Abstract
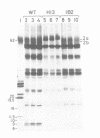
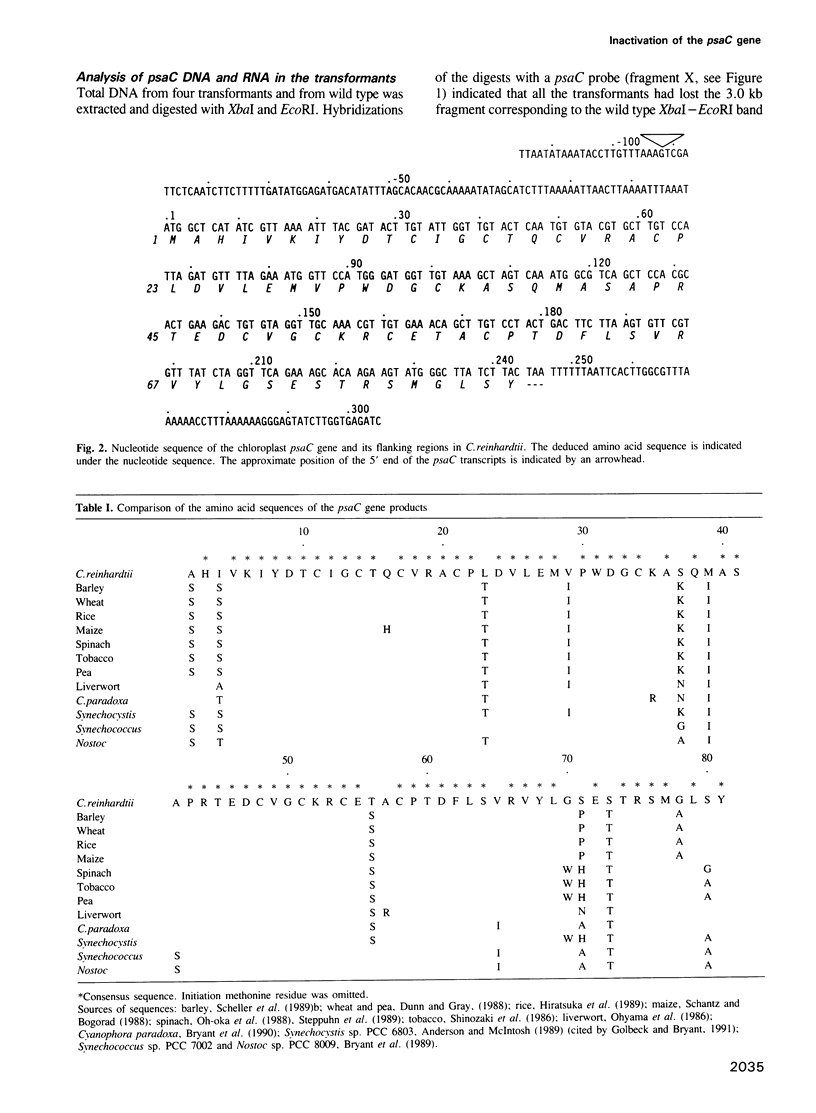
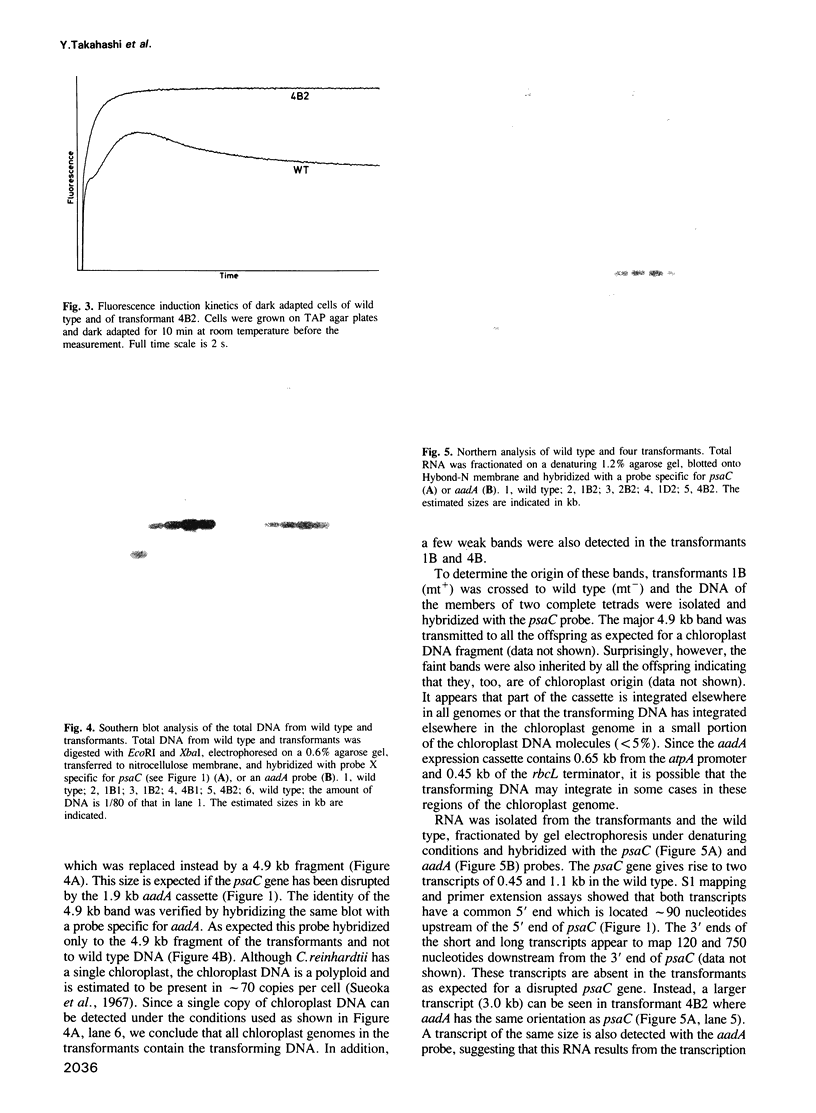
The chloroplast gene psaC encoding the iron sulfur protein of photosystem I (PSI) from the green alga Chlamydomonas reinhardtii has been cloned and characterized. The deduced amino acid sequence is highly related to that of higher plants and cyanobacteria. Using a particle gun, wild type C. reinhardtii cells have been transformed with a plasmid carrying the psaC gene disrupted by an aadA gene cassette designed to express spectinomycin/streptomycin resistance in the chloroplast. Transformants selected on plates containing acetate as a reduced carbon source and spectinomycin are unable to grow on minimal medium lacking acetate and are deficient in PSI activity. Southern blot analysis of total cell DNA of the transformants shows that the wild type psaC gene has been replaced by the interrupted psaC gene through homologous recombination. While authentic transcripts of the psaC gene are no longer detected, aadA gives rise to a few transcripts in the transformants. Biochemical analysis indicates that neither PSI reaction center subunits nor the seven small subunits belonging to PSI accumulate stably in the thylakoid membranes of the transformants. Pulse-chase labeling of cell proteins shows that the PSI reaction center subunits are synthesized normally but turn over rapidly in the transformants. We conclude that the iron sulfur binding protein encoded by the psaC gene is an essential component, both for photochemical activity and for stable assembly of PSI. The present study suggests that any chloroplast gene encoding a component of the photosynthetic apparatus can be disrupted in C. reinhardtii using the strategy described.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengis C., Nelson N. Subunit structure of chloroplast photosystem I reaction center. J Biol Chem. 1977 Jul 10;252(13):4564–4569. [PubMed] [Google Scholar]
- Blowers A. D., Bogorad L., Shark K. B., Sanford J. C. Studies on Chlamydomonas chloroplast transformation: foreign DNA can be stably maintained in the chromosome. Plant Cell. 1989 Jan;1(1):123–132. doi: 10.1105/tpc.1.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton J. E., Gillham N. W., Harris E. H., Hosler J. P., Johnson A. M., Jones A. R., Randolph-Anderson B. L., Robertson D., Klein T. M., Shark K. B. Chloroplast transformation in Chlamydomonas with high velocity microprojectiles. Science. 1988 Jun 10;240(4858):1534–1538. doi: 10.1126/science.2897716. [DOI] [PubMed] [Google Scholar]
- Burnap R. L., Sherman L. A. Deletion mutagenesis in Synechocystis sp. PCC6803 indicates that the Mn-stabilizing protein of photosystem II is not essential for O2 evolution. Biochemistry. 1991 Jan 15;30(2):440–446. doi: 10.1021/bi00216a020. [DOI] [PubMed] [Google Scholar]
- Chitnis P. R., Reilly P. A., Miedel M. C., Nelson N. Structure and targeted mutagenesis of the gene encoding 8-kDa subunit of photosystem I from the cyanobacterium Synechocystis sp. PCC 6803. J Biol Chem. 1989 Nov 5;264(31):18374–18380. [PubMed] [Google Scholar]
- Chitnis P. R., Reilly P. A., Nelson N. Insertional inactivation of the gene encoding subunit II of photosystem I from the cyanobacterium Synechocystis sp. PCC 6803. J Biol Chem. 1989 Nov 5;264(31):18381–18385. [PubMed] [Google Scholar]
- Choquet Y., Goldschmidt-Clermont M., Girard-Bascou J., Kück U., Bennoun P., Rochaix J. D. Mutant phenotypes support a trans-splicing mechanism for the expression of the tripartite psaA gene in the C. reinhardtii chloroplast. Cell. 1988 Mar 25;52(6):903–913. doi: 10.1016/0092-8674(88)90432-1. [DOI] [PubMed] [Google Scholar]
- Chua N. H., Bennoun P. Thylakoid membrane polypeptides of Chlamydomonas reinhardtii: wild-type and mutant strains deficient in photosystem II reaction center. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2175–2179. doi: 10.1073/pnas.72.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua N. H., Matlin K., Bennoun P. A chlorophyll-protein complex lacking in photosystem I mutants of Chlamydomonas reinhardtii. J Cell Biol. 1975 Nov;67(2PT1):361–377. doi: 10.1083/jcb.67.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fling S. P., Gregerson D. S. Peptide and protein molecular weight determination by electrophoresis using a high-molarity tris buffer system without urea. Anal Biochem. 1986 May 15;155(1):83–88. doi: 10.1016/0003-2697(86)90228-9. [DOI] [PubMed] [Google Scholar]
- Franzén L. G., Frank G., Zuber H., Rochaix J. D. Isolation and characterization of cDNA clones encoding photosystem I subunits with molecular masses 11.0, 10.0 and 8.4 kDa from Chlamydomonas reinhardtii. Mol Gen Genet. 1989 Oct;219(1-2):137–144. doi: 10.1007/BF00261169. [DOI] [PubMed] [Google Scholar]
- Girard-Bascou J., Choquet Y., Schneider M., Delosme M., Dron M. Characterization of a chloroplast mutation in the psaA2 gene of Chlamydomonas reinhardtii. Curr Genet. 1987;12(7):489–495. doi: 10.1007/BF00419557. [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont M., Girard-Bascou J., Choquet Y., Rochaix J. D. Trans-splicing mutants of Chlamydomonas reinhardtii. Mol Gen Genet. 1990 Sep;223(3):417–425. doi: 10.1007/BF00264448. [DOI] [PubMed] [Google Scholar]
- Gorman D. S., Levine R. P. Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1665–1669. doi: 10.1073/pnas.54.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida N., Matsubayashi T., Shinozaki K., Sugiura M., Inoue K., Hiyama T. The gene for the 9 kd polypeptide, a possible apoprotein for the iron-sulfur centers A and B of the photosystem I complex, in tobacco chloroplast DNA. Curr Genet. 1987;12(4):247–250. doi: 10.1007/BF00435285. [DOI] [PubMed] [Google Scholar]
- Hiratsuka J., Shimada H., Whittier R., Ishibashi T., Sakamoto M., Mori M., Kondo C., Honji Y., Sun C. R., Meng B. Y. The complete sequence of the rice (Oryza sativa) chloroplast genome: intermolecular recombination between distinct tRNA genes accounts for a major plastid DNA inversion during the evolution of the cereals. Mol Gen Genet. 1989 Jun;217(2-3):185–194. doi: 10.1007/BF02464880. [DOI] [PubMed] [Google Scholar]
- Hovanessian A. G., Galabru J., Rivière Y., Montagnier L. Efficiency of poly(A).poly(U) as an adjuvant. Immunol Today. 1988 Jun;9(6):161–162. doi: 10.1016/0167-5699(88)91288-1. [DOI] [PubMed] [Google Scholar]
- Høj P. B., Svendsen I., Scheller H. V., Møller B. L. Identification of a chloroplast-encoded 9-kDa polypeptide as a 2[4Fe-4S] protein carrying centers A and B of photosystem I. J Biol Chem. 1987 Sep 15;262(26):12676–12684. [PubMed] [Google Scholar]
- Koike H., Ikeuchi M., Hiyama T., Inoue Y. Identification of photosystem I components from the cyanobacterium, Synechococcus vulcanus by N-terminal sequencing. FEBS Lett. 1989 Aug 14;253(1-2):257–263. doi: 10.1016/0014-5793(89)80971-8. [DOI] [PubMed] [Google Scholar]
- Kück U., Choquet Y., Schneider M., Dron M., Bennoun P. Structural and transcription analysis of two homologous genes for the P700 chlorophyll a-apoproteins in Chlamydomonas reinhardii: evidence for in vivo trans-splicing. EMBO J. 1987 Aug;6(8):2185–2195. doi: 10.1002/j.1460-2075.1987.tb02489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Pakrasi H. B., Williams J. G., Arntzen C. J. Targeted mutagenesis of the psbE and psbF genes blocks photosynthetic electron transport: evidence for a functional role of cytochrome b559 in photosystem II. EMBO J. 1988 Feb;7(2):325–332. doi: 10.1002/j.1460-2075.1988.tb02816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przibilla E., Heiss S., Johanningmeier U., Trebst A. Site-specific mutagenesis of the D1 subunit of photosystem II in wild-type Chlamydomonas. Plant Cell. 1991 Feb;3(2):169–174. doi: 10.1105/tpc.3.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller H. V., Okkels J. S., Høj P. B., Svendsen I., Roepstorff P., Møller B. L. The primary structure of a 4.0-kDa photosystem I polypeptide encoded by the chloroplast psaI gene. J Biol Chem. 1989 Nov 5;264(31):18402–18406. [PubMed] [Google Scholar]
- Scheller H. V., Svendsen I., Møller B. L. Amino acid sequence of the 9-kDa iron-sulfur protein of photosystem I in barley. Carlsberg Res Commun. 1989;54(1):11–15. doi: 10.1007/BF02910468. [DOI] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Shepherd H. S., Boynton J. E., Gillham N. W. Mutations in nine chloroplast loci of Chlamydomonas affecting different photosynthetic functions. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1353–1357. doi: 10.1073/pnas.76.3.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K., Ohme M., Tanaka M., Wakasugi T., Hayashida N., Matsubayashi T., Zaita N., Chunwongse J., Obokata J., Yamaguchi-Shinozaki K. The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J. 1986 Sep;5(9):2043–2049. doi: 10.1002/j.1460-2075.1986.tb04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steppuhn J., Hermans J., Nechushtai R., Herrmann G. S., Herrmann R. G. Nucleotide sequences of cDNA clones encoding the entire precursor polypeptide for subunit VI and of the plastome-encoded gene for subunit VII of the photosystem I reaction center from spinach. Curr Genet. 1989 Aug;16(2):99–108. doi: 10.1007/BF00393402. [DOI] [PubMed] [Google Scholar]
- Sueoka N., Chiang K. S., Kates J. R. Deoxyribonucleic acid replication in meiosis of Chlamydomonas reinhardi. I. Isotopic transfer experiments with a strain producing eight zoospores. J Mol Biol. 1967 Apr 14;25(1):47–66. doi: 10.1016/0022-2836(67)90278-1. [DOI] [PubMed] [Google Scholar]
- Vermaas W. F., Williams J. G., Rutherford A. W., Mathis P., Arntzen C. J. Genetically engineered mutant of the cyanobacterium Synechocystis 6803 lacks the photosystem II chlorophyll-binding protein CP-47. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9474–9477. doi: 10.1073/pnas.83.24.9474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks D. P., Beerman N., Griffith O. M. A small-scale five-hour procedure for isolating multiple samples of CsCl-purified DNA: application to isolations from mammalian, insect, higher plant, algal, yeast, and bacterial sources. Anal Biochem. 1986 Feb 1;152(2):376–385. doi: 10.1016/0003-2697(86)90423-9. [DOI] [PubMed] [Google Scholar]
- Zanetti G., Merati G. Interaction between photosystem I and ferredoxin. Identification by chemical cross-linking of the polypeptide which binds ferredoxin. Eur J Biochem. 1987 Nov 16;169(1):143–146. doi: 10.1111/j.1432-1033.1987.tb13591.x. [DOI] [PubMed] [Google Scholar]