Abstract
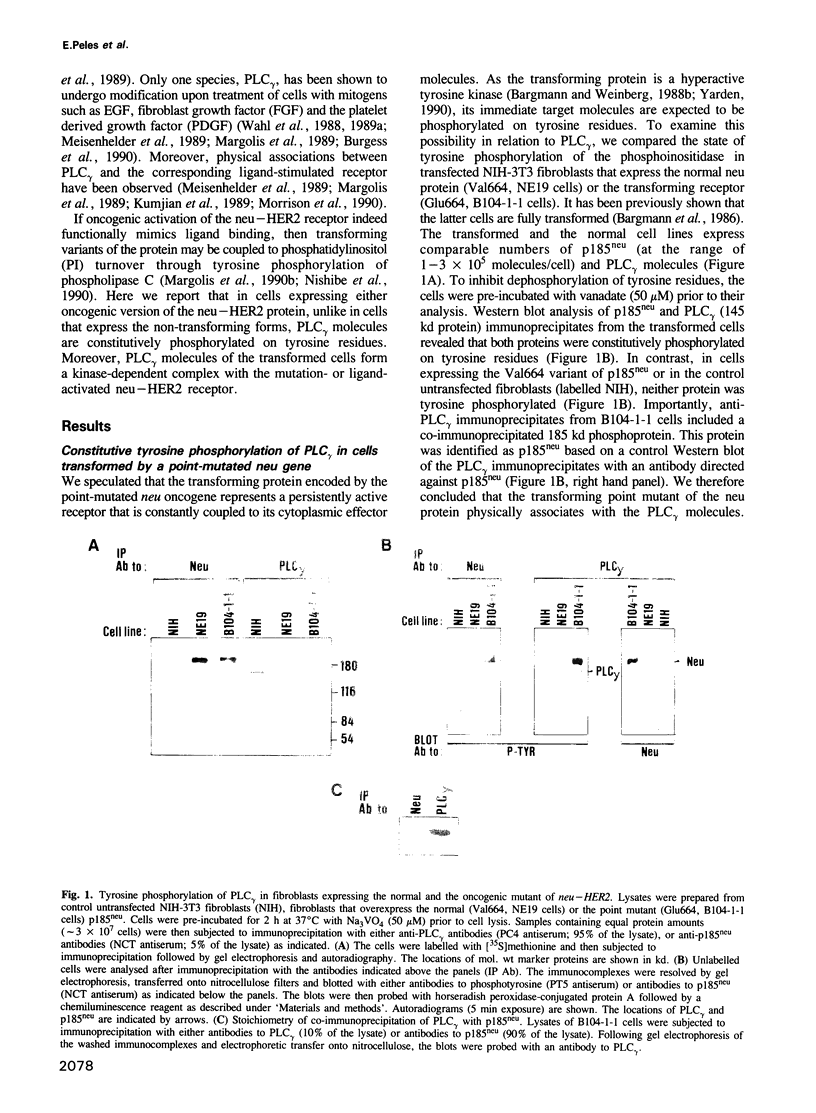
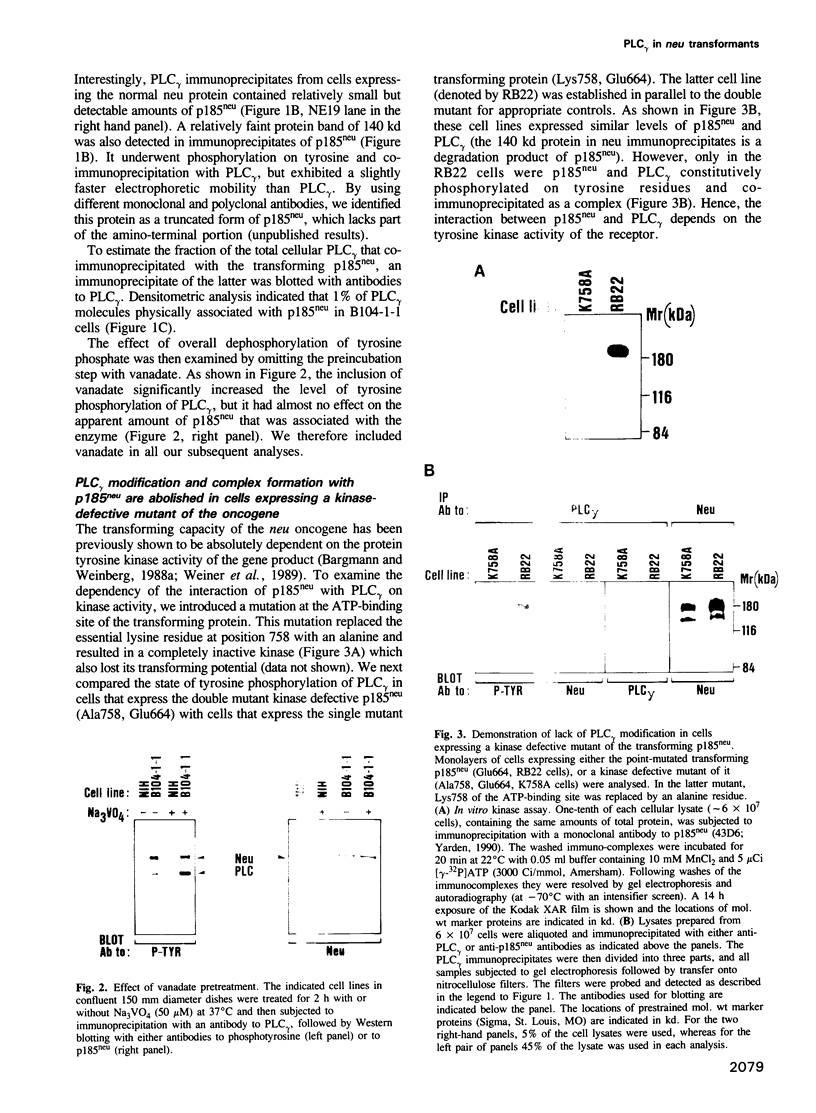
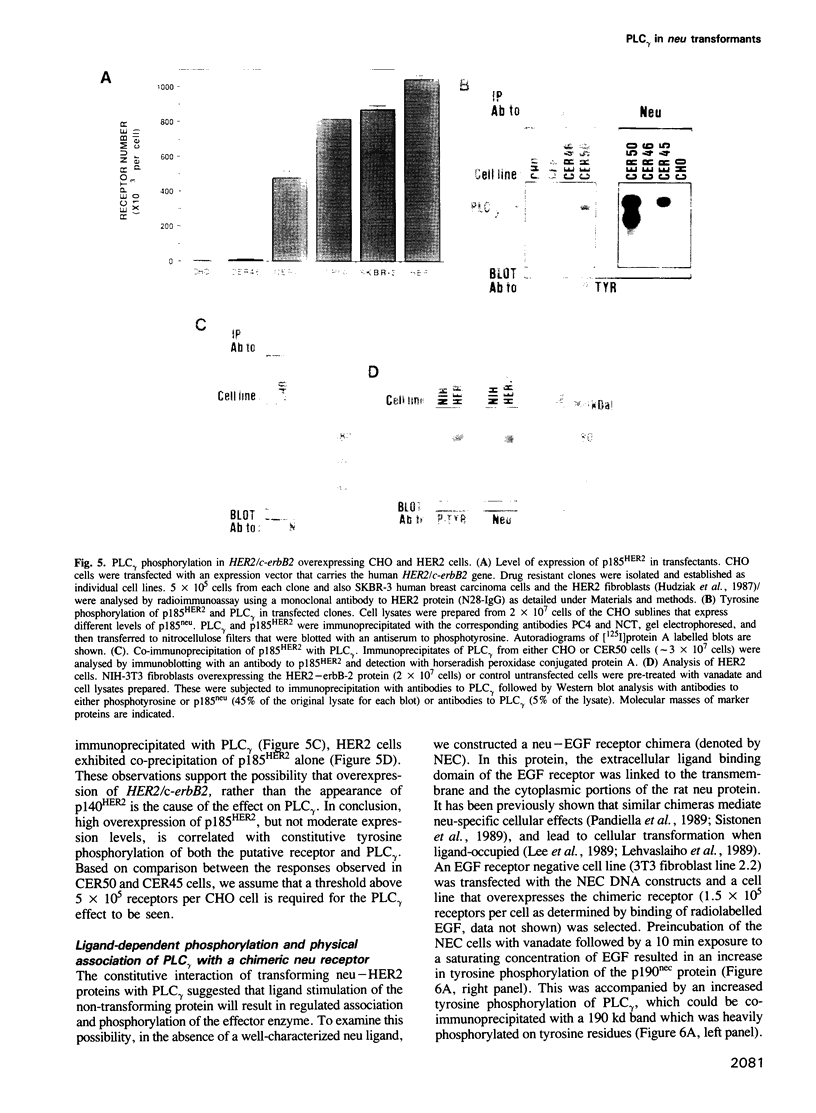
The neu/HER2 proto-oncogene encodes a transmembrane tyrosine kinase homologous to receptors for polypeptide growth factors. The oncogenic potential for the presumed receptor is released through multiple genetic mechanisms including a specific point mutation, truncation at the extracellular domain and overexpression of the protooncogene. Here we show that all these modes of oncogenic activation result in a constitutively phosphorylated neu protein and an increase in tyrosine phosphorylation of a phosphatidylinositol-specific phospholipase (PLC gamma). The examined transforming neu/HER2 proteins, unlike the normal gene product, also co-immunoprecipitated with PLC gamma molecules. A kinase-defective mutant of a transforming neu failed to mediate both tyrosine phosphorylation and association with PLC gamma, suggesting direct interaction of the neu kinase with PLC gamma. This possibility was examined by employing a chimeric protein composed of the extracellular ligand-binding domain of the epidermal growth factor receptor and the neu cytoplasmic portion. The chimeric receptor mediated rapid ligand-dependent modification of PLC gamma on tyrosine residues. It also physically associated, in a ligand-dependent manner, with the phosphoinositidase. Based on the presented results we suggest that the mechanism of cellular transformation by the neu/HER2 receptor involves tyrosine phosphorylation and activation of PLC gamma.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama T., Sudo C., Ogawara H., Toyoshima K., Yamamoto T. The product of the human c-erbB-2 gene: a 185-kilodalton glycoprotein with tyrosine kinase activity. Science. 1986 Jun 27;232(4758):1644–1646. doi: 10.1126/science.3012781. [DOI] [PubMed] [Google Scholar]
- Bargmann C. I., Hung M. C., Weinberg R. A. Multiple independent activations of the neu oncogene by a point mutation altering the transmembrane domain of p185. Cell. 1986 Jun 6;45(5):649–657. doi: 10.1016/0092-8674(86)90779-8. [DOI] [PubMed] [Google Scholar]
- Bargmann C. I., Weinberg R. A. Increased tyrosine kinase activity associated with the protein encoded by the activated neu oncogene. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5394–5398. doi: 10.1073/pnas.85.15.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann C. I., Weinberg R. A. Oncogenic activation of the neu-encoded receptor protein by point mutation and deletion. EMBO J. 1988 Jul;7(7):2043–2052. doi: 10.1002/j.1460-2075.1988.tb03044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M. S., Locher G. W., Saurer S., Gullick W. J., Waterfield M. D., Groner B., Hynes N. E. Correlation of c-erbB-2 gene amplification and protein expression in human breast carcinoma with nodal status and nuclear grading. Cancer Res. 1988 Mar 1;48(5):1238–1243. [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Burgess W. H., Dionne C. A., Kaplow J., Mudd R., Friesel R., Zilberstein A., Schlessinger J., Jaye M. Characterization and cDNA cloning of phospholipase C-gamma, a major substrate for heparin-binding growth factor 1 (acidic fibroblast growth factor)-activated tyrosine kinase. Mol Cell Biol. 1990 Sep;10(9):4770–4777. doi: 10.1128/mcb.10.9.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens L., Yang-Feng T. L., Liao Y. C., Chen E., Gray A., McGrath J., Seeburg P. H., Libermann T. A., Schlessinger J., Francke U. Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene. Science. 1985 Dec 6;230(4730):1132–1139. doi: 10.1126/science.2999974. [DOI] [PubMed] [Google Scholar]
- Di Fiore P. P., Pierce J. H., Kraus M. H., Segatto O., King C. R., Aaronson S. A. erbB-2 is a potent oncogene when overexpressed in NIH/3T3 cells. Science. 1987 Jul 10;237(4811):178–182. doi: 10.1126/science.2885917. [DOI] [PubMed] [Google Scholar]
- Di Marco E., Pierce J. H., Knicley C. L., Di Fiore P. P. Transformation of NIH 3T3 cells by overexpression of the normal coding sequence of the rat neu gene. Mol Cell Biol. 1990 Jun;10(6):3247–3252. doi: 10.1128/mcb.10.6.3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R., Levy R. B., Peles E., Yarden Y. Heterodimerization of the erbB-1 and erbB-2 receptors in human breast carcinoma cells: a mechanism for receptor transregulation. Biochemistry. 1990 Dec 18;29(50):11024–11028. doi: 10.1021/bi00502a002. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Gullick W. J., Berger M. S., Bennett P. L., Rothbard J. B., Waterfield M. D. Expression of the c-erbB-2 protein in normal and transformed cells. Int J Cancer. 1987 Aug 15;40(2):246–254. doi: 10.1002/ijc.2910400221. [DOI] [PubMed] [Google Scholar]
- Hudziak R. M., Schlessinger J., Ullrich A. Increased expression of the putative growth factor receptor p185HER2 causes transformation and tumorigenesis of NIH 3T3 cells. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7159–7163. doi: 10.1073/pnas.84.20.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung M. C., Schechter A. L., Chevray P. Y., Stern D. F., Weinberg R. A. Molecular cloning of the neu gene: absence of gross structural alteration in oncogenic alleles. Proc Natl Acad Sci U S A. 1986 Jan;83(2):261–264. doi: 10.1073/pnas.83.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamps M. P., Sefton B. M. Identification of multiple novel polypeptide substrates of the v-src, v-yes, v-fps, v-ros, and v-erb-B oncogenic tyrosine protein kinases utilizing antisera against phosphotyrosine. Oncogene. 1988 Apr;2(4):305–315. [PubMed] [Google Scholar]
- Kawamoto T., Sato J. D., Le A., Polikoff J., Sato G. H., Mendelsohn J. Growth stimulation of A431 cells by epidermal growth factor: identification of high-affinity receptors for epidermal growth factor by an anti-receptor monoclonal antibody. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1337–1341. doi: 10.1073/pnas.80.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. W., Sim S. S., Kim U. H., Nishibe S., Wahl M. I., Carpenter G., Rhee S. G. Tyrosine residues in bovine phospholipase C-gamma phosphorylated by the epidermal growth factor receptor in vitro. J Biol Chem. 1990 Mar 5;265(7):3940–3943. [PubMed] [Google Scholar]
- Kraus M. H., Popescu N. C., Amsbaugh S. C., King C. R. Overexpression of the EGF receptor-related proto-oncogene erbB-2 in human mammary tumor cell lines by different molecular mechanisms. EMBO J. 1987 Mar;6(3):605–610. doi: 10.1002/j.1460-2075.1987.tb04797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumjian D. A., Wahl M. I., Rhee S. G., Daniel T. O. Platelet-derived growth factor (PDGF) binding promotes physical association of PDGF receptor with phospholipase C. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8232–8236. doi: 10.1073/pnas.86.21.8232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Dull T. J., Lax I., Schlessinger J., Ullrich A. HER2 cytoplasmic domain generates normal mitogenic and transforming signals in a chimeric receptor. EMBO J. 1989 Jan;8(1):167–173. doi: 10.1002/j.1460-2075.1989.tb03361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehväslaiho H., Lehtola L., Sistonen L., Alitalo K. A chimeric EGF-R-neu proto-oncogene allows EGF to regulate neu tyrosine kinase and cell transformation. EMBO J. 1989 Jan;8(1):159–166. doi: 10.1002/j.1460-2075.1989.tb03360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupu R., Colomer R., Zugmaier G., Sarup J., Shepard M., Slamon D., Lippman M. E. Direct interaction of a ligand for the erbB2 oncogene product with the EGF receptor and p185erbB2. Science. 1990 Sep 28;249(4976):1552–1555. doi: 10.1126/science.2218496. [DOI] [PubMed] [Google Scholar]
- Margolis B., Bellot F., Honegger A. M., Ullrich A., Schlessinger J., Zilberstein A. Tyrosine kinase activity is essential for the association of phospholipase C-gamma with the epidermal growth factor receptor. Mol Cell Biol. 1990 Feb;10(2):435–441. doi: 10.1128/mcb.10.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis B., Rhee S. G., Felder S., Mervic M., Lyall R., Levitzki A., Ullrich A., Zilberstein A., Schlessinger J. EGF induces tyrosine phosphorylation of phospholipase C-II: a potential mechanism for EGF receptor signaling. Cell. 1989 Jun 30;57(7):1101–1107. doi: 10.1016/0092-8674(89)90047-0. [DOI] [PubMed] [Google Scholar]
- Margolis B., Zilberstein A., Franks C., Felder S., Kremer S., Ullrich A., Rhee S. G., Skorecki K., Schlessinger J. Effect of phospholipase C-gamma overexpression on PDGF-induced second messengers and mitogenesis. Science. 1990 May 4;248(4955):607–610. doi: 10.1126/science.2333512. [DOI] [PubMed] [Google Scholar]
- Meisenhelder J., Suh P. G., Rhee S. G., Hunter T. Phospholipase C-gamma is a substrate for the PDGF and EGF receptor protein-tyrosine kinases in vivo and in vitro. Cell. 1989 Jun 30;57(7):1109–1122. doi: 10.1016/0092-8674(89)90048-2. [DOI] [PubMed] [Google Scholar]
- Morrison D. K., Kaplan D. R., Rhee S. G., Williams L. T. Platelet-derived growth factor (PDGF)-dependent association of phospholipase C-gamma with the PDGF receptor signaling complex. Mol Cell Biol. 1990 May;10(5):2359–2366. doi: 10.1128/mcb.10.5.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishibe S., Wahl M. I., Hernández-Sotomayor S. M., Tonks N. K., Rhee S. G., Carpenter G. Increase of the catalytic activity of phospholipase C-gamma 1 by tyrosine phosphorylation. Science. 1990 Nov 30;250(4985):1253–1256. doi: 10.1126/science.1700866. [DOI] [PubMed] [Google Scholar]
- Nishibe S., Wahl M. I., Rhee S. G., Carpenter G. Tyrosine phosphorylation of phospholipase C-II in vitro by the epidermal growth factor receptor. J Biol Chem. 1989 Jun 25;264(18):10335–10338. [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Pandiella A., Lehvaslaiho H., Magni M., Alitalo K., Meldolesi J. Activation of an EGFR/neu chimeric receptor: early intracellular signals and cell proliferation responses. Oncogene. 1989 Nov;4(11):1299–1305. [PubMed] [Google Scholar]
- Rhee S. G., Suh P. G., Ryu S. H., Lee S. Y. Studies of inositol phospholipid-specific phospholipase C. Science. 1989 May 5;244(4904):546–550. doi: 10.1126/science.2541501. [DOI] [PubMed] [Google Scholar]
- Schechter A. L., Stern D. F., Vaidyanathan L., Decker S. J., Drebin J. A., Greene M. I., Weinberg R. A. The neu oncogene: an erb-B-related gene encoding a 185,000-Mr tumour antigen. Nature. 1984 Dec 6;312(5994):513–516. doi: 10.1038/312513a0. [DOI] [PubMed] [Google Scholar]
- Segatto O., King C. R., Pierce J. H., Di Fiore P. P., Aaronson S. A. Different structural alterations upregulate in vitro tyrosine kinase activity and transforming potency of the erbB-2 gene. Mol Cell Biol. 1988 Dec;8(12):5570–5574. doi: 10.1128/mcb.8.12.5570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sistonen L., Hölttä E., Lehväslaiho H., Lehtola L., Alitalo K. Activation of the neu tyrosine kinase induces the fos/jun transcription factor complex, the glucose transporter and ornithine decarboxylase. J Cell Biol. 1989 Nov;109(5):1911–1919. doi: 10.1083/jcb.109.5.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamon D. J., Clark G. M., Wong S. G., Levin W. J., Ullrich A., McGuire W. L. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987 Jan 9;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- Slamon D. J., Godolphin W., Jones L. A., Holt J. A., Wong S. G., Keith D. E., Levin W. J., Stuart S. G., Udove J., Ullrich A. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989 May 12;244(4905):707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- Stahl M. L., Ferenz C. R., Kelleher K. L., Kriz R. W., Knopf J. L. Sequence similarity of phospholipase C with the non-catalytic region of src. Nature. 1988 Mar 17;332(6161):269–272. doi: 10.1038/332269a0. [DOI] [PubMed] [Google Scholar]
- Stern D. F., Heffernan P. A., Weinberg R. A. p185, a product of the neu proto-oncogene, is a receptorlike protein associated with tyrosine kinase activity. Mol Cell Biol. 1986 May;6(5):1729–1740. doi: 10.1128/mcb.6.5.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda Y., Aizawa S., Furuta Y., Yagi T., Ikawa Y., Saitoh K., Yamada Y., Toyoshima K., Yamamoto T. Induction of a variety of tumors by c-erbB2 and clonal nature of lymphomas even with the mutated gene (Val659----Glu659). EMBO J. 1990 Jan;9(1):181–190. doi: 10.1002/j.1460-2075.1990.tb08094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Urlaub G., Chasin L. A. Isolation of Chinese hamster cell mutants deficient in dihydrofolate reductase activity. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4216–4220. doi: 10.1073/pnas.77.7.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varley J. M., Swallow J. E., Brammar W. J., Whittaker J. L., Walker R. A. Alterations to either c-erbB-2(neu) or c-myc proto-oncogenes in breast carcinomas correlate with poor short-term prognosis. Oncogene. 1987;1(4):423–430. [PubMed] [Google Scholar]
- Venter D. J., Tuzi N. L., Kumar S., Gullick W. J. Overexpression of the c-erbB-2 oncoprotein in human breast carcinomas: immunohistological assessment correlates with gene amplification. Lancet. 1987 Jul 11;2(8550):69–72. doi: 10.1016/s0140-6736(87)92736-x. [DOI] [PubMed] [Google Scholar]
- Wahl M. I., Daniel T. O., Carpenter G. Antiphosphotyrosine recovery of phospholipase C activity after EGF treatment of A-431 cells. Science. 1988 Aug 19;241(4868):968–970. doi: 10.1126/science.2457254. [DOI] [PubMed] [Google Scholar]
- Wahl M. I., Nishibe S., Kim J. W., Kim H., Rhee S. G., Carpenter G. Identification of two epidermal growth factor-sensitive tyrosine phosphorylation sites of phospholipase C-gamma in intact HSC-1 cells. J Biol Chem. 1990 Mar 5;265(7):3944–3948. [PubMed] [Google Scholar]
- Wahl M. I., Nishibe S., Suh P. G., Rhee S. G., Carpenter G. Epidermal growth factor stimulates tyrosine phosphorylation of phospholipase C-II independently of receptor internalization and extracellular calcium. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1568–1572. doi: 10.1073/pnas.86.5.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl M. I., Olashaw N. E., Nishibe S., Rhee S. G., Pledger W. J., Carpenter G. Platelet-derived growth factor induces rapid and sustained tyrosine phosphorylation of phospholipase C-gamma in quiescent BALB/c 3T3 cells. Mol Cell Biol. 1989 Jul;9(7):2934–2943. doi: 10.1128/mcb.9.7.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner D. B., Kokai Y., Wada T., Cohen J. A., Williams W. V., Greene M. I. Linkage of tyrosine kinase activity with transforming ability of the p185neu oncoprotein. Oncogene. 1989 Oct;4(10):1175–1183. [PubMed] [Google Scholar]
- Whitman M., Cantley L. Phosphoinositide metabolism and the control of cell proliferation. Biochim Biophys Acta. 1989 Feb;948(3):327–344. doi: 10.1016/0304-419x(89)90005-x. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Ikawa S., Akiyama T., Semba K., Nomura N., Miyajima N., Saito T., Toyoshima K. Similarity of protein encoded by the human c-erb-B-2 gene to epidermal growth factor receptor. Nature. 1986 Jan 16;319(6050):230–234. doi: 10.1038/319230a0. [DOI] [PubMed] [Google Scholar]
- Yarden Y. Agonistic antibodies stimulate the kinase encoded by the neu protooncogene in living cells but the oncogenic mutant is constitutively active. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2569–2573. doi: 10.1073/pnas.87.7.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden Y., Peles E. Biochemical analysis of the ligand for the neu oncogenic receptor. Biochemistry. 1991 Apr 9;30(14):3543–3550. doi: 10.1021/bi00228a027. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Weinberg R. A. Experimental approaches to hypothetical hormones: detection of a candidate ligand of the neu protooncogene. Proc Natl Acad Sci U S A. 1989 May;86(9):3179–3183. doi: 10.1073/pnas.86.9.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota J., Yamamoto T., Toyoshima K., Terada M., Sugimura T., Battifora H., Cline M. J. Amplification of c-erbB-2 oncogene in human adenocarcinomas in vivo. Lancet. 1986 Apr 5;1(8484):765–767. doi: 10.1016/s0140-6736(86)91782-4. [DOI] [PubMed] [Google Scholar]
- Zhou D., Battifora H., Yokota J., Yamamoto T., Cline M. J. Association of multiple copies of the c-erbB-2 oncogene with spread of breast cancer. Cancer Res. 1987 Nov 15;47(22):6123–6125. [PubMed] [Google Scholar]
- van de Vijver M., van de Bersselaar R., Devilee P., Cornelisse C., Peterse J., Nusse R. Amplification of the neu (c-erbB-2) oncogene in human mammmary tumors is relatively frequent and is often accompanied by amplification of the linked c-erbA oncogene. Mol Cell Biol. 1987 May;7(5):2019–2023. doi: 10.1128/mcb.7.5.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]