Summary
Rho family GTPases regulate diverse processes in human melanoma ranging from tumor formation to metastasis and chemoresistance. In this study, a combination of in vitro and in vivo approaches was utilized to determine whether RHOJ, a CDC42 homologue that regulates melanoma chemoresistance, also controls melanoma migration. Depletion or overexpression of RHOJ altered cellular morphology, implicating a role for RHOJ in modulating actin cytoskeletal dynamics. RHOJ depletion inhibited melanoma cell migration and invasion in vitro and melanoma tumor growth and lymphatic spread in mice. Molecular studies revealed that RHOJ alters actin cytoskeletal dynamics by inducing the phosphorylation of LIMK, cofilin, and p41-ARC (ARP2/3 complex subunit) in a PAK1-dependent manner in vitro and in tumor xenografts. Taken together, these observations identify RHOJ as a melanoma linchpin determinant that regulates both actin cytoskeletal dynamics and chemoresistance by activating PAK1.
Keywords: RHOJ, PAK1, melanoma, migration, invasion, cytoskeleton
Introduction
Melanoma cells are resistant to DNA damage and invade the adjacent stroma at early stages of tumor evolution, making these cancers difficult to treat (Gaggioli and Sahai, 2007; Soengas and Lowe, 2003). Recent studies have utilized genomic approaches to identify the molecular regulators of melanoma proliferation, invasion, and chemoresistance (Arozarena et al., 2011; Gaggioli and Sahai, 2007; Scott et al., 2011). These studies identified BRAF as a gene that is mutated in 50% of melanomas (Davies et al., 2002). Pharmacologic inhibitors of mutant BRAF are effective at slowing the proliferation of BRAF mutant human tumors (Chapman et al., 2011), demonstrating the power of the genetic approach to identify new therapies for melanoma. More recent studies have determined that subsets of melanoma tumors harbor mutations in the Rho family GTPase Rac1 (Hodis et al., 2012; Krauthammer et al., 2012) or putative upstream activators of Rac1 (PREX2) (Berger et al., 2012). Functional studies have revealed that Rho and CDC42 family GTPases, which include Rac1, regulate melanoma morphology and invasion (Calvo et al., 2011; Sanz-Moreno et al., 2008). Consistent with these observations, upstream activators of Rac GTPases are also required for melanoma migration and metastasis in vivo (Lindsay et al., 2011), identifying a central role for these GTPases in tumor migration. Recent work from our group identified the CDC42 GTPase Ras Homolog family member J (RHOJ), a gene that is upregulated in advanced melanoma, as a novel regulator of melanoma chemoresistance (Ho et al., 2012). Additional studies revealed that RHOJ modulates melanoma chemoresistance by activating p21 protein (Cdc42/Rac)-activated kinase 1 (PAK1), a kinase that is also known to modulate cell migration (Delorme-Walker et al., 2011) and tumor invasion (Ong et al., 2011). In this study, we investigate whether RhoJ and Pak1 not only regulate melanoma chemoresistance but also modulate melanoma invasion.
Results
RhoJ modulates cell migration and invasion in vitro
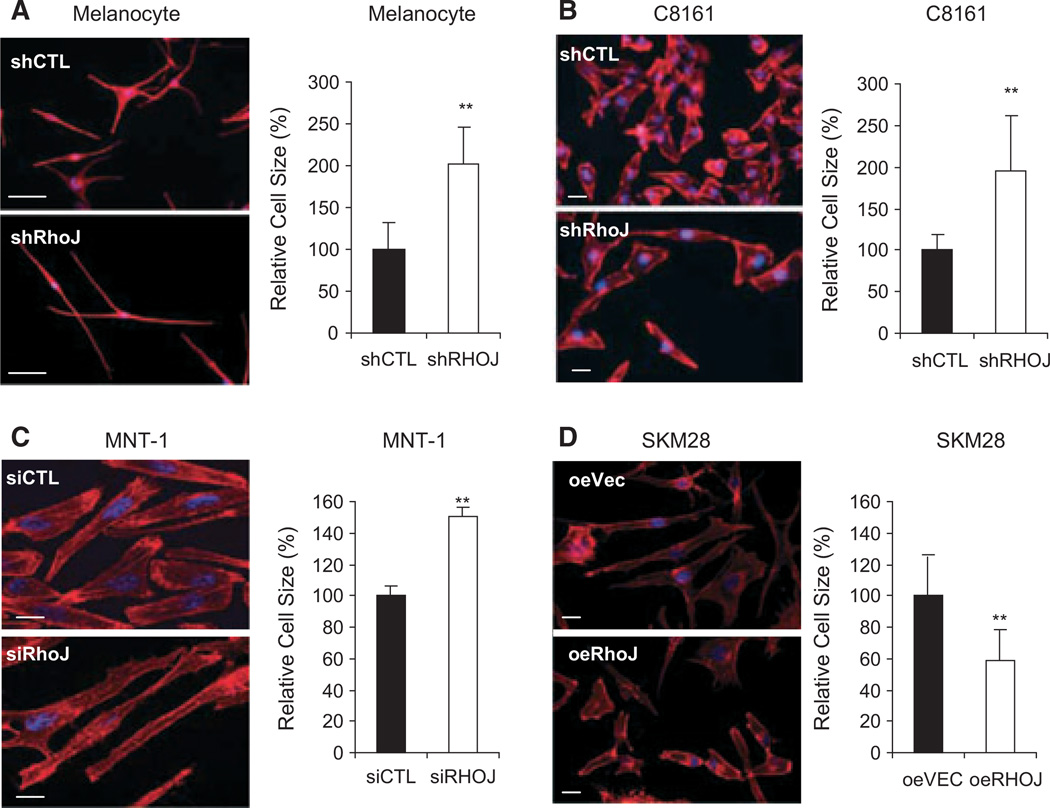
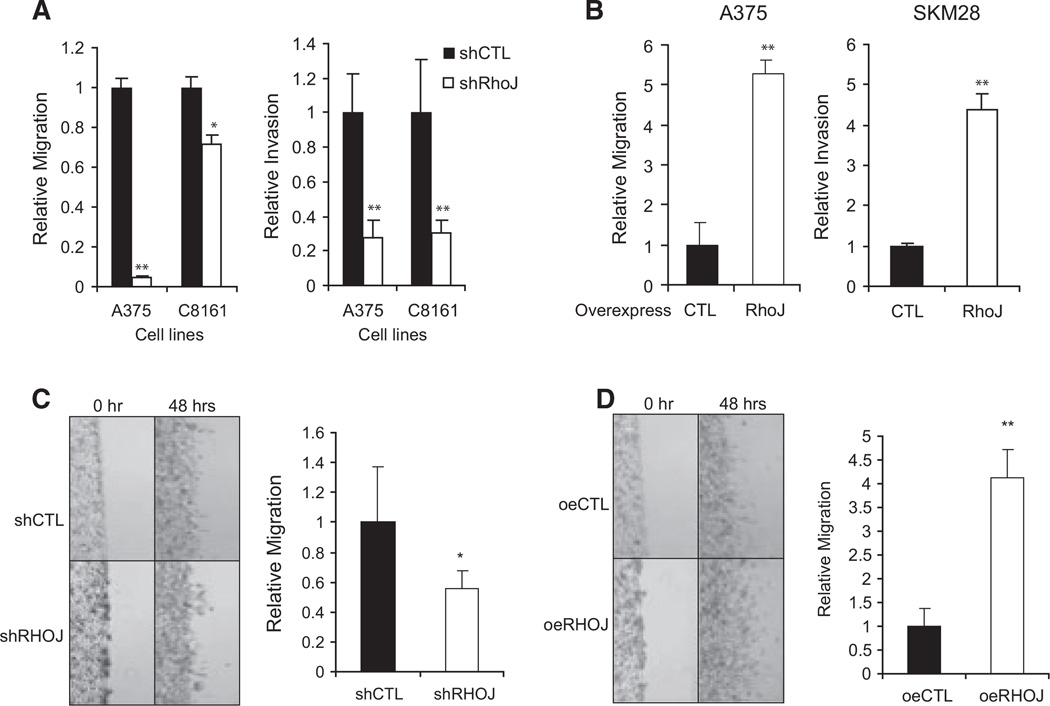
Initial studies sought to determine whether RHOJ, like other Rho family GTPases (Kolyada et al., 2003; Liebig et al., 2009; Otani et al., 2011; Thirone et al., 2009), regulates melanoma cell size and shape. RHOJ depletion (see Figure S1 for knockdown validation) induced an increase in cell length in melanocytes, C8161, and MNT-1 melanoma cells (Figure 1A–C). In contrast, RHOJ overexpression (see Figure S1 for overexpression validation) induced a decrease in cell length in SK-Mel-28 melanoma cells (Figure 1D). As Rho proteins are known to regulate melanoma migration and invasion by controlling actin cytoskeletal dynamics (Calvo et al., 2011; Lindsay et al., 2011; Sanz-Moreno et al., 2008), we next sought to determine whether RhoJ regulates melanoma cell migration and invasion in vitro. RHOJ depletion suppressed the migration of melanoma cells through polycarbonate membranes, indicating that RHOJ regulates melanoma cell migration (Figure 2A). Additional studies revealed that RHOJ-depleted cells migrated slower through collagen Icoated transwells (Figure 2A), a transwell system that closely approximates the physiologic barrier that cancer cells have to traverse in order to invade surrounding tissues (Sodek et al., 2008). In contrast, RhoJ overexpression was sufficient to accelerate melanoma cell migration across polycarbonate membranes and accelerate the invasion of melanoma cells across collagen I-coated transwells (Figure 2B). Additional experiments revealed that RHOJ-depleted cells migrated slower and RHOJ-overexpressing cells migrated faster in scratch assays performed in the presence of the proliferation inhibitor mitomycin C (Figure 2C, D). Taken together, these results indicate that RHOJ modulates cell migration independent of its effect on proliferation presumably by promoting melanoma cell movement. These observations are consistent with the established roles of Rho proteins in melanoma cell movement and invasion (Estecha et al., 2009; Goetz et al., 2011; Katiyar and Aplin, 2011).
Figure 1.
RHOJ modulates cell length. Control- or RHOJ-shRNA-expressing (A) human primary melanocytes, (B) C8161 melanoma cells, (C) MNT-1 human melanoma cells, or (D) RHOJ-overexpressing SK-Mel-28 human melanoma cells were stained with rhodamine-conjugated phalloidin and DAPI. Images were recorded using a fluorescent microscope. The length of cells in their greatest dimension was measured using ImageJ, and the data were normalized to the respective control (n = 10). **P-value < 0.01. Scale bar, 10 µm.
Figure 2.
RHOJ controls melanoma migration and invasion in vitro. (A) Fifty thousand melanoma cells expressing RHOJ or control-shRNAs (B) or overexpressing vector or RHOJ were starved for 24 h in serum-free medium and plated in serum-free medium in the upper chamber. Serum-containing medium was then added to the bottom chamber. Twenty-four hours later, cells that migrated through the membrane were counted as described in the manufacturer’s protocol (Cell Biolabs, Inc.). The relative migration was calculated by normalizing each cell line to their corresponding controls, n = 3. For the invasion assay, collagen I-matrix-coated upper chambers were used. *P < 0.05, **P < 0.01 using Student’s t-test. (C) RHOJ-shRNA- or control-shRNA-expressing or (D) RHOJ- or control-overexpressing A375 melanoma cells were plated and serum-starved overnight before being treated with mitomycin C. The area of cells that migrated into the scraped zone after 48 h was measured and the results were normalized to the respective controls (n = 4). *P < 0.05 by Student’s t-test. **P < 0.01 by Student’s t-test.
RhoJ modulates melanoma tumor growth in vivo
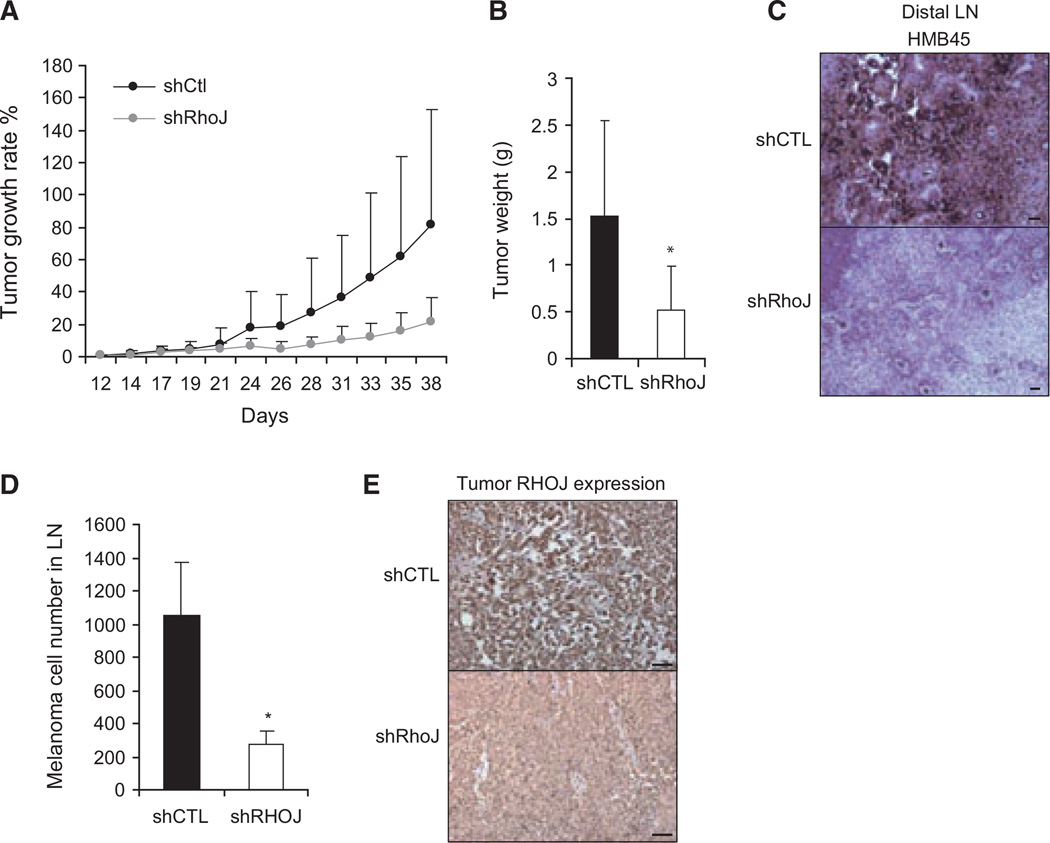
Once we had determined that RhoJ regulates melanoma cell migration and invasion in vitro, we next sought to determine whether RhoJ regulates melanoma tumor growth and invasion in vivo. Control- or RHOJ-shRNA-expressing A375 melanoma cells were injected subcutaneously into the bilateral flanks of nude mice, and the rate of tumor growth and tumor weight was recorded. RHOJ-shRNA-expressing tumors grew slower than control-shRNA-expressing tumors as determined by repeated-measures anova type III, P < 0.05 (Figure 3A). RHOJ-shRNA-expressing tumors weighed significantly less than control-shRNA-expressing tumors after 38 days of growth (Figure 3B). In addition, we observed that while control-shRNA-expressing tumor cells effectively migrated to both the immediate draining lymph node basin and the brachial nodal basin, RhoJ-shRNA-expressing cells were only able to migrate to the immediate draining lymph node basin (Figure 3C, D). Immunohistochemistry studies demonstrated that RhoJ-shRNAs effectively inhibited the expression of RhoJ in xenografted tumors (Figure 3E). While xenograft models are not ideal systems to study tumor invasion and metastasis because the generated tumors are not autochthonous, our results nonetheless suggest that RhoJ modulates not only melanoma tumor growth but melanoma migration in vivo.
Figure 3.
RHOJ controls melanoma migration and invasion in vivo. (A) Two million RHOJ-shRNA- or control-shRNA-expressing A375 melanoma cells were subcutaneously injected to J:NU nude mice on their bilateral flanks. Tumor sizes were measured using calipers every 2–3 days (n = 8) and were normalized to each tumor’s starting size at day 12. (B) Tumors weights were measured at day 38 after injection (n = 8). *P-value < 0.05 using Student’s t-test. (C) Brachial lymph nodes (LN) from the same mice above were stained with human melanoma marker HMB45 and counterstained with hematoxylin. Scale bar, 100 µm. (D) Eight-micrometer sections of brachial lymph nodes from mice carrying RHOJ-shRNA-expressing tumors or control-shRNA-expressing tumors were prepared and stained with an HMB45 antibody. The total number of HMB45+ cells in each lymph node was measured by summing the number of HMB45 cells in each section. *P < 0.05, Student’s t-test. (E) Control- or RhoJ-shRNA-expressing xenografts were collected 38 days post-injection and stained with RhoJ antibody (hematoxylin counterstain). Scale bar, 100 µm.
RhoJ modulates melanoma cell migration by activating PAK1
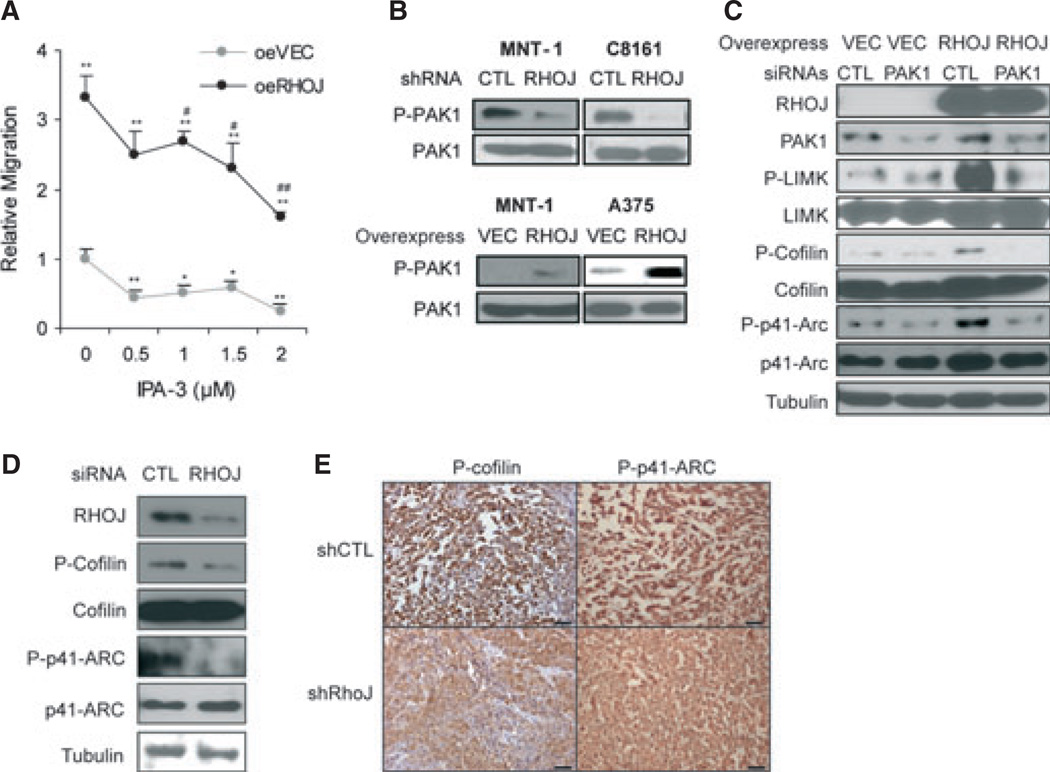
Next, we sought to determine whether RhoJ modulates melanoma cell migration by activating PAK kinases. RhoJ, like other CDC42 homologues, can bind to and activate group I PAK kinases (PAK1, PAK2, PAK3) that contain Pak autoinhibitory domains (Vignal et al., 2000). Rho GTPase binding inhibits the ability of PAKs to dimerize and autophosphorylate (phospho-Ser199/204 for PAK1/3 or Ser192/197 for PAK2; Dummler et al., 2009). As RHOJ is already known to activate PAK1 in response to DNA damage (Ho et al., 2012), we next examined whether RHOJ activates PAK1 to control cell migration. First, we utilized a group I PAK inhibitor (IPA-3) to determine whether Pak inhibition suppressed melanoma cell migration. IPA-3 inhibited A375 melanoma cell migration in a dose-dependent manner (Figure 4A), indicating that group I PAKs control melanoma cell migration. These experiments were performed in the presence of mitomycin C, and the number of migrating cells was normalized to the total number of cells present in each well to exclude the possibility that the effects were secondary to an impact of IPA-3 on cellular proliferation (Figure S2). Next, we asked whether group I PAK kinases are activated by RHOJ in the absence of DNA damage stimuli. IPA-3 inhibited the migration of RHOJ-overexpressing cells (Figure 4A), indicating RhoJ regulates melanoma invasion by activating group I PAKs. Using an antibody that can recognize phospho-Ser199/204 PAK1/3 or Ser192/197 PAK2, we observed that RHOJ depletion inhibited the accumulation of the band corresponding to p-PAK1/PAK3 in both MNT-1 and C8161 melanoma cells, while RHOJ overexpression induced the accumulation of p-PAK1/PAK3 in these cell lines (Figure 4B). Previous studies had established that PAK3 is not expressed in the cell lines studied (Ho et al., 2012), indicating that RhoJ controls melanoma invasion by activating PAK1.
Figure 4.
RHOJ/PAK1 control melanoma cell migration by modulating actin cytoskeletal dynamics. (A) The relative migration of A375 melanoma cells overexpressing RhoJ or a control vector in the presence and absence of IPA-3 was measured using the modified Boyden chamber assay described in Figure 1(A). *P < 0.05; **P < 0.01 versus vehicle-treated overexpressing vector cells; #P < 0.05; ##P < 0.01 versus vehicle-treated RHOJ-overexpressing cells using Student’s t-test. (B) Melanoma cells expressing control or RHOJ-shRNA (upper panel), or vector and RHOJ overexpression constructs (lower panel) were subjected to immunoblotting with phospho-Ser-199/204 PAK1/3 and PAK1 antibodies. (C) A375 melanoma cells overexpressing RHOJ or control vector were transfected with PAK1 siRNA for 72 h. Cell lysates were subjected to immunoblotting with the indicated antibodies. (D) A375 melanoma cells were transfected with control or RHOJ siRNA for 72 h and subjected to immunoblotting with the indicated antibodies. (E) Control- or RhoJ-shRNA-expressing tumor xenografts were collected 38 days post-injection. Tumors were fixed and subjected to immunohistochemistry with the indicated antibodies. Scale bar, 100 µm.
RhoJ/PAK1 modulates melanoma cell migration by altering actin cytoskeletal dynamics
Next, we sought to determine how PAK1 regulates actin cytoskeletal dynamics in melanoma cells. PAK1 is known to regulate cell movement by controlling actin cytoskeletal dynamics in multiple different ways (Delorme-Walker et al., 2011). PAK1 phosphorylates LIM domain containing protein kinase (LIMK), which phosphorylates cofilin and inactivates its F-actin depolymerization activity (Edwards et al., 1999). P41-ARC is another PAK1 target that stimulates the formation of an actin-related protein 2/3 protein complex (ARP2/3 protein complex), which initiates actin filament assembly (Vadlamudi et al., 2004). Finally, PAK1 can also activate myosin light chain kinase and regulatory light chains of myosin (R-MLC), which are responsible for the formation of focal adhesion and lamellipodia extensions during tumor cells’ migration and invasion (Coniglio et al., 2008). In order to determine how RHOJ/PAK1 regulates melanoma invasion, we examined whether phosphorylated LIMK, P41-ARC, or R-MLC accumulate in RHOJ-overexpressing cells. Initial studies verified that the antibodies used in this study were specific for phosphorylated LIMK, P41-ARC, and R-MLC (Figure S3A). While we did not observe the accumulation of phospho-R-MLC in RHOJ-overexpressing cells (Figure S3B), we did observe that RHOJ overexpression induced the accumulation of phospho-LIMK and p-p41-ARC (Figure 4C). PAK1 depletion inhibited the accumulation of p-LIMK and p-p41-ARC in the context of RHOJ overexpression (Figure 4C), indicating that RHOJ activates PAK1 in order to phosphorylate these downstream targets. Once activated by PAK1, LIMK can then phosphorylate cofilin on serine 3, which inactivates its filament-severing activity (Edwards et al., 1999). As expected, RHOJ overexpression induced the accumulation of phospho-serine 3-cofilin in A375 melanoma cells, while RHOJ depletion in vitro (Figure 4D) or in vivo (Figure 4E) suppressed cofilin phosphorylation. In addition, we observed that RHOJ overexpression induces the phosphorylation of cofilin in a PAK1-dependent manner (Figure 4C). Additional studies indicated that RHOJ/PAK1 also controls the activation of the Arp2/3 complex: RhoJ depletion in vitro (Figure 4D) or in vivo (Figure 4E) suppressed p41-ARC phosphorylation. Taken together, these results indicate that PAK1 modulates actin cytoskeletal dynamics by phosphorylating specific downstream targets.
Discussion
Melanoma cells acquire the ability to invade adjacent tissues and resist agents that induce DNA damage (chemotherapy or radiotherapy) early during their evolution (Gaggioli and Sahai, 2007; Soengas and Lowe, 2003), making these tumors very aggressive and treatment resistant. Our recently published work determined that RhoJ activates its downstream kinase PAK1 in response to DNA damage (Ho et al., 2012). Functional studies have revealed that CDC42 family GTPases, which include Rac1 and RhoJ, can be mutated in melanoma (Hodis et al., 2012; Krauthammer et al., 2012) and regulate melanoma morphology and invasion (Calvo et al., 2011; Sanz-Moreno et al., 2008). In this study, we examined whether RHOJ and its downstream kinase PAK1 regulate melanoma migration and invasion. Initial studies revealed that RHOJ modulates cell shape, cellular migration, and cellular invasion in vitro. Additional studies determined that RhoJ modulates these phenotypes via activation of PAK1, consistent with previous observations that PAK1 induces invasion and metastasis in multiple types of cancer cells by stimulating cell motility (Kamai et al., 2010; Kumar et al., 2006; Li et al., 2010, 2012; Ong et al., 2011; Rettig et al., 2012). Additional studies revealed that PAK1 modulates cellular invasion by phosphorylating LIMK and p41-ARC, leading to enhanced cellular protrusion (Delorme et al., 2007), invadopodium formation (Oser et al., 2009; Wang et al., 2007), and tumor cell migration (Quintela-Fandino et al., 2010; Vadlamudi et al., 2004; see model Figure 5). Other studies have identified new PAK inhibitors and determined that these inhibitors can inhibit the growth of melanoma xenografts (Murray et al., 2010). Taken together, these studies forward the novel concept that melanoma invasion and chemoresistance are coordinately regulated and identify PAK inhibitors as agents that could both sensitize melanoma tumors to DNA damage agents and also inhibit melanoma invasion and metastasis.
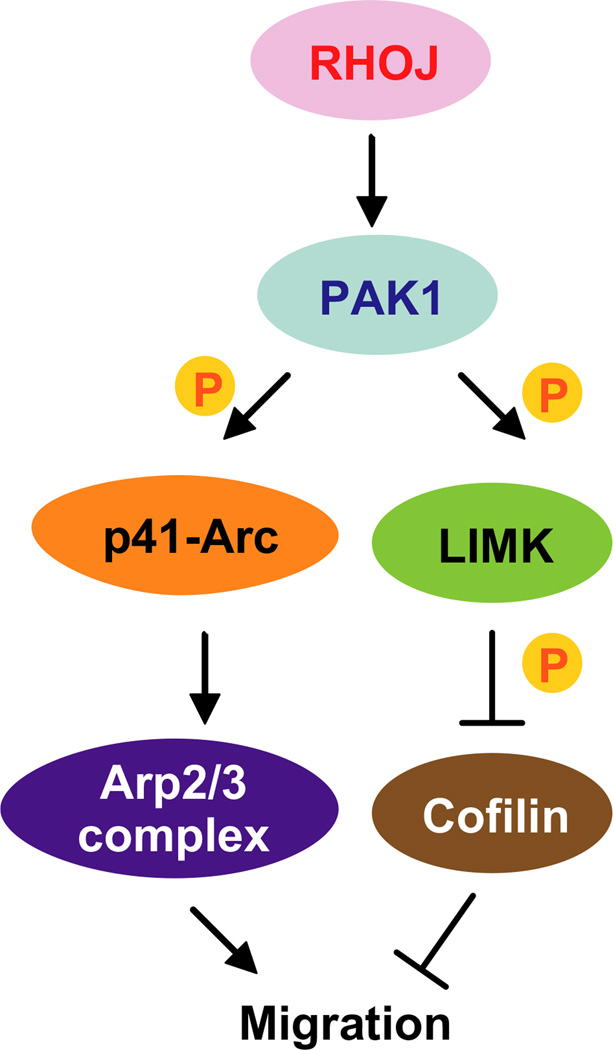
Figure 5.
RhoJ Modulates Melanoma Invasion by altering actin cytoskeletal dynamics. RhoJ regulates melanoma migration and invasion by activating PAK1. PAK1 then phosphorylates p-41-Arc and LIMK, which both promote melanoma migration by modulating actin cytoskeletal dynamics.
Methods
Materials
MNT-1 (from M. Marks, University of Pennsylvania) and A375 (from ATCC) melanoma cells were cultured in DMEM medium with 10% fetal bovine serum. C8161 (from Frank Meyskens, University of California, Irvine) and SK-Mel-28 (from ATCC) melanoma cells were cultured in RPMI medium with 10% fetal bovine serum. Darkly pigmented normal human foreskin melanocytes were purchased from Cascade Biologics (Portland, OR) and cultured as recommended by the manufacturer. Cell line verification was performed by Powerplex genotyping before use. IPA-3 was purchased from Tocris Biosciences (Minneapolis, MN).
Antibodies
α-/β-Tubulin, cofilin (D3F9), phospho-ser3-cofilin (77G2), LIMK2 (8C11), phosphor-LIMK1(Thr508)/LIMK2(Thr505), PAK1 and Phospho-PAK1,3 (Ser199/204)/PAK2 (Ser192/197) were purchased from Cell Signaling Technology (Danvers, MA). P41-Arc and phosphor- Thr21-p41-Arc were purchased from ECM Biosciences. RhoJ (M01) antibody was from Abnova. Anti-MLC2 was purchased from Cell Signaling Technology. Anti-P-R-MLC was from ECM Biosciences.
RNAi
Control- or RhoJ shRNA (pLKO.1 backbone vector; Open Biosystem (Huntsville, Alabama)-expressing C8161, A375, MNT-1 cells and melanocytes were established using low-titer lentiviral transduction protocols (Ho et al., 2012). RhoJ- or vector-overexpressing (pEz-Lv105 backbone vector; Genecopoeia) SK-Mel-28, A375, MNT-1, C8161 cells were also established using low-titer lentiviral transduction protocols (Ho et al., 2012). For all siRNA experiments, A375 or MNT-1 cells were transfected with 50 nM of the indicated ON-TARGETplus SMARTpool siRNAs (Thermo scientific) using Dharmafect3 and Dharmafect2, respectively, as described in the manufacturer’s instruction.
Actin staining and image quantification
Melanocytes expressing the indicated shRNAs were trypsinized and seeded on cover slides for 2 weeks before fixation. Similarly, C8161 or SK-Mel-28 cells expressing the indicated shRNAs were seeded on cover slides 24 h before fixation. MNT-1 cells were transfected with the indicated siRNAs for 72 h on cover slides before fixation. After fixation in paraformaldehyde, cells were permeabilized with 0.1% Triton X-100, followed by blocking with 1% BSA and 0.1% Tween 10 for 1 h. Coverslips were then incubated with 165 nM rhodamine phalloidin in the same buffer for 1 h. After three washes, the fixed cells were counterstained with DAPI and images were acquired using a LSM-510 meta confocal multiphoton microscope Zeiss (Thornwood, NY). Maximum cell length (longest distance between the two ends of a cell) was measured using ImageJ.
Migration and invasion assays
We purchased Boyden chambers (polycarbonate membrane, Cytoselect 24-well Migration assay) and collagen I invasion chambers (Collagen I-coated, Cytoselect 24-well Migration assay) from Cell Biolabs (San Diego, CA). A million melanoma cells were starved in serum-free medium for 24 h before plating in serum-free medium into the top well of the respective chambers. The lower wells of the chambers were then filled with growth medium containing 10% serum. Twenty-four hours after plating, the cells remaining inside the chamber were removed using established protocols and the number of cells that migrated to the other side of the membrane was quantified (n = 4 for each experiment performed in triplicate). For the IPA-3 experiments, melanoma cells were first incubated with 3.5 µg/ml mitomycin C for 2 h before seeding with indicated concentrations of IPA-3. For each scratch assay, 4 million RHOJ- or vector-overexpressing A375 melanoma cells were plated in six-well plates and treated with 3.5 µg/ml mitomycin C for 2 h. Horizontal scrapes were made, and cell surfaces were rinsed and refreshed with growth medium. Images were taken right after the scratch (0 h) and 24 h later using a Nikon-Ti (Melville, NY) microscope, and the area occupied by the invading cells was measured using imageJ and normalized to the respective control.
Melanoma xenograft mouse model
Two million RHOJ-shRNA- or control-shRNA-expressing A375 melanoma cells were subcutaneously injected to J:NU nude mice on their bilateral flanks. Tumor sizes were measured using calipers every 2–3 days (n = 8), and each tumor’s weight was measured 38 days after injection. Brachial lymph nodes from the same mice above were collected, stained with human melanoma marker HMB45, and counterstained with hematoxylin.
Immunohistochemistry
Tumor xenografts or draining lymph nodes from tumor xenografts were fixed in 10% formalin at 4°C for 48 h, then were dehydrated by 25, 50, 75 and 80% ethanol sequentially followed by paraffin embedding. Eight-micrometer sections were cut from paraffin blocks and were adhered to glass slides. Paraffin was removed with xylene, and sections were rehydrated and autoclaved at 100°C for 10 min in Target Retrival Solution (Dako (Carpinteria, CA)). Sections were then treated with Dual Enzyme Block (Dako), permeabilized with 0.2% Triton X-100 for 5 min, blocked by Protein Block (Dako) for 30 min, and treated with primary antibody (RhoJ, HMB45, p-cofilin, or p-p41-Arc at 1:100 dilution). Slides were then washed three times in PBS, treated with biotinylated secondary antibodies (1:500; Vector Inc. (Burlingame, CA)) for 30 min, treated with ABC–streptavidin–peroxidase for 30 min, and developed with DAB for 30 s. Slides were then counterstained with 1:5 hematoxylin and dehydrated before mounting.
Immunoblotting
Vector- or RhoJ-overexpressing cells were transfected with indicated siRNA for 72 h and were lysed in sample buffer (Bioland Inc. (Paramount, CA)) containing proteases and a phosphatase inhibitors cocktail (Thermo Scientific). Lysates were then subjected to SDS-PAGE, transferred to PVDF membranes, followed by incubation with the indicated primary antibody followed by incubation with the appropriate HRP-secondary antibody as previously described (Ho et al., 2012).
Supplementary Material
Significance.
Melanoma cells metastasize at an early stage of tumor development and are profoundly resistant to chemotherapy, radiotherapy, or immunotherapy, making these tumors particularly difficult to treat. In this study, we investigated whether melanoma invasion and chemoresistance are coordinately regulated. RHOJ and its downstream kinase PAK1 regulate melanoma chemoresistance by suppressing DNA damage sensing. In this study, we determined that RHOJ and PAK1 also regulate melanoma migration by controlling actin cytoskeletal dynamics. Taken together, these studies nominate RHOJ/PAK1 as ideal targets for the rational design of melanoma therapeutics because they are critical regulators of both invasion and chemoresistance.
Acknowledgements
This work was supported by the NIH (1K08AR056001 to AKG), the UC Cancer Research Coordinating Committee, and the American Cancer Society (121540-RSG-11-128-01-CSM to AKG). The microscopy studies were supported by P41-RR01192. This work was also partially supported by UL1 RR031985 from the National Center for Research Resources (NCRR) and by P30CA062203 from the NCI. Amelia Hopkin is supported by an NCI training grant (5T32CA009054-34).
Footnotes
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Confirmation of RHOJ overexpression and depletion.
Figure S2. IPA-3 when coupled with mitomycin C does not impact cellular proliferation.
Figure S3. (A) Validation of phospho-specific antibodies. Lysates from A375 melanoma cells were incubated in the presence and absence of calf intestine phosphatase (CIP) 1 unit/µg protein at 37°C for 1 h according to the CIP manufacturer’s protocol (New England Biolab). Lysates were then subjected to SDS-PAGE, followed by immunoblotting with the indicated phospho-specific antibodies that specifically recognize the respective PAK1 phosphorylation sites. Note that the antibodies specifically recognize the samples that were not treated with CIP. (B) RHOJ overexpression does not alter the phosphorylation of regulatory myosin light chain. A375 melanoma cells overexpressing RHOJ or control vector were transfected with PAK1 siRNA for 72 h. Cell lysates were prepared and subjected to immunoblotting with the indicated antibodies.
References
- Arozarena I, Sanchez-Laorden B, Packer L, Hidalgo-Carcedo C, Hayward R, Viros A, Sahai E, Marais R. Oncogenic BRAF induces melanoma cell invasion by downregulating the cGMP-specific phosphodiesterase PDE5A. Cancer Cell. 2011;19:45–57. doi: 10.1016/j.ccr.2010.10.029. [DOI] [PubMed] [Google Scholar]
- Berger MF, Hodis E, Heffernan TP, et al. Melanoma genome sequencing reveals frequent PREX2 mutations. Nature. 2012;485:502–506. doi: 10.1038/nature11071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo F, Sanz-Moreno V, Agudo-Ibanez L, Wallberg F, Sahai E, Marshall CJ, Crespo P. RasGRF suppresses Cdc42-mediated tumour cell movement, cytoskeletal dynamics and transformation. Nat. Cell Biol. 2011;13:819–826. doi: 10.1038/ncb2271. [DOI] [PubMed] [Google Scholar]
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coniglio SJ, Zavarella S, Symons MH. Pak1 and Pak2 mediate tumor cell invasion through distinct signaling mechanisms. Mol. Cell. Biol. 2008;28:4162–4172. doi: 10.1128/MCB.01532-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- Delorme V, Machacek M, Dermardirossian C, Anderson KL, Wittmann T, Hanein D, Waterman-Storer C, Danuser G, Bokoch GM. Cofilin activity downstream of Pak1 regulates cell protrusion efficiency by organizing lamellipodium and lamella actin networks. Dev. Cell. 2007;13:646–662. doi: 10.1016/j.devcel.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme-Walker VD, Peterson JR, Chernoff J, Waterman CM, Danuser G, Dermardirossian C, Bokoch GM. Pak1 regulates focal adhesion strength, myosin IIA distribution, and actin dynamics to optimize cell migration. J. Cell Biol. 2011;193:1289–1303. doi: 10.1083/jcb.201010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dummler B, Ohshiro K, Kumar R, Field J. Pak protein kinases and their role in cancer. Cancer Metastasis Rev. 2009;28:51–63. doi: 10.1007/s10555-008-9168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DC, Sanders LC, Bokoch GM, Gill GN. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat. Cell Biol. 1999;1:253–259. doi: 10.1038/12963. [DOI] [PubMed] [Google Scholar]
- Estecha A, Sanchez-Martin L, Puig-Kroger A, Bartolome RA, Teixido J, Samaniego R, Sanchez-Mateos P. Moesin orchestrates cortical polarity of melanoma tumour cells to initiate 3D invasion. J. Cell Sci. 2009;122:3492–3501. doi: 10.1242/jcs.053157. [DOI] [PubMed] [Google Scholar]
- Gaggioli C, Sahai E. Melanoma invasion – current knowledge and future directions. Pigment Cell Res. 2007;20:161–172. doi: 10.1111/j.1600-0749.2007.00378.x. [DOI] [PubMed] [Google Scholar]
- Goetz JG, Minguet S, Navarro-Lerida I, et al. Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. Cell. 2011;146:148–163. doi: 10.1016/j.cell.2011.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho H, Aruri J, Kapadia R, Mehr H, White MA, Ganesan AK. RhoJ and Pak kinases regulate melanoma chemoresistance by suppressing pathways that sense DNA damage. Cancer Res. 2012;72:5516–5528. doi: 10.1158/0008-5472.CAN-12-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodis E, Watson IR, Kryukov GV, et al. A landscape of driver mutations in melanoma. Cell. 2012;150:251–263. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamai T, Shirataki H, Nakanishi K, Furuya N, Kambara T, Abe H, Oyama T, Yoshida K. Increased Rac1 activity and Pak1 overexpression are associated with lymphovascular invasion and lymph node metastasis of upper urinary tract cancer. BMC Cancer. 2010;10:164. doi: 10.1186/1471-2407-10-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katiyar P, Aplin AE. FOXD3 regulates migration properties and Rnd3 expression in melanoma cells. Mol. Cancer Res. 2011;9:545–552. doi: 10.1158/1541-7786.MCR-10-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolyada AY, Riley KN, Herman IM. Rho GTPase signaling modulates cell shape and contractile phenotype in an isoactin-specific manner. Am. J. Physiol. Cell Physiol. 2003;285:C1116–C1121. doi: 10.1152/ajpcell.00177.2003. [DOI] [PubMed] [Google Scholar]
- Krauthammer M, Kong Y, Ha BH, et al. Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nat. Genet. 2012;44(9):1006–1014. doi: 10.1038/ng.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Gururaj AE, Barnes CJ. p21-activated kinases in cancer. Nat. Rev. Cancer. 2006;6:459–471. doi: 10.1038/nrc1892. [DOI] [PubMed] [Google Scholar]
- Li LH, Zheng MH, Luo Q, Ye Q, Feng B, Lu AG, Wang ML, Chen XH, Su LP, Liu BY. P21-activated protein kinase 1 induces colorectal cancer metastasis involving ERK activation and phosphorylation of FAK at Ser-910. Int. J. Oncol. 2010;37:951–962. [PubMed] [Google Scholar]
- Li LH, Luo Q, Zheng MH, Pan C, Wu GY, Lu YZ, Feng B, Chen XH, Liu BY. P21-activated protein kinase 1 is overexpressed in gastric cancer and induces cancer metastasis. Oncol. Rep. 2012;27:1435–1442. doi: 10.3892/or.2012.1664. [DOI] [PubMed] [Google Scholar]
- Liebig T, Erasmus J, Kalaji R, Davies D, Loirand G, Ridley A, Braga VM. RhoE Is required for keratinocyte differentiation and stratification. Mol. Biol. Cell. 2009;20:452–463. doi: 10.1091/mbc.E07-11-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay CR, Lawn S, Campbell AD, et al. P-Rex1 is required for efficient melanoblast migration and melanoma metastasis. Nat. Commun. 2011;2:555. doi: 10.1038/ncomms1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray BW, Guo C, Piraino J, et al. Small-molecule p21-activated kinase inhibitor PF-3758309 is a potent inhibitor of oncogenic signaling and tumor growth. Proc. Natl Acad. Sci. U.S.A. 2010;107:9446–9451. doi: 10.1073/pnas.0911863107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong CC, Jubb AM, Zhou W, Haverty PM, Harris AL, Belvin M, Friedman LS, Koeppen H, Hoeflich KP. p21-activated kinase 1: PAK’ed with potential. Oncotarget. 2011;2:491–496. doi: 10.18632/oncotarget.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oser M, Yamaguchi H, Mader CC, Bravo-Cordero JJ, Arias M, Chen X, Desmarais V, Van Rheenen J, Koleske AJ, Condeelis J. Cortactin regulates cofilin and N-WASp activities to control the stages of invadopodium assembly and maturation. J. Cell Biol. 2009;186:571–587. doi: 10.1083/jcb.200812176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani H, Yoshioka K, Nishikawa H, Inagaki C, Nakamura T. Involvement of protein kinase C and RhoA in protease-activated receptor 1-mediated F-actin reorganization and cell growth in rat cardiomyocytes. J. Pharmacol. Sci. 2011;115:135–143. doi: 10.1254/jphs.10197fp. [DOI] [PubMed] [Google Scholar]
- Quintela-Fandino M, Arpaia E, Brenner D, et al. HUNK suppresses metastasis of basal type breast cancers by disrupting the interaction between PP2A and cofilin-1. Proc. Natl Acad. Sci. U.S.A. 2010;107:2622–2627. doi: 10.1073/pnas.0914492107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettig M, Trinidad K, Pezeshkpour G, Frost P, Sharma S, Moatamed F, Tamanoi F, Mortazavi F. PAK1 kinase promotes cell motility and invasiveness through CRK-II serine phosphorylation in non-small cell lung cancer cells. PLoS ONE. 2012;7:e42012. doi: 10.1371/journal.pone.0042012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz-Moreno V, Gadea G, Ahn J, Paterson H, Marra P, Pinner S, Sahai E, Marshall CJ. Rac activation and inactivation control plasticity of tumor cell movement. Cell. 2008;135:510–523. doi: 10.1016/j.cell.2008.09.043. [DOI] [PubMed] [Google Scholar]
- Scott KL, Nogueira C, Heffernan TP, et al. Proinvasion metastasis drivers in early-stage melanoma are oncogenes. Cancer Cell. 2011;20:92–103. doi: 10.1016/j.ccr.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodek KL, Brown TJ, Ringuette MJ. Collagen I but not Matrigel matrices provide an MMP-dependent barrier to ovarian cancer cell penetration. BMC Cancer. 2008;8:223. doi: 10.1186/1471-2407-8-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soengas MS, Lowe SW. Apoptosis and melanoma chemoresistance. Oncogene. 2003;22:3138–3151. doi: 10.1038/sj.onc.1206454. [DOI] [PubMed] [Google Scholar]
- Thirone AC, Speight P, Zulys M, Rotstein OD, Szaszi K, Pedersen SF, Kapus A. Hyperosmotic stress induces Rho/Rho kinase/LIM kinase-mediated cofilin phosphorylation in tubular cells: key role in the osmotically triggered F-actin response. Am. J. Physiol. Cell Physiol. 2009;296:C463–C475. doi: 10.1152/ajpcell.00467.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadlamudi RK, Li F, Barnes CJ, Bagheri-Yarmand R, Kumar R. p41-Arc subunit of human Arp2/3 complex is a p21-activated kinase-1-interacting substrate. EMBO Rep. 2004;5:154–160. doi: 10.1038/sj.embor.7400079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignal E, De Toledo M, Comunale F, Ladopoulou A, Gauthier-Rouviere C, Blangy A, Fort P. Characterization of TCL, a new GTPase of the rho family related to TC10 and Ccdc42. J. Biol. Chem. 2000;275:36457–36464. doi: 10.1074/jbc.M003487200. [DOI] [PubMed] [Google Scholar]
- Wang W, Eddy R, Condeelis J. The cofilin pathway in breast cancer invasion and metastasis. Nat. Rev. Cancer. 2007;7:429–440. doi: 10.1038/nrc2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.