Abstract
Variation in environmental factors such as day length and social context greatly affects reproductive behavior and the brain areas that regulate these behaviors. One such behavior is song in songbirds, which males use to attract a mate during the breeding season. In these species the absence of a potential mate leads to an increase in the number of songs produced, while the presence of a mate greatly diminishes singing. Interestingly, although long days promote song behavior, producing song itself can promote the incorporation of new neurons in brain regions controlling song output. Social context can also affect such neuroplasticity in these song control nuclei. The goal of the present study was to investigate in canaries (Serinus canaria), a songbird species, how photoperiod and social context affect song and the incorporation of new neurons, as measured by the microtubule-associated protein, doublecortin (DCX), in HVC, a key vocal production brain region of the song control system. We show that long days increased HVC size and singing activity. In addition, male canaries paired with a female for two weeks showed enhanced DCX-immunoreactivity in HVC relative to birds housed alone. Strikingly, however, paired males sang fewer songs that exhibited a reduction in acoustic features such as song complexity and energy, compared to birds housed alone, which sang prolifically. These results show that social presence plays a significant role in the regulation of neural and behavioral plasticity in songbirds and can exert these effects in opposition to what might be expected based on activity-induced neurogenesis.
Keywords: neurogenesis, songbird, canary, plasticity
Introduction
The social environment causes robust changes in behavior (Cacioppo et al, 2000; Catchpole and Slater, 2003; Conaway, 1971; Lehrman et al,, 1961). For instance, in many species male songbirds sing more songs when in the absence of a potential mate as compared to when the female is present (Orr & Hansell, 1975; Cuthill, 1985; Sockman et al., 2005; Carere et al., 2006; Alward et al., 2013). This suggests that once a male attracts a mate, it is economically favorable to reduce courtship singing (Sockman et al, 2005).
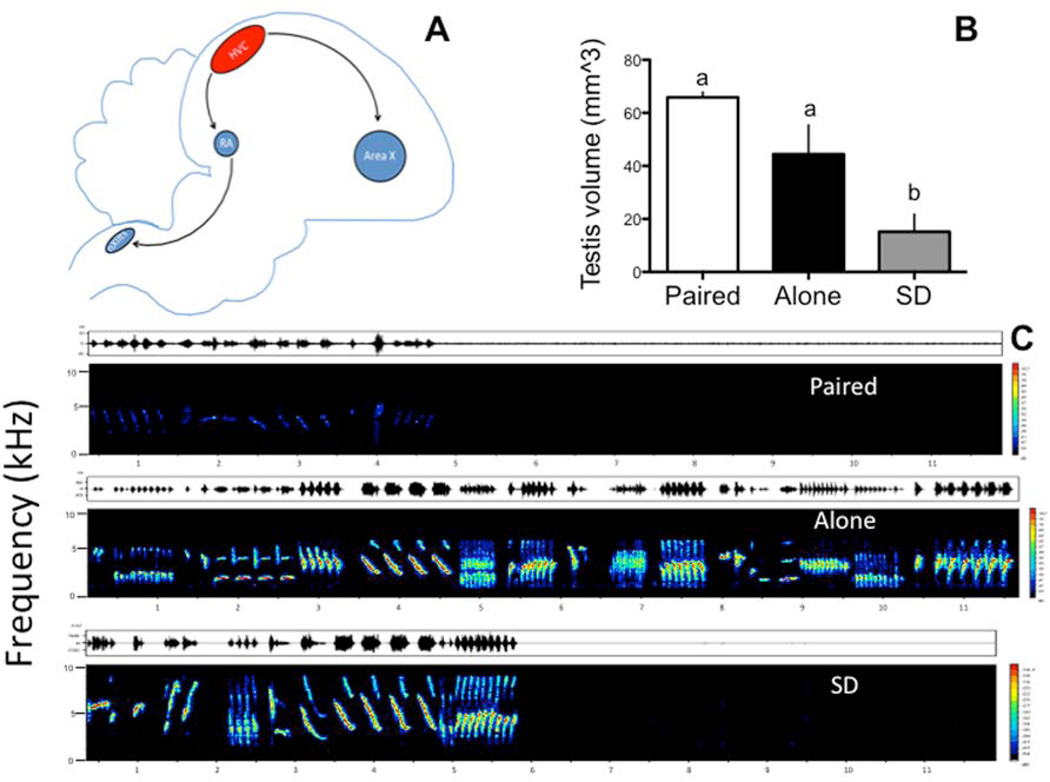
Songbirds possess an interconnected system of brain nuclei that orchestrate the learning and production of song (Figure 1A; reviewed in Nottebohm, 1996; Bottjer & Johnson, 1997; Brenowitz et al., 1997; Fee & Scharff, 2010). The sensorimotor nucleus HVC projects to the robust nucleus of the arcopallium (RA), which is part of the pathway required for song production. HVC also projects to Area X (in the basal ganglia; Bottjer & Johnson, 1997; Fee & Scharff, 2010; Fee & Goldberg, 2011), which is part of the pathway required for song learning and auditory feedback. These nuclei show remarkable plasticity in response to seasonally changing testosterone (T) (Brenowitz & Lent, 2002; Nottebohm, 1981; Thompson, Bentley, & Brenowitz, 2007; Tramontin & Brenowitz, 2000; Ball, 1999). There is also evidence that singing activity (Alvarez-Borda & Nottebohm, 2002; Ball et al 2006; Larson et al., 2013; Nottebohm, 2002; Sartor & Ball, 2005), social cues (Boseret et al 2006; Tramontin et al 1999b), and photoperiod (Bernard et al 1997; Gulledge & Deviche, 1998) can contribute to this seasonal neuroplasticity in the song control system (SCS) independently of T. For example, Boseret and colleagues (2006) have shown that males housed with a female possess larger HVC with a larger volume relative to males housed with a male. Males housed with a female also had more cells in HVC labeled with doublecortin (DCX), a largely specific marker of new neurons during their first 30–40 days of post-mitotic life (Balthazart et al., 2008), as compared to those housed with a male, suggesting changes in the rate of neurogenesis. However, this experiment was not designed to allow one to determine whether the female partner enhanced or the male partner attenuated the incorporation of these new neurons (Balthazart et al., 2008). In canaries, singing per se also induces neurogenesis and enhances the size of the SCS nuclei (Alward et al 2013; Li et al., 2000) but observed changes in neurogenesis could not be driven by singing activity since in the previous experiments males housed with a female sang much less than males housed with another male (Boseret et al 2006).
Figure 1.
A) Schematic of HVC and its main synaptic targets in RA and Area X. B) Effects of treatment on breeding condition as measured via the volume of the testis. Bar graphs are means ± SEM. Bars with a different letter are significantly different for p≤0.05, bars with a same letter are not different. C). Representative songs from each treatment group.
In the present study, we took advantage of the variation in socially modified singing behavior and neuroplasticity in canaries to examine the relationship between photoperiod, social context, behavioral plasticity, and new neuron incorporation. Specifically, we employed behavioral and immunohistochemical techniques to evaluate the effects of photoperiod and social context on song output, acoustic complexity, and DCX-immunoreactivity (-ir) in HVC of male canaries.
Methods And Materials
Subjects
A total of fifteen male and five female border canaries were purchased from a local supplier in July 2010 when they were naturally photorefractory (based on the fact that they were experiencing the ambient photoperiod). They were brought into the laboratory and group housed on short day lengths (8L:16D; SD) for six weeks in cages (1m by 0.5m by 0.5m) in either male or female groups. This photoperiodic treatment has been shown to induce photosensitivity in photorefractory starlings and canaries (Dawson et al, 2001) Hurley et al., 2008). Food and water were provided ad libitum for the duration of the experiment. After two weeks in the lab, all birds were laparotomized under isoflurane (3–4% induction, then 1–2% maintenance) to confirm their sex and evaluate their gonadal state; all birds were observed to have regressed testes/oviduct. At the termination of each experiment, the body cavities were examined to confirm gonadal state. The protocols and procedures were approved by the Johns Hopkins University Animal Care and Use Committee and in accordance with the guidelines of the National Institutes of Health.
Experimental procedure
This experiment was performed with five stimulus females and fifteen males divided into three treatment groups. Two groups consisted of male canaries placed individually on long days (16L:8D; LD) in sound-attenuated testing chambers (0.94m × 0.56m × 0.56m) for two weeks to stimulate reproductive development (Dawson et al., 2001; Hurley et al., 2008) while the other group remained on SD. In one LD group, each male was paired with a female (Paired, n=5) on the day he was transferred to LD, and in another LD group, males were housed alone (Alone, n=5). The third group consisted of male birds housed alone, but kept on SD (n=5). Auditory recordings were collected during the first 2 hours of the morning period on days 1, 2, 3, 7, and 14 after the transfer of the birds to the sound-attenuated chambers. On the final day, the birds were euthanized with an overdose injection of secobarbital (60 mg/kg, i.m.) followed by rapid decapitation. The length and width of the largest testis was measured following decapitation to determine the breeding conditions of the birds. Testis volume was determined using the equation for an ellipsoid (oblate spheroid): V=4/3πa2b, where a is half the width and b is half the length (Stevenson & Ball, 2010).
Song analysis
Each isolation chamber was fitted with a microphone (BT-MP8087 Mini microphone from B&H) and camera (KPC-600 Pinhole Camera 3.6mm from B&H) that was connected to a computer running DVRserver (V6.33b; Mammoth Technologies, Austin, TX) designed for real-time full-motion video-capture and high-speed recording. Each day, the DVRserver captured the dawn singing behavior produced between the hours of 7:30 AM and 9:30 AM in .wav files sampled at 22050Hz which translated to a frequency range of 0 kHz to 11 kHz. Song files were run through a highpass filter set to a threshold of 900Hz to remove low frequency noise and converted to a digital format using Goldwavetm (Version 5.55; GoldWave, St. John’s, Newfoundland, Canada) before they were visualized into sound spectrograms using Avisoft (SASlab Pro, Berlin Germany), a Windows application for investigating animal acoustic communication by increasing the efficiency in extensive sound analysis projects. For the spectrograms, the fast Fourier transforms length was set to 512 with an overlap of 75% for the temporal resolution. The song parameters of interest for each bird were exported into Excel after being counted by Avisoft with automated parameter measurements. The parameters selected for song analysis were based on previously published work in canaries (Alward et al 2013; Voigt & Leitner, 2008) with slight modifications based on past observations in our laboratory. In brief, songs were defined as having a duration greater than one second of continuous notes with gaps no longer than half a second. Each song was verified by looking at the original sonograms to further eliminate noise that escaped the filter. For each bird the song measures included song rate (total number of songs divided by the total recording time; i.e., 10 hours) and the average length of songs.
In addition to the above measures, we were interested in particular features of song such as how loudly the bird sings and song complexity. Previous work suggests that the social context and breeding conditions can modulate acoustic features of song such as amplitude (Cynx & Gell, 2004). To quantify these features, we used Avisoft Sound Analysis Software to compute the following:
Energy: This is the sum of the squared amplitude of a song multiplied by its sampling time (volt2*second). It quantifies how loud or intense the song is.
Entropy variance: Entropy is a measure of the spectral width and uniformity of a signal. It is a pure number (i.e. unitless) with 0 being a pure tone (e.g., a uniform sinusoidal wave) and 1 being noise (i.e. a non-uniform random signal) (Tchernichovski et al., 2000). The variance of this measure collapsed across a single bout of singing is indicative of the diversity of vocalization types included. High entropy variance tends to indicate songs with a high degree vocal variability caused by the inclusion of transitions between multiple different syllable/phrase types in individual songs.
Brain extraction and sectioning
The brains were quickly extracted and fixed in 0.5% acrolein solution (9.5 ml 1M phosphate buffered saline, PBS, and 500 µl acrolein) for 2 hours at room temperature. The brains were washed four times for fifteen minutes each in 1M PBS and then transferred to 30% sucrose solution overnight. Once saturated, brains were flash frozen in powdered dry ice for 5 minutes, then placed at −80°C until brain sectioning. Brains were sectioned in the coronal plane by a single individual to ensure consistency in orientation of sections with a cryostat at 30 µm thickness into three series and we collected every section.
Doublecortin immunocytochemistry and quantification
We used a DCX immunocytochemistry protocol previously used in canary brains (Yamamura et al., 2011). Sections were washed in 0.01M PBS three times, once in 0.1% sodium borohydride in 0.01M PBS, and 3 times in 0.01M PBS with 1% Triton X (PBST). Endogenous peroxidases were blocked using 0.6% H2O2 in PBST for 20 minutes which was followed by three washes in PBST and additional blocking using 10% normal horse serum (NHS) in PBST for 30 minutes. Sections were incubated at 4°C in 2% NHS and PBST and primary antibody (1:5,000 Horse-anti goat, Doublecortin, Santa Cruz, cat#; sc-8066). Sections were washed three times in PBST, then incubated in avidin biotin horseradish-peroxidase complex (Vectastain ABC Elite Kit, 1:200) for 1 hour, and washed three times in PBST. The peroxidase was then visualized using diamniobenzidine (Sigma Fast DAB) for 5 minutes and sections were subsequently washed in 0.01M PBS and mounted onto gelatin-coated microscope slides. Slides were serially dehydrated in ethanol and placed in xylene for 10 min before being coverslipped using Permount (Fisher, Fair Lawn, NJ).
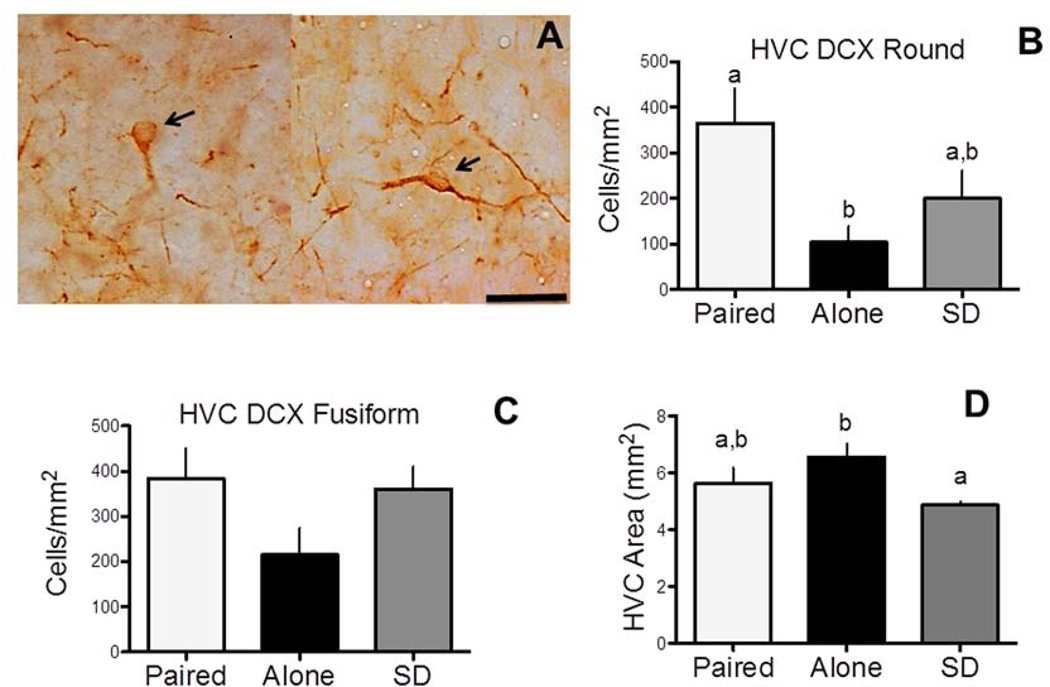
We counted the two different types of DCX-ir cells (Figure 3A)--round and fusiform--in HVC. The round cells are new neurons that have migrated to their final site and begun differentiating while the fusiform cells are still migrating and have not begun differentiating. The counting method was similar to that used by others (Balthazart et al., 2008; Yamamura et al., 2011). Specifically, DCX-ir cells were counted at three different rostro-caudal levels in three separate fields positioned in the center of HVC and in the adjacent nidopallium lateral and ventral to HVC (see Figure 1 in Balthazart et al., 2008). These three rostro-caudal levels of HVC were roughly equally spaced in the nucleus to provide an overall representation of DCX-ir cells in this structure (Balthazart et al., 2008) and they show significant levels of neuronal incorporation in adulthood (Kirn et al., 1999; Boseret et al., 2007; Balthazart et al., 2008; Yamamura et al., 2011). Immunoreactive cells were manually counted on images digitized through the microscope (20 × objective) in a standardized square area (200 × 200 µm) in each brain region of interest. The Cell Counter function of ImageJ software (version 1.40g; Wayne Rasband, National Institutes of Health) was used to identify DCX-ir cells, which were then classified as round or fusiform by a human observer. The area used for quantification was positioned within the structure of interest in a standard manner using clearly defined brain landmarks as previously described in detail (Balthazart et al., 2008; Yamamura et al., 2011). All immunoreactive cells that contained a clear unstained nucleus surrounded by stained cytoplasm were manually labeled. Cells were counted on one side of the brain that was randomly chosen. We also counted the two cell types in comparable brain regions, lateral and ventral to HVC, to confirm specificity of changes in HVC. Cells counts in each area (HVC, lateral or ventral to this nucleus) were added across the three rostro-caudal level (i.e., in three 200 X 200 µm fields or 3×0.04= 0.12 mm2) and expressed as numbers of cells per mm2.
Figure 3.
The effects of treatment on DCX immunoreactivity in HVC and on HVC area. A) Representative photomicrograph depicting DCX round (left panel) and fusiform (right panel) cells. Magnification bar=50 µm B) Effects of treatment on the number DCX round cells per mm2 in HVC. C) Effects of treatment on DCX fusiform cells per mm2 in HVC. D) Effects of treatment on HVC area at its largest extension. Bar graphs are means ± SEM. Bars with a different letter are significantly different for p≤0.05, bars with a same letter are not different.
Nissl staining and HVC and POM area reconstruction
One series of sections was used for a Nissl stain. To obtain an estimate of HVC size, we traced the borders of the HVC at its largest point using Image J (NIH) and computed the corresponding area. The focus of this paper is to assess the effects of social environment and photoperiod on new neuron incorporation as assessed by DCX immunoreactivity. However, we also thought an estimate of HVC size among the groups would be useful. Due to some damage to the dorsal surface of the Nissl-stained sections, we were not able to reliably assess total HVC volume but we could reliably assess HVC size using an area estimate at its largest extent. We also determined the size of the medial preoptic nucleus (POM) as a proxy for central exposure to testosterone (Riters et al., 2000; Charlier et al., 2008; Alward et al., 2013). We traced the borders of the POM at the level of the anterior commissure where it fully intersects with the occipitomesencephalicus (OM) tract and computed its area with the use of the Image J software. We confirmed consistency in the planes of sections of all brains by measuring the volume of the nucleus rotundus, a nucleus that has been shown not to change as a function of endogenous or exogenous changes, at the same rostro-caudal level as where the POM was measured. We confirmed that its size was not different across all treatment groups (One-way ANOVA, F(2,14)=0.1, p>0.9).
Statistical analyses
All data including the song parameters mentioned above, the number of DCX-ir round and fusiform cells, and the HVC areas were analyzed using one- or two-way ANOVAs. Tukey’s post-hoc analyses were used to make pairwise comparisons when appropriate. Differences were considered significant for p≤0.05.
Results
Effects of treatment on breeding physiology
There was a main effect of treatment on testis volume (F(2,14)=11.43; p=0.002; Figure 1B). Specifically SD males had smaller testis than Alone (p=0.001) and Paired (p=0.04) males. Alone and Paired males were not statistically different from one another (p=0.15). Moreover, there was a main effect of treatment on the area of the POM (F(2,14)=7.34; p<0.05), such that SD birds on average possessed smaller POM (1.73±0.09 mm2) than Paired (3.22 ±0.46 mm2) and Alone birds (2.84±0.23 mm2) (p<0.05 for both comparisons), which did not differ from one another (p>0.05). Therefore, our photoperiodic treatments were successful in inducing a physiological condition characteristic of a breeding (Paired and Alone) and non-breeding (SD) state. Additionally, the endocrine state of the two LD groups was not significantly different based on the volume of their testes and on the measure of their brain exposure to testosterone as reflected by the size of the POM.
Song rate and acoustic features
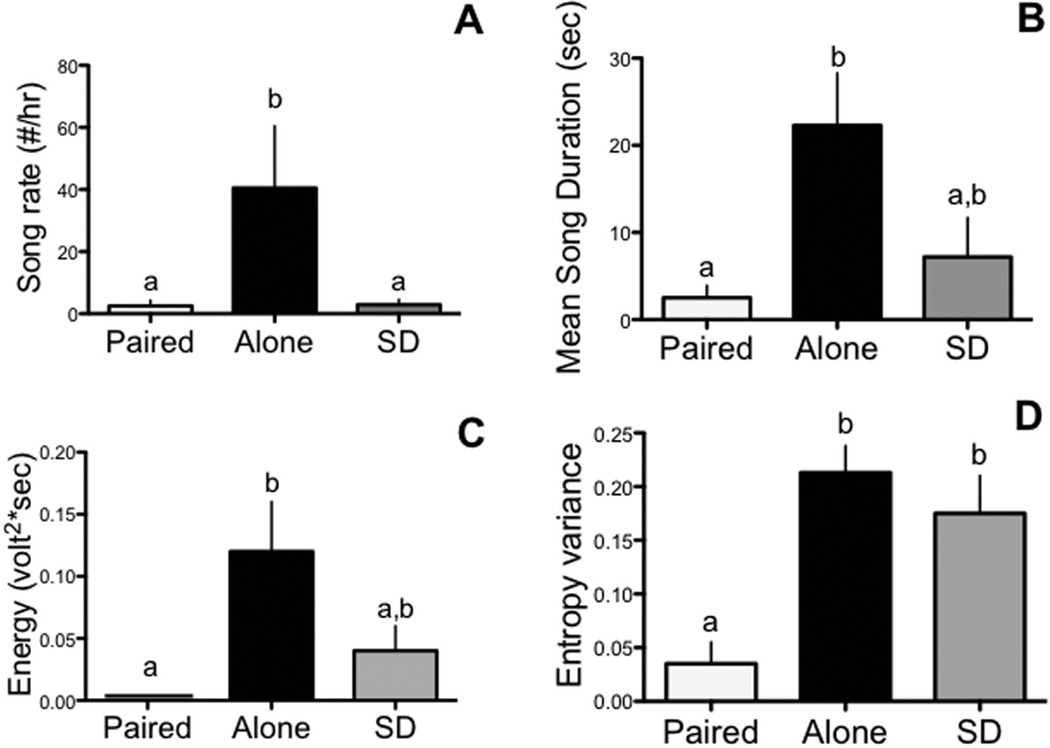
We first analyzed the song data by two-way ANOVAs with the three experimental groups as independent variable and the successive days as repeated variable and did not find a significant interaction between these two factors for all of the variables studied (p > 0.1 for all interaction components of the Omnibus ANOVAs). Based on the lack of statistical effects associated with these interactions, we focused our analyses on the main effects of treatments on all parameters for concision and clarity of data presentation. These data were thus analyzed by one-way ANOVAs focusing on the average/total measures across the entire experimental period. There was a significant effect of the different treatments on song rate (F(2,14) = 6.52; p < 0.01; Figure 2A). Males that were housed alone sang significantly more songs per hour as compared to SD (p < 0.05) and Paired (p < 0.05) birds. There was no significant difference in the song rate between Paired and SD (p >0.5). There was also a significant effect of treatments on the average song duration (F(2,14) = 7.25; p < 0.01; Figure 2B). Males that were housed alone sang significantly longer songs compared to SD (p < 0.01) and Paired (p < 0.05) birds and there was no significant difference in song duration between these last two groups (p = 0.65).
Figure 2.
Effects of treatment on song output (A. Song rate (number per hour); B. Mean song duration) and its acoustic features (C. Energy; D. Entropy variance, see text for additional explanations) in male canaries. Bar graphs are means ± SEM. Bars with a different letter are significantly different for p≤0.05, bars with a same letter are not different.
Similarly we also detected significant treatment effects on the entropy variance (F(2,14)=11.68; p<0.005, Figure 1C) and energy (F(2,14)=4.77; p<0.05, Figures 2C–D). Compared to paired birds, males housed alone sang with more energy (p<0.05) and with a higher entropy variance (p<0.005). Compared to SD birds, however, birds housed alone did not differ in any of these acoustic measures (p>0.17 for all paired comparisons). SD birds sang with more entropy variance (p<0.05) than paired birds but did not differ from them with respect to the energy of their songs (p>0.5).
HVC area
There was a significant effect of treatment on the area of HVC at its largest extension (F(2,14)=4.70, p<0.05; Figure 3D), such that the Alone birds possessed larger HVC than SD birds (p<0.05). Paired birds were indistinguishable from both Alone and SD birds.
DCX immunoreactive cells in HVC
We found a significant difference between groups in the number of DCX round cells in HVC (F(2,14) = 4.71, p< 0.05; Figure 3B). Male canaries that were paired with a female had significantly more DCX round cells compared to males housed alone (p< 0.05). There was no significant difference between paired and SD males (p=0.18) and between Alone and SD males (p=0.49). In contrast, we did not find a significant effect of treatments on the number of fusiform cells in HVC (F(2,14) = 2.36, p= 0.13; Figure 3C). There was also no effect of treatments on the number of round cells or fusiform cells in the region lateral (round: F(2,14) = 0.65, P = 0.54; fusiform: F(2,14) = 1.03, P = 0.38) or ventral (round: F(2,14) = 1.17, P = 0.34; fusiform: F(2,14) = 0.1, P = 0.91) to HVC.
Discussion
In this study, we assessed the effects of photoperiod and female presence on the neural and behavioral plasticity among males of a well-studied songbird species, the canary. We found that males housed alone in a breeding condition sing prolifically and with more acoustic complexity than males that were paired with a female. We also found that males housed with a female exhibited more DCX-ir round cells in HVC relative to birds on long days housed alone. These data suggest that the social context can regulate the incorporation of new neurons into HVC as well as modulate features of song that are used by males to attract a potential mate.
These observations regarding the effects of social context on singing behavior are in line with the well-established hypothesis that song is used by males to attract a potential mate (Catchpole and Slater, 2003; Kroodsma & Byers, 1991). Males that were housed alone and in a breeding condition sang a larger number of songs with a greater length than birds that already possessed a female, supporting the idea that once a male has attracted a mate, it is economically favorable to reduce this form of courtship behavior (Sockman et al., 2005). Previous studies have tended not to test specifically the effects of a female on changes in song complexity. It has of course been shown that females do prefer more complex songs in a number of songbird species (Leitão et al., 2006). Other studies, however, have indicated the importance of emphasizing song features such as loudness to convey efficiently this reproductive signal to its receivers (Brumm & Todt, 2002; Brumm, 2004, 2013; Brumm & Slater, 2006), including females (Cynx & Gell, 2004). Our results indicate that male songbirds in search of a mate may sing with more complexity amid a loud signal to further increase the probability that they will attract a mate.
SD birds were similar to paired long day birds with respect to the number of songs, their duration, and their loudness. The presence of the female thus completely suppressed these song characteristics to the level observed under short day conditions. However SD birds were indistinguishable from long day birds housed alone with respect song complexity. In canaries during the non-breeding season (i.e. between late summer and early winter) canaries are modifying their song by adding and deleting syllables until the song is eventually crystallized (Nottebohm, 1984; Voigt & Leitner, 2008). This must lead to increased variability in the song (i.e., increased entropy variance in the entire song) as birds are producing a variety of songs that they will use during the breeding season to attract a potential mate. Our entropy measure indeed quantifies variance in the entire song taking into account the changes from one syllable type to another. In the end, while long day birds housed alone and short day birds housed alone may be similar in this song measure, their reason for enhancing their song complexity may differ: in one case they are modifying their song before it eventually reaches its crystallized form (SD) while in the other they would be trying to attract a mate (Alone).
Previous work in canaries has shown that male canaries housed with a female exhibited enhanced HVC size relative to males housed with other males (Boseret et al., 2006). Here we found that males housed on long days with a female were not statistically different from birds housed alone on long days. It is, however, possible that, in the study by Boseret and colleagues (Boseret et al., 2006), males housed with females possessed larger HVC than those housed with a male because the presence of a male actually suppressed HVC size due to the stress associated with aggression, not because the presence of a female enhanced HVC size. Effects of stress and corticosterone on HVC size have indeed been observed in a number of studies in several songbird species (zebra finches: Buchanan et al, 2004; song sparrows: Newman et al, 2010).
Although the paired birds had the same HVC size as the alone groups, they had significantly more DCX-ir cells in HVC despite the fact that they sang much less. This replicates a finding of the Boseret and colleagues studies in which males paired with a female sang less but had more DCX-ir cells in HVC than males paired with another male (Boseret et al., 2006; Balthazart et al., 2008). These data lead to two important conclusions. Firstly they indicate that the size of HVC does not necessarily correlate with the number of DCX-ir cells. Such a correlation would be expected and was observed at some time points when the size of the nucleus and the rate of neurogenesis are dynamically changing (e.g. See Balthazart et al., 2008; Yamamura et al., 2011). It is also conceivable that changes in these two variables are not necessarily synchronous: after a given stimulation, the size may change later than the numbers of DCX-positive cells and this would obviously result at specific time points in discrepancies between these two variables as observed here. This prediction is in line with previous studies showing that the dramatic seasonal increase in the size of HVC does not occur until several days after the incorporation of new neurons (Nottebohm, 1984; Alvarez-Buylla et al., 1992; Kirn, 2010).
Secondly the present data demonstrate that the density of DCX-ir cells in HVC is controlled by a variety of factors acting in a semi-independent manner and including exposure to testosterone, singing activity and presence or absence of a female partner. Previous studies in canaries revealed that the production of song by itself leads to enhanced expression of trophic factors such as brain derived neurotrophic factor (BDNF) in HVC, which causes an increase in the incorporation of new neurons in this brain region (Li et al., 2000; Alvarez-Borda & Nottebohm, 2002). Therefore, one may have expected to observe a larger number of DCX-ir cells in birds from the alone group (who sang at a high rate) as compared to the paired birds (that were singing very rarely), but the opposite result was observed. It is important to note in this context that, regardless of the social context (Paired vs. Alone), birds housed on long days possessed similarly enlarged testis size and POM nuclei of similar sizes, suggesting that both were in a similar breeding condition and exposed to similarly high circulating T concentrations. It is therefore unlikely (although cannot be completely excluded) that differences in circulating T led to the observed differences in DCX-ir cells, especially given that HVC size (a T-dependent variable) was equal in both the Paired and Alone groups. The large number of DCX-ir cells in the Paired group thus seems to relate specifically to the presence of the female independently of the singing activity and of the endocrine (plasma T) condition. It remains nevertheless possible that long day birds that were housed alone may have experienced some form of stress, which could have reduced the number of DCX-immunoreactive round cells in HVC (see above and Buchanan et al, 2004; Newman et al, 2010).
Overall, the results of this study support the importance of one of the well known proposed functions of birdsong as applying to canaries, mate attraction, and suggest that male songbirds emphasize specific features of song to accomplish this. These data also suggest that the social context may have a very substantial effect on neurogenesis, and in some contexts at least possibly more important than the production of song. This increased number of round DCX-ir neurons present in HVC at 14 days after the placement in experimental conditions provides an integrated view of the dynamic changes in neurogenesis that took place during this period. It does not however allow us to determine with certainty whether these changes are linked to modifications in cell proliferation, migration, recruitment, differentiation and survival. It provides a result that reflects the sum of these measures. By counting separately the fusiform (presumably still migrating) and round (currently differentiating) DCX-ir cells we obtain, however, a view of two cell populations that are at different stages of their ontogeny and were thus in all likelihood born at different times before brain collection. This suggests that the presence of a female rapidly affected neurogenesis so that 14 days later, there was an effect on cells that has already been recruited and had initiated their differentiation in HVC. Future studies combining BrdU injections and DCX immunohistochemistry should now be performed to dissect the interesting phenomenon.
The adaptive consequence of neurogenesis in HVC in paired birds remains to be investigated, but the present results suggest that if paired birds do sing, such as during later stages of the reproductive cycle (Hinde & Matthews, 1957; Hinde & Steel, 1976; Alward et al., 2013), they will have an increased capacity for producing attractive, high quality song (Leitner & Catchpole, 2004).
Acknowledgements
This work was supported by an NIH/NINDS RO1 35467 to GFB and an Interuniversity Attraction Pole (IAP) grant number SSTC PAI P7/17 from the Belgian Science Policy Office (BELSPO) to JB and GFB. TJS was supported by NSERC-PGSD 334570.
Footnotes
Disclosure statement: The authors have nothing to disclose.
References
- Alvarez-Borda B, Nottebohm F. Gonads and singing play separate, additive roles in new neuron recruitment in adult canary brain. J. Neurosci. 2002;22:8684–8690. doi: 10.1523/JNEUROSCI.22-19-08684.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla a, Ling CY, Nottebohm F. High vocal center growth and its relation to neurogenesis, neuronal replacement and song acquisition in juvenile canaries. J. Neurobiol. 1992;23:396–406. doi: 10.1002/neu.480230406. [DOI] [PubMed] [Google Scholar]
- Alward BA, Balthazart J, Ball GF. Differential effects of global versus local testosterone on singing behavior and its underlying neural substrate. Proc. Natl. Acad. Sci. U. S. A. 2013;110:19573–19578. doi: 10.1073/pnas.1311371110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball GF, Auger CJ, Bernard DJ, Charlier TD, Sartor JJ, Riters LV, Balthazart J. Seasonal plasticity in the song control system: multiple brain sites of steroid hormone action and the importance of variation in song behavior. Ann. N. Y. Acad. Sci. 2006;1016:586–610. doi: 10.1196/annals.1298.043. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Boseret G, Konkle ATM, Hurley LL, Ball GF. Doublecortin as a marker of adult neuroplasticity in the canary song control nucleus HVC. Eur. J. Neurosci. 2008;27:801–817. doi: 10.1111/j.1460-9568.2008.06059.x. [DOI] [PubMed] [Google Scholar]
- Bernard DJ, Wilson FE, Ball GF. Testis-dependent and -independent effects of photoperiod on volumes of song control nuclei in American tree sparrows (Spizella arborea) Brain Res. 1997;760:163–169. doi: 10.1016/s0006-8993(97)00277-1. [DOI] [PubMed] [Google Scholar]
- Boseret G, Ball GF, Balthazart J. The microtubule-associated protein doublecortin is broadly expressed in the telencephalon of adult canaries. J. Chem. Neuroanat. 2007;33:140–154. doi: 10.1016/j.jchemneu.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottjer SW, Johnson F. Circuits, hormones, and learning: vocal behavior in songbirds. J. Neurobiol. 1997;33:602–618. doi: 10.1002/(sici)1097-4695(19971105)33:5<602::aid-neu8>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Brenowitz Ea, Lent K. Act locally and think globally: intracerebral testosterone implants induce seasonal-like growth of adult avian song control circuits. Proc. Natl. Acad. Sci. U. S. A. 2002;99:12421–12426. doi: 10.1073/pnas.192308799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz Ea, Margoliash D, Nordeen KW. An introduction to birdsong and the avian song system. J. Neurobiol. 1997;33:495–500. [PubMed] [Google Scholar]
- Brumm H. Male-male vocal interactions and the adjustment of song amplitude in a territorial bird. Anim. Behav. 2004;67:281–286. [Google Scholar]
- Brumm H. The impact of environmental noise on song amplitude in a territorial bird. J. Anim. Ecol. 2013;73:434–440. [Google Scholar]
- Brumm H, Slater PJB. Animals can vary signal amplitude with receiver distance: evidence from zebra finch song. Anim. Behav. 2006;72:699–705. [Google Scholar]
- Brumm H, Todt D. Noise-dependent song amplitude regulation in a territorial songbird. Anim. Behav. 2002;63:891–897. [Google Scholar]
- Buchanan KL, Leitner S, Spencer KA, Goldsmith AR, Catchpole CK. Developmental stress selectively affects the song control nucleus HVC in the zebra finch. Proc. Biol. Sci. 2004;271:2381–2386. doi: 10.1098/rspb.2004.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG, Sheridan JF, McClintock MK. Multilevel integrative analyses of human behavior: social neuroscience and the complementing nature of social and biological approaches. Psychol. Bull. 2000;126:829–843. doi: 10.1037/0033-2909.126.6.829. [DOI] [PubMed] [Google Scholar]
- Boseret G, Carere C, Ball GF, Balthazart J. Social context affects testosterone-induced singing and the volume of song control nuclei in male canaries (Serinus canaria) J. Neurobiol. 2006;66:1044–1060. doi: 10.1002/neu.20268. [DOI] [PubMed] [Google Scholar]
- Catchpole CK, S PJ. Bird Song: Biological Themes and Variations. Cambridge, UK: Cambridge University Press; 2003. [Google Scholar]
- Charlier TD, Ball GF, Balthazart J. Rapid action on neuroplasticity precedes behavioral activation by testosterone. Horm. Behav. 2008;54:488–495. doi: 10.1016/j.yhbeh.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conaway CH. Ecological adaptation and mammalian reproduction. Biol. Reprod. 1971;4:239–247. doi: 10.1093/biolreprod/4.3.239. [DOI] [PubMed] [Google Scholar]
- Cuthill IHA. Increase in starling song activity with removal of mate. J. Exp. Biol. 1985;33:326–335. [Google Scholar]
- Cynx J, Gell C. Social mediation of vocal amplitude in a songbird, Taeniopygia guttata. Anim. Behav. 2004;67:451–455. [Google Scholar]
- Dawson A, King VM, Bentley GE, Ball GF. Photoperiodic control of seasonality in birds. J. Biol. Rhythms. 2001;16:365–380. doi: 10.1177/074873001129002079. [DOI] [PubMed] [Google Scholar]
- Fee MS, Goldberg JH. A hypothesis for basal ganglia-dependent reinforcement learning in the songbird. Neuroscience. 2011;198:152–170. doi: 10.1016/j.neuroscience.2011.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fee MS, Scharff C. The songbird as a model for the generation and learning of complex sequential behaviors. ILAR J. 2010;51:362–377. doi: 10.1093/ilar.51.4.362. [DOI] [PubMed] [Google Scholar]
- Gulledge CC, Deviche P. Photoperiod and testosterone independently affect vocal control region volumes in adolescent male songbirds. J. Neurobiol. 1998;36:550–558. [PubMed] [Google Scholar]
- Hinde RA, Steel E. The effect of male song on an estrogen-dependent behavior pattern in the female canary (Serinus canarius) Horm. Behav. 1976;7:293–304. doi: 10.1016/0018-506x(76)90035-0. [DOI] [PubMed] [Google Scholar]
- Hinde RA, Matthews LH. The Nest-Building Behaviour of Domesticated Canaries. Proc. Zool. Soc. London. 1957;131:1–48. [Google Scholar]
- Hurley LL, Wallace AM, Sartor JJ, Ball GF. Photoperiodic induced changes in reproductive state of border canaries (Serinus canaria) are associated with marked variation in hypothalamic gonadotropin-releasing hormone immunoreactivity and the volume of song control regions. Gen. Comp. Endocrinol. 2008;158:10–19. doi: 10.1016/j.ygcen.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirn JR. The relationship of neurogenesis and growth of brain regions to song learning. Brain Lang. 2010;115:29–44. doi: 10.1016/j.bandl.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirn JR, Fishman Y, Sasportas K, Alvarez-Buylla a, Nottebohm F. Fate of new neurons in adult canary high vocal center during the first 30 days after their formation. J. Comp. Neurol. 1999;411:487–494. [PubMed] [Google Scholar]
- Kroodsma DE, Byers BE. The function(s) of bird song. Am. Zool. 1991;31:318–328. [Google Scholar]
- Larson TA, Wang T, Gale SD, Miller KE, Thatra NM, Caras ML, Perkel DJ, Brenowitz EA. Postsynaptic neural activity regulates neuronal addition in the adult avian song control system. Proc. Natl. Acad. Sci. U. S. A. 2013;110:16640–16644. doi: 10.1073/pnas.1310237110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrman DS, Brody PN, Wortis RP. The presence of the mate and of nesting material as stimuli for the development of incubation behavior and for gonadotropin secretion in the ring dove (Streptopelia risoria) Endocrinology. 1961;68:507–516. doi: 10.1210/endo-68-3-507. [DOI] [PubMed] [Google Scholar]
- Leitão A, ten Cate C, Riebel K. Within-song complexity in a songbird is meaningful to both male and female receivers. Anim. Behav. 2006;71:1289–1296. [Google Scholar]
- Leitner S, Catchpole CK. Syllable repertoire and the size of the song control system in captive canaries (Serinus canaria) J. Neurobiol. 2004;60:21–27. doi: 10.1002/neu.10331. [DOI] [PubMed] [Google Scholar]
- Li XC, Jarvis ED, Alvarez-Borda B, Lim Da, Nottebohm F. A relationship between behavior, neurotrophin expression, and new neuron survival. Proc. Natl. Acad. Sci. U. S. A. 2000;97:8584–8589. doi: 10.1073/pnas.140222497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AEM, MacDougall-Shackleton SA, An YS, Kriengwatana B, Soma KK. Corticosterone and dehydroepiandrosterone have opposing effects on adult neuroplasticity in the avian song control system. J. Comp. Neurol. 2010;518:3662–3678. doi: 10.1002/cne.22395. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. A Brain for All Seasons : Cyclical anatomical changes in song control nuclei of the canary brain. Science. 1981;214:1368–1370. doi: 10.1126/science.7313697. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. Birdsong as a model in which to study brain processes related to learning. Condor. 1984;86:227–236. [Google Scholar]
- Nottebohm F. A white canary on Mount Acropolis. J. Comp. Physiol. A. 1996;179:149–156. doi: 10.1007/BF00222782. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. Why are some neurons replaced in adult brain? J. Neurosci. 2002;22:624–628. doi: 10.1523/JNEUROSCI.22-03-00624.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr L, Hansell M. Effect of removal of mate on the singing behaviour of great tits. Anim. Behav. 1975;29:635–637. [Google Scholar]
- Riters LV, Eens M, Pinxten R, Duffy DL, Balthazart J, Ball GF. Seasonal changes in courtship song and the medial preoptic area in male European starlings (Sturnus vulgaris) Horm. Behav. 2000;38:250–261. doi: 10.1006/hbeh.2000.1623. [DOI] [PubMed] [Google Scholar]
- Sartor JJ, Ball GF. Social suppression of song is associated with a reduction in volume of a song-control nucleus in European starlings (Sturnus vulgaris) Behav. Neurosci. 2005;119:233–244. doi: 10.1037/0735-7044.119.1.233. [DOI] [PubMed] [Google Scholar]
- Sockman KW, Sewall KB, Ball GF, Hahn TP. Economy of mate attraction in the Cassin’s finch. Biol. Lett. 2005;1:34–37. doi: 10.1098/rsbl.2004.0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson TJ, Ball GF. Photoperiodic differences in a forebrain nucleus involved in vocal plasticity: enkephalin immunoreactivity reveals volumetric variation in song nucleus lMAN but not NIf in male European starlings (Sturnus vulgaris) Dev. Neurobiol. 2010;70:751–763. doi: 10.1002/dneu.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchernichovski O, Nottebohm F, Ho C, Pesaran B, Mitra P. A procedure for an automated measurement of song similarity. Anim. Behav. 2000;59:1167–1176. doi: 10.1006/anbe.1999.1416. [DOI] [PubMed] [Google Scholar]
- Thompson CK, Bentley GE, Brenowitz EA. Rapid seasonal-like regression of the adult avian song control system. Proc. Natl. Acad. Sci. U. S. A. 2007;104:15520–15525. doi: 10.1073/pnas.0707239104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramontin aD, Brenowitz EA. Seasonal plasticity in the adult brain. Trends Neurosci. 2000;23:251–258. doi: 10.1016/s0166-2236(00)01558-7. [DOI] [PubMed] [Google Scholar]
- Tramontin AD, Wingfield JC, Brenowitz EA. Contributions of social cues and photoperiod to seasonal plasticity in the adult avian song control system. J. Neurosci. 1999;19:476–483. doi: 10.1523/JNEUROSCI.19-01-00476.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt C, Leitner S. Seasonality in song behaviour revisited: seasonal and annual variants and invariants in the song of the domesticated canary (Serinus canaria) Horm. Behav. 2008;54:373–378. doi: 10.1016/j.yhbeh.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Yamamura T, Barker JM, Balthazart J, Ball GF. Androgens and estrogens synergistically regulate the expression of doublecortin and enhance neuronal recruitment in the song system of adult female canaries. J. Neurosci. 2011;31:9649–9657. doi: 10.1523/JNEUROSCI.0088-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]