1176/appi.ajp.2009.09020149) [DOI] [PubMed] [Google Scholar]
- 10.Taylor MJ, Freemantle N, Geddes JR, Bhagwagar Z. 2006. Early onset of selective serotonin reuptake inhibitor antidepressant action: systematic review and meta-analysis. Arch. Gen. Psychiatry 63, 1217–1223. ( 10.1001/archpsyc.63.11.1217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roiser JP, Elliott R, Sahakian BJ. 2012. Cognitive mechanisms of treatment in depression. Neuropsychopharmacology 37, 117–136. ( 10.1038/npp.2011.183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bradley BP, Mogg K, Williams R. 1995. Implicit and explicit memory for emotion-congruent information in clinical depression and anxiety. Behav. Res. Ther. 33, 755–770. ( 10.1016/0005-7967(95)00029-W) [DOI] [PubMed] [Google Scholar]
- 13.Bourke C, Douglas K, Porter R. 2010. Processing of facial emotion expression in major depression: a review. Aust. N. Z. J. Psychiatry 44, 681–696. ( 10.3109/00048674.2010.496359) [DOI] [PubMed] [Google Scholar]
- 14.Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. 2012. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of baseline activation and neural response data. Am. J. Psychiatry 169, 693–703. ( 10.1176/appi.ajp.2012.11071105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Disner SG, Beevers CG, Haigh EAP, Beck AT. 2011. Neural mechanisms of the cognitive model of depression. Nat. Rev. Neurosci. 12, 467–477. ( 10.1038/nrn3027) [DOI] [PubMed] [Google Scholar]
- 16.Gonul AS, Kula M, Bilgin AG, Tutus A, Oguz A. 2004. The regional cerebral blood flow changes in major depressive disorder with and without psychotic features. Prog. Neuropsychopharmacol. Biol. Psychiatry 28, 1015–1021. ( 10.1016/j.pnpbp.2004.05.036) [DOI] [PubMed] [Google Scholar]
- 17.Suslow T, et al. 2010. Automatic mood-congruent amygdala responses to masked facial expressions in major depression. Biol. Psychiatry 67, 155–160. ( 10.1016/j.biopsych.2009.07.023) [DOI] [PubMed] [Google Scholar]
- 18.Teasdale JD. 1988. Cognitive vulnerability to persistent depression. Cogn. Emotion 2, 247–274. ( 10.1080/02699938808410927) [DOI] [Google Scholar]
- 19.Bouhuys AL, Geerts E, Gordijn MC. 1999. Depressed patients’ perceptions of facial emotions in depressed and remitted states are associated with relapse: a longitudinal study. J. Nerv. Ment. Dis. 187, 595–602. ( 10.1097/00005053-199910000-00002) [DOI] [PubMed] [Google Scholar]
- 20.Browning M, Holmes EA, Charles M, Cowen PJ, Harmer CJ. 2012. Using attentional bias modification as a cognitive vaccine against depression. Biol. Psychiatry 72, 572–579. ( 10.1016/j.biopsych.2012.04.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacLeod C, Rutherford E, Campbell L, Ebsworthy G, Holker L. 2002. Selective attention and emotional vulnerability: assessing the causal basis of their association through the experimental manipulation of attentional bias. J. Abnorm. Psychol. 111, 107–123. ( 10.1037/0021-843X.111.1.107) [DOI] [PubMed] [Google Scholar]
- 22.Harmer CJ, Shelley NC, Cowen PJ, Goodwin GM. 2004. Increased positive versus negative affective perception and memory in healthy volunteers following selective serotonin and norepinephrine reuptake inhibition. Am. J. Psychiatry 161, 1256–1263. ( 10.1176/appi.ajp.161.7.1256) [DOI] [PubMed] [Google Scholar]
- 23.Harmer CJ, Mackay CE, Reid CB, Cowen PJ, Goodwin GM. 2006. Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biol. Psychiatry 59, 816–820. ( 10.1016/j.biopsych.2005.10.015) [DOI] [PubMed] [Google Scholar]
- 24.Murphy SE, Yiend J, Lester KJ, Cowen PJ, Harmer CJ. 2009. Short-term serotonergic but not noradrenergic antidepressant administration reduces attentional vigilance to threat in healthy volunteers. Int. J. Neuropsychopharmacol. 12, 169–179. ( 10.1017/s1461145708009164) [DOI] [PubMed] [Google Scholar]
- 25.Murphy SE, Longhitano C, Ayres RE, Cowen PJ, Harmer CJ. 2006. Tryptophan supplementation induces a positive bias in the processing of emotional material in healthy female volunteers. Psychopharmacology 187, 121–130. ( 10.1007/s00213-006-0401-8) [DOI] [PubMed] [Google Scholar]
- 26.Harmer CJ, de Bodinat C, Dawson GR, Dourish CT, Waldenmaier L, Adams S, Cowen PJ, Goodwin GM. 2011. Agomelatine facilitates positive versus negative affective processing in healthy volunteer models. J. Psychopharmacol. 25, 1159–1167. ( 10.1177/0269881110376689) [DOI] [PubMed] [Google Scholar]
- 27.Shiroma PR, Thuras P, Johns B, Lim KO. 2014. Emotion recognition processing as early predictor of response to 8-week citalopram treatment in late-life depression. Int. J. Geriatr. Psychiatry. 29, 1132–1139. ( 10.1002/gps.4104) [DOI] [PubMed] [Google Scholar]
- 28.Tranter R, Bell D, Gutting P, Harmer C, Healy D, Anderson IM. 2009. The effect of serotonergic and noradrenergic antidepressants on face emotion processing in depressed patients. J. Affect. Disord. 118, 87–93. ( 10.1016/j.jad.2009.01.028) [DOI] [PubMed] [Google Scholar]
- 29.Di Simplicio M, Doallo S, Costoloni G, Rohenkohl G, Nobre AC, Harmer CJ. 2014. ‘Can you look me in the face?’ Short-term SSRI administration reverts avoidant ocular face exploration in subjects at risk for psychopathology. Neuropsychopharmacology 39, 3059–3066. ( 10.1038/npp.2014.159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy SE, Norbury R, O'Sullivan U, Cowen PJ, Harmer CJ. 2009. Effect of a single dose of citalopram on amygdala response to emotional faces. Br. J. Psychiatry 194, 535–540. ( 10.1192/bjp.bp.108.056093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Browning M, Reid C, Cowen PJ, Goodwin GM, Harmer CJ. 2007. A single dose of citalopram increases fear recognition in healthy subjects. J. Psychopharmacol. 21, 684–690. ( 10.1177/0269881106074062) [DOI] [PubMed] [Google Scholar]
- 32.Miskowiak K, Papadatou-Pastou M, Cowen PJ, Goodwin GM, Norbury R, Harmer CJ. 2007. Single dose antidepressant administration modulates the neural processing of self-referent personality trait words. NeuroImage 37, 904–911. ( 10.1016/j.neuroimage.2007.05.036) [DOI] [PubMed] [Google Scholar]
- 33.Harmer CJ, Heinzen J, O'Sullivan U, Ayres RA, Cowen PJ. 2008. Dissociable effects of acute antidepressant drug administration on subjective and emotional processing measures in healthy volunteers. Psychopharmacology 199, 495–502. ( 10.1007/s00213-007-1058-7) [DOI] [PubMed] [Google Scholar]
- 34.Arnone D, Horder J, Cowen PJ, Harmer CJ. 2009. Early effects of mirtazapine on emotional processing. Psychopharmacology 203, 685–691. ( 10.1007/s00213-008-1410-6) [DOI] [PubMed] [Google Scholar]
- 35.Norbury R, Taylor MJ, Selvaraj S, Murphy SE, Harmer CJ, Cowen PJ. 2009. Short-term antidepressant treatment modulates amygdala response to happy faces. Psychopharmacology 206, 197–204. ( 10.1007/s00213-009-1597-1) [DOI] [PubMed] [Google Scholar]
- 36.Norbury R, Mackay CE, Cowen PJ, Goodwin GM, Harmer CJ. 2007. Short-term antidepressant treatment and facial processing. Functional magnetic resonance imaging study. Br. J. Psychiatry 190, 531–532. ( 10.1192/bjp.bp.106.031393) [DOI] [PubMed] [Google Scholar]
- 37.Godlewska BR, Norbury R, Selvaraj S, Cowen PJ, Harmer CJ. 2012. Short-term SSRI treatment normalises amygdala hyperactivity in depressed patients. Psychol. Med. 42, 2609–2617. ( 10.1017/s0033291712000591) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di Simplicio M, Norbury R, Reinecke A, Harmer CJ. 2014. Paradoxical effects of short-term antidepressant treatment in fMRI emotional processing models in volunteers with high neuroticism. Psychol. Med. 44, 241–252. ( 10.1017/S0033291713000731) [DOI] [PubMed] [Google Scholar]
- 39.Anderson IM, Juhasz G, Thomas E, Downey D, McKie S, Deakin JF, Elliott R. 2011. The effect of acute citalopram on face emotion processing in remitted depression: a pharmacoMRI study. Eur. Neuropsychopharmacol. 21, 140–148. ( 10.1016/j.euroneuro.2010.06.008) [DOI] [PubMed] [Google Scholar]
- 40.Grady CL, Siebner HR, Hornboll B, Macoveanu J, Paulson OB, Knudsen GM. 2013. Acute pharmacologically induced shifts in serotonin availability abolish emotion-selective responses to negative face emotions in distinct brain networks. Eur. Neuropsychopharmacol. 23, 368–378. ( 10.1016/j.euroneuro.2012.06.003) [DOI] [PubMed] [Google Scholar]
- 41.Anderson IM, Del-Ben CM, McKie S, Richardson P, Williams SR, Elliott R, Deakin JF. 2007. Citalopram modulation of neuronal responses to aversive face emotions: a functional MRI study. Neuroreport 18, 1351–1355. ( 10.1097/WNR.0b013e3282742115) [DOI] [PubMed] [Google Scholar]
- 42.Del-Ben CM, Deakin JF, McKie S, Delvai NA, Williams SR, Elliott R, Dolan M, Anderson IM. 2005. The effect of citalopram pretreatment on neuronal responses to neuropsychological tasks in normal volunteers: an fMRI study. Neuropsychopharmacology 30, 1724–1734. ( 10.1038/sj.npp.1300728) [DOI] [PubMed] [Google Scholar]
- 43.Bigos KL, Pollock BG, Aizenstein HJ, Fisher PM, Bies RR, Hariri AR. 2008. Acute 5-HT reuptake blockade potentiates human amygdala reactivity. Neuropsychopharmacology 33, 3221–3225. ( 10.1038/npp.2008.52) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rawlings NB, Norbury R, Cowen PJ, Harmer CJ. 2010. A single dose of mirtazapine modulates neural responses to emotional faces in healthy people. Psychopharmacology 212, 625–634. ( 10.1007/s00213-010-1983-8) [DOI] [PubMed] [Google Scholar]
- 45.Harmer CJ. 2012. Emotional processing and antidepressant action. Curr. Top. Behav. Neurosci. 14, 209–222. ( 10.1007/7854_2012_210) [DOI] [PubMed] [Google Scholar]
- 46.Ma Y. 2014. Neuropsychological mechanism underlying antidepressant effect: a systematic meta-analysis. Mol. Psychiatry. 20, 311–319. ( 10.1038/mp.2014.24) [DOI] [PubMed] [Google Scholar]
- 47.Norbury R, Mackay CE, Cowen PJ, Goodwin GM, Harmer CJ. 2008. The effects of reboxetine on emotional processing in healthy volunteers: an fMRI study. Mol. Psychiatry 13, 1011–1020. ( 10.1038/sj.mp.4002091) [DOI] [PubMed] [Google Scholar]
- 48.Eyding D, Lelgemann M, Grouven U, Härter M, Kromp M, Kaiser T, Kerekes MF, Gerken M, Wieseler B. 2010. Reboxetine for acute treatment of major depression: systematic review and meta-analysis of published and unpublished placebo and selective serotonin reuptake inhibitor controlled trials. BMJ 341 ( 10.1136/bmj.c4737) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wiles NJ, Fischer K, Cowen P, Nutt D, Peters TJ, Lewis G, White IR. 2014. Allowing for non-adherence to treatment in a randomized controlled trial of two antidepressants (citalopram versus reboxetine): an example from the GENPOD trial. Psychol. Med. 44, 2855–2866. ( 10.1017/S0033291714000221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Craske MG, Wolitzky-Taylor KB, Mineka S, Zinbarg R, Waters AM, Vrshek-Schallhorn S, Epstein A, Naliboff B, Ornitz E. 2012. Elevated responding to safe conditions as a specific risk factor for anxiety versus depressive disorders: evidence from a longitudinal investigation. J. Abnorm. Psychol. 121, 315–324. ( 10.1037/a0025738) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mogg K, Bradley B. 2005. Attentional bias in generalized anxiety disorder versus depressive disorder. Cogn. Ther. Res. 29, 29–45. ( 10.1007/s10608-005-1646-y) [DOI] [Google Scholar]
- 52.Griebel G, Moreau JL, Jenck F, Misslin R, Martin JR. 1994. Acute and chronic treatment with 5-HT reuptake inhibitors differentially modulate emotional responses in anxiety models in rodents. Psychopharmacology 113, 463–470. ( 10.1007/BF02245224) [DOI] [PubMed] [Google Scholar]
- 53.Pringle A, McCabe C, Cowen PJ, Harmer CJ. 2013. Antidepressant treatment and emotional processing: can we dissociate the roles of serotonin and noradrenaline? J. Psychopharmacol. 27, 719–731. ( 10.1177/0269881112474523) [DOI] [PubMed] [Google Scholar]
- 54.Golinkoff M, Sweeney JA. 1989. Cognitive impairments in depression. J. Affect. Disord. 17, 105–112. ( 10.1016/0165-0327(89)90032-3) [DOI] [PubMed] [Google Scholar]
- 55.Castaneda AE, Tuulio-Henriksson A, Marttunen M, Suvisaari J, Lonnqvist J. 2008. A review on cognitive impairments in depressive and anxiety disorders with a focus on young adults. J. Affect. Disord. 106, 1–27. ( 10.1016/j.jad.2007.06.006) [DOI] [PubMed] [Google Scholar]
- 56.Fu CH, et al. 2004. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Arch. Gen. Psychiatry 61, 877–889. ( 10.1001/archpsyc.61.9.877) [DOI] [PubMed] [Google Scholar]
- 57.Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. 2001. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol. Psychiatry 50, 651–658. ( 10.1016/S0006-3223(01)01263-X) [DOI] [PubMed] [Google Scholar]
- 58.Fu CH, et al. 2007. Neural responses to happy facial expressions in major depression following antidepressant treatment. Am. J. Psychiatry 164, 599–607. ( 10.1176/appi.ajp.164.4.599) [DOI] [PubMed] [Google Scholar]
- 59.Hirschfeld RM, Klerman GL, Lavori P, Keller MB, Griffith P, Coryell W. 1989. Premorbid personality assessments of first onset of major depression. Arch. Gen. Psychiatry 46, 345–350. ( 10.1001/archpsyc.1989.01810040051008) [DOI] [PubMed] [Google Scholar]
- 60.Saklofske DH, Kelly IW, Janzen BL. 1995. Neuroticism, depression, and depression proneness. Person. Individual Diff. 18, 27–31. ( 10.1016/0191-8869(94)00128-F) [DOI] [Google Scholar]
- 61.Chan SW, Goodwin GM, Harmer CJ. 2007. Highly neurotic never-depressed students have negative biases in information processing. Psychol. Med. 37, 1281–1291. ( 10.1017/S0033291707000669) [DOI] [PubMed] [Google Scholar]
- 62.Chan SW, Harmer CJ, Goodwin GM, Norbury R. 2008. Risk for depression is associated with neural biases in emotional categorisation. Neuropsychologia 46, 2896–2903. ( 10.1016/j.neuropsychologia.2008.05.030) [DOI] [PubMed] [Google Scholar]
- 63.Jonassen R, Chelnokova O, Harmer C, Leknes S, Landro NI. 2014. A single dose of antidepressant alters eye-gaze patterns across face stimuli in healthy women. Psychopharmacology. 232, 953–958. ( 10.1007/s00213-014-3729-5) [DOI] [PubMed] [Google Scholar]
- 64.Di Simplicio M, Norbury R, Harmer CJ. 2012. Short-term antidepressant administration reduces negative self-referential processing in the medial prefrontal cortex in subjects at risk for depression. Mol. Psychiatry 17, 503–510. ( 10.1038/mp.2011.16) [DOI] [PubMed] [Google Scholar]
- 65.Shaw K, Turner J, Del Mar C. 2002. Tryptophan and 5-hydroxytryptophan for depression. Cochrane Database Syst. Rev. 1, Cd003198 ( 10.1002/14651858.cd003198) [DOI] [PubMed] [Google Scholar]
- 66.Quera Salva MA, Vanier B, Laredo J, Hartley S, Chapotot F, Moulin C, Lofaso F, Guilleminault C. 2007. Major depressive disorder, sleep EEG and agomelatine: an open-label study. Int. J. Neuropsychopharmacol. 10, 691–696. ( 10.1017/s1461145707007754) [DOI] [PubMed] [Google Scholar]
- 67.Terman M, Terman JS. 2006. Controlled trial of naturalistic dawn simulation and negative air ionization for seasonal affective disorder. Am. J. Psychiatry 163, 2126–2133. ( 10.1176/appi.ajp.163.12.2126) [DOI] [PubMed] [Google Scholar]
- 68.Harmer CJ, Charles M, McTavish S, Favaron E, Cowen PJ. 2012. Negative ion treatment increases positive emotional processing in seasonal affective disorder. Psychol. Med. 42, 1605–1612. ( 10.1017/S0033291711002820) [DOI] [PubMed] [Google Scholar]
- 69.Shiozawa P, Fregni F, Bensenor IM, Lotufo PA, Berlim MT, Daskalakis JZ, Cordeiro Q, Brunoni AR. 2014. Transcranial direct current stimulation for major depression: an updated systematic review and meta-analysis. Int. J. Neuropsychopharmacol. 17, 1443–1452. ( 10.1017/s1461145714000418) [DOI] [PubMed] [Google Scholar]
- 70.Nitsche MA, Koschack J, Pohlers H, Hullemann S, Paulus W, Happe S. 2012. Effects of frontal transcranial direct current stimulation on emotional state and processing in healthy humans. Front. Psychiatry 3, 58 ( 10.3389/fpsyt.2012.00058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ironside M, O'Shea J, Cowen PJ, Harmer CJ. In press. Frontal cortex stimulation reduces vigilance to threat: implications for the treatment of depression and anxiety. Biol. Psych. ( 10.1016/j.biopsych.2015.06.012) [DOI] [PubMed] [Google Scholar]
- 72.Brunoni AR, Zanao TA, Vanderhasselt MA, Valiengo L, de Oliveira JF, Boggio PS, Lotufo PA, Bensenor IM, Fregni F. 2014. Enhancement of affective processing induced by bifrontal transcranial direct current stimulation in patients with major depression. Neuromodulation 17, 138–142. ( 10.1111/ner.12080) [DOI] [PubMed] [Google Scholar]
- 73.Hilimire MR, Mayberg HS, Holtzheimer PE, Broadway JM, Parks NA, DeVylder JE, Corballis PM. 2015. Effects of subcallosal cingulate deep brain stimulation on negative self-bias in patients with treatment-resistant depression. Brain Stimul. 8, 185–191. ( 10.1016/j.brs.2014.11.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lamers F, et al. 2011. Comorbidity patterns of anxiety and depressive disorders in a large cohort study: the Netherlands Study of Depression and Anxiety (NESDA). J. Clin. Psychiatry 72, 341–348. ( 10.4088/JCP.10m06176blu) [DOI] [PubMed] [Google Scholar]
- 75.Mogg K, Bradley BP. 2002. Selective orienting of attention to masked threat faces in social anxiety. Behav. Res. Ther. 40, 1403–1414. ( 10.1016/S0005-7967(02)00017-7) [DOI] [PubMed] [Google Scholar]
- 76.Mogg K, Baldwin DS, Brodrick P, Bradley BP. 2004. Effect of short-term SSRI treatment on cognitive bias in generalised anxiety disorder. Psychopharmacology 176, 466–470. ( 10.1007/s00213-004-1902-y) [DOI] [PubMed] [Google Scholar]
- 77.Blair RJ, Curran HV. 1999. Selective impairment in the recognition of anger induced by diazepam. Psychopharmacology 147, 335–338. ( 10.1007/s002130051177) [DOI] [PubMed] [Google Scholar]
- 78.Zangara A, Blair RJ, Curran HV. 2002. A comparison of the effects of a beta-adrenergic blocker and a benzodiazepine upon the recognition of human facial expressions. Psychopharmacology 163, 36–41. ( 10.1007/s00213-002-1120-4) [DOI] [PubMed] [Google Scholar]
- 79.Murphy SE, Downham C, Cowen PJ, Harmer CJ. 2008. Direct effects of diazepam on emotional processing in healthy volunteers. Psychopharmacology 199, 503–513. ( 10.1007/s00213-008-1082-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. 2007. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet 370, 1706–1713. ( 10.1016/s0140-6736(07)61721-8) [DOI] [PubMed] [Google Scholar]
- 81.Horder J, Cowen PJ, Di Simplicio M, Browning M, Harmer CJ. 2009. Acute administration of the cannabinoid CB1 antagonist rimonabant impairs positive affective memory in healthy volunteers. Psychopharmacology 205, 85–91. ( 10.1007/s00213-009-1517-4) [DOI] [PubMed] [Google Scholar]
- 82.Horder J, Browning M, Di Simplicio M, Cowen PJ, Harmer CJ. 2012. Effects of 7 days of treatment with the cannabinoid type 1 receptor antagonist, rimonabant, on emotional processing. J. Psychopharmacol. 26, 125–132. ( 10.1177/0269881111400649) [DOI] [PubMed] [Google Scholar]
- 83.Zarate CA, Jr, Singh JB, Quiroz JA, De Jesus G, Denicoff KK, Luckenbaugh DA, Manji HK, Charney DS. 2006. A double-blind, placebo-controlled study of memantine in the treatment of major depression. Am. J. Psychiatry 163, 153–155. ( 10.1176/appi.ajp.163.1.153) [DOI] [PubMed] [Google Scholar]
- 84.Pringle A, Parsons E, Cowen LG, McTavish SF, Cowen PJ, Harmer CJ. 2012. Using an experimental medicine model to understand the antidepressant potential of the N-methyl-d-aspartic acid (NMDA) receptor antagonist memantine. J. Psychopharmacol. 26, 1417–1423. ( 10.1177/0269881112446535) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Keller M, et al. 2006. Lack of efficacy of the substance P (Neurokinin1 receptor) antagonist aprepitant in the treatment of major depressive disorder. Biol. Psychiatry 59, 216–223. ( 10.1016/j.biopsych.2005.07.013) [DOI] [PubMed] [Google Scholar]
- 86.Kramer MS, et al. 2004. Demonstration of the efficacy and safety of a novel substance P (NK1) receptor antagonist in major depression. Neuropsychopharmacology 29, 385–392. ( 10.1038/sj.npp.1300260) [DOI] [PubMed] [Google Scholar]
- 87.McCabe C, Cowen PJ, Harmer CJ. 2009. NK1 receptor antagonism and the neural processing of emotional information in healthy volunteers. Int. J. Neuropsychopharmacol. 12, 1261–1274. ( 10.1017/s1461145709990150) [DOI] [PubMed] [Google Scholar]
- 88.Chandra P, Hafizi S, Massey-Chase RM, Goodwin GM, Cowen PJ, Harmer CJ. 2010. NK1 receptor antagonism and emotional processing in healthy volunteers. J. Psychopharmacol. 24, 481–487. ( 10.1177/0269881109103101) [DOI] [PubMed] [Google Scholar]
- 89.Pringle A, McTavish SF, Williams C, Smith R, Cowen PJ, Harmer CJ. 2011. Short-term NK1 receptor antagonism and emotional processing in healthy volunteers. Psychopharmacology 215, 239–246. ( 10.1007/s00213-010-2133-z) [DOI] [PubMed] [Google Scholar]
- 90.Harmer CJ, et al. 2013. Combined NK1 antagonism and serotonin reuptake inhibition: effects on emotional processing in humans. J. Psychopharmacol. 27, 435–443. ( 10.1177/0269881112472558) [DOI] [PubMed] [Google Scholar]
- 91.Godlewska BR, Browning M, Norbury R, Cowen PJ, Harmer CJ. 2014. Early changes in neural response to emotional stimuli predict clinical response to SSRI treatment in depression. J. Psychopharmacol. (Suppl.) 28, A45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Trivedi MH, et al. 2006. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am. J. Psychiatry 163, 28–40. ( 10.1176/appi.ajp.163.1.28) [DOI] [PubMed] [Google Scholar]
- 93.Porcelli S, Fabbri C, Serretti A. 2012. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with antidepressant efficacy. Eur. Neuropsychopharmacol. 22, 239–258. ( 10.1016/j.euroneuro.2011.10.003) [DOI] [PubMed] [Google Scholar]
- 94.Outhred T, Das P, Dobson-Stone C, Felmingham KL, Bryant RA, Nathan PJ, Malhi GS, Kemp AH. 2014. The impact of 5-HTTLPR on acute serotonin transporter blockade by escitalopram on emotion processing: preliminary findings from a randomised, crossover fMRI study. Aust. N. Z. J. Psychiatry 48, 1115–1125. ( 10.1177/0004867414533837) [DOI] [PubMed] [Google Scholar]
- 95.de Quervain DJF, Kolassa I-T, Ertl V, Onyut PL, Neuner F, Elbert T, Papassotiropoulos A. 2007. A deletion variant of the α2b-adrenoceptor is related to emotional memory in Europeans and Africans. Nat. Neurosci. 10, 1137–1139. ( 10.1038/nn1945) [DOI] [PubMed] [Google Scholar]
- 96.Gibbs AA, Bautista CE, Mowlem FD, Naudts KH, Duka T. 2013. α2B adrenoceptor genotype moderates effect of reboxetine on negative emotional memory bias in healthy volunteers. J. Neurosci. 33, 17 023–17 028. ( 10.1523/jneurosci.2124-13.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rush AJ, et al. 2006. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am. J. Psychiatry 163, 1905–1917. ( 10.1176/appi.ajp.163.11.1905) [DOI] [PubMed] [Google Scholar]
- 98.Alural B, Duran GA, Tufekci KU, Allmer J, Onkal Z, Tunali D, Genc K, Genc S. 2014. EPO mediates neurotrophic, neuroprotective, anti-oxidant, and anti-apoptotic effects via downregulation of mir-451 and miR-885–5p in SH-SY5Y neuron-like cells. Front. Immunol. 5, 475 ( 10.3389/fimmu.2014.00475) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Miskowiak K, O'Sullivan U, Harmer CJ. 2007. Erythropoietin reduces neural and cognitive processing of fear in human models of antidepressant drug action. Biol. Psychiatry 62, 1244–1250. ( 10.1016/j.biopsych.2007.01.011) [DOI] [PubMed] [Google Scholar]
- 100.Miskowiak K, Inkster B, Selvaraj S, Wise R, Goodwin GM, Harmer CJ. 2008. Erythropoietin improves mood and modulates the cognitive and neural processing of emotion 3 days post administration. Neuropsychopharmacology 33, 611–618. ( 10.1038/sj.npp.1301439) [DOI] [PubMed] [Google Scholar]
- 101.Miskowiak K, Favaron E, Hafizi S, Inkster B, Goodwin G, Cowen P, Harmer C. 2009. Effects of erythropoietin on emotional processing biases in patients with major depression: an exploratory fMRI study. Psychopharmacology 207, 133–142. ( 10.1007/s00213-009-1641-1) [DOI] [PubMed] [Google Scholar]
- 102.Miskowiak KW, Vinberg M, Christensen EM, Bukh JD, Harmer CJ, Ehrenreich H, Kessing LV. 2014. Recombinant human erythropoietin for treating treatment-resistant depression: a double-blind, randomized, placebo-controlled phase 2 trial. Neuropsychopharmacology 39, 1399–1408. ( 10.1038/npp.2013.335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Diamond PR, Farmery AD, Atkinson S, Haldar J, Williams N, Cowen PJ, Geddes JR, McShane R. 2014. Ketamine infusions for treatment resistant depression: a series of 28 patients treated weekly or twice weekly in an ECT clinic. J. Psychopharmacol. 28, 536–544. ( 10.1177/0269881114527361) [DOI] [PubMed] [Google Scholar]
- 104.Murrough JW, et al. 2013. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol. Psychiatry 74, 250–256. ( 10.1016/j.biopsych.2012.06.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Abel KM, Allin MP, Kucharska-Pietura K, David A, Andrew C, Williams S, Brammer MJ, Phillips ML. 2003. Ketamine alters neural processing of facial emotion recognition in healthy men: an fMRI study. Neuroreport 14, 387–391. ( 10.1097/01.wnr.0000058031.29600.31) [DOI] [PubMed] [Google Scholar]
- 106.Deakin JFW, Williams S, Downey D, McKie S, Goodwin GM, Rylands A, Harmer CJ, Craig KJ, Dourish CT, Dawson GR, McCarthy CJ, Smith MA.2012. PharmacoMRI and cognitive effects of the low-trapping NMDA channel blocker AZD6765 compared with ketamine in untreated major depressive disorder. 28th CINP World Congress of Neuropsychopharmacology, Stockholm, Sweden, 3–7 June 2012.
- 107.Stuart SA, Butler P, Nutt DJ, Robinson ESJ. 2012. Investigating the mechanism underlying delayed versus rapid antidepressant action using a novel rodent model of affective state-induced memory bias. J. Psychopharmacol. (Suppl.) 26, A46. [Google Scholar]
- 108.Hajszan T, MacLusky NJ, Leranth C. 2005. Short-term treatment with the antidepressant fluoxetine triggers pyramidal dendritic spine synapse formation in rat hippocampus. Eur. J. Neurosci. 21, 1299–1303. ( 10.1111/j.1460-9568.2005.03968.x) [DOI] [PubMed] [Google Scholar]
- 109.Musazzi L, Cattaneo A, Tardito D, Barbon A, Gennarelli M, Barlati S, Racagni G, Popoli M. 2009. Early raise of BDNF in hippocampus suggests induction of posttranscriptional mechanisms by antidepressants. BMC Neurosci. 10, 48 ( 10.1186/1471-2202-10-48) [DOI] [PMC free article] [PubMed] [Google Scholar]